The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children
Abstract
:1. Introduction
2. Materials and Methods
3. Results
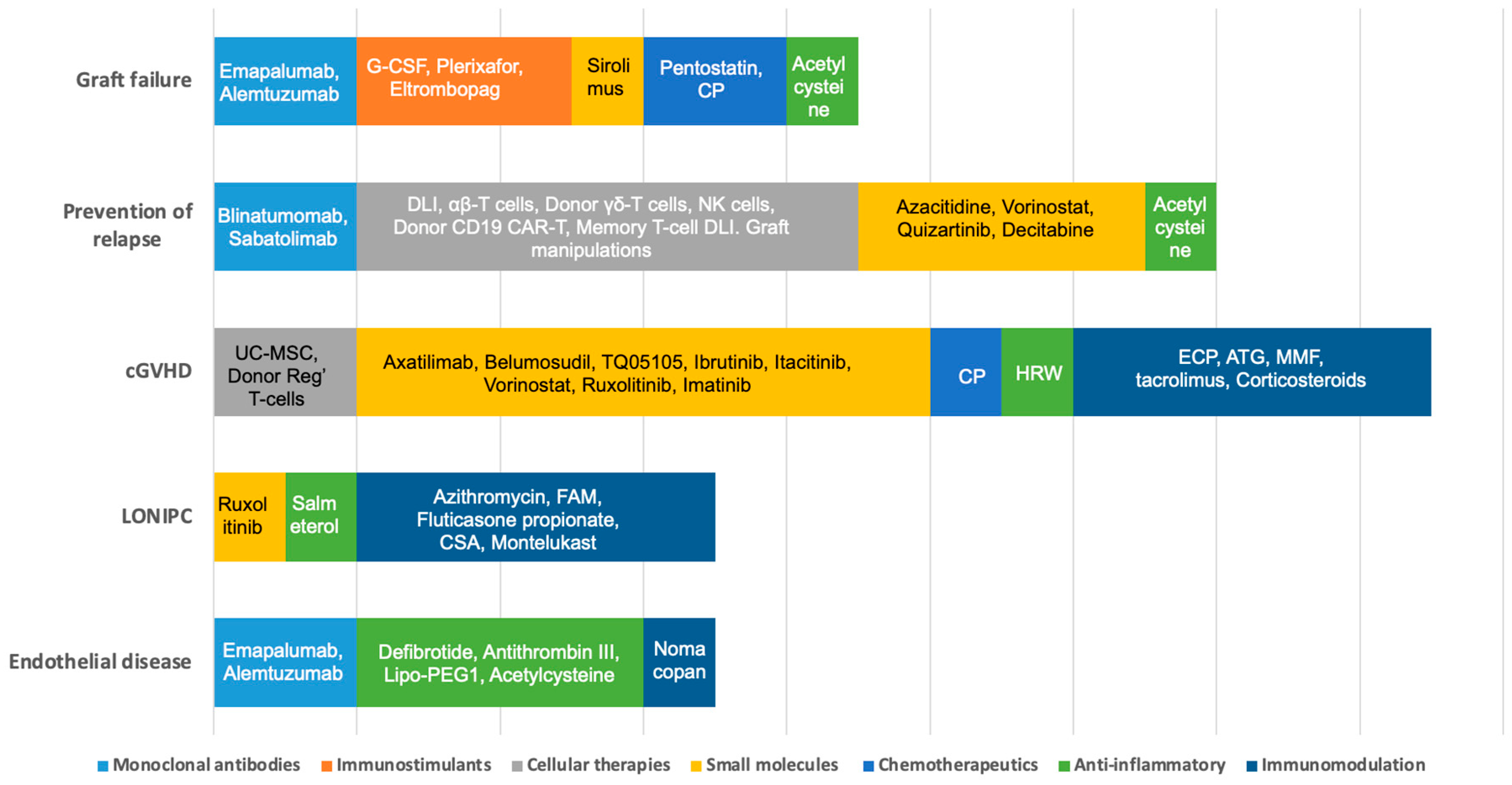
3.1. Graft Failure
3.2. Prevention of Relapse
3.2.1. Cellular Therapies
3.2.2. Hypomethylating Agents (HMA)
3.2.3. Targeted Therapy
Tyrosine Kinase Inhibition
Monoclonal Antibodies/Bispecific T-Cell Engagers
3.3. Chronic Graft vs. Host Disease (cGVHD)
3.3.1. Cellular Therapies
3.3.2. Targeted Therapies
3.3.3. Chemotherapeutics
3.3.4. Other Therapies
3.4. Non-Infectious Pulmonary Complications
3.4.1. Bronchiolitis Obliterans Syndrome (BOS)
3.4.2. Cryptogenetic Organizing Pneumonia (COP)
3.4.3. Idiopathic Pneumonia Syndrome (IPS)
3.4.4. Diffuse Alveolar Hemorrhage (DAH)
3.5. Complications of Endothelial Origin
3.5.1. Veno-Occlusive Disease (VOD) or Sinusoidal Obstruction Syndrome (SOS)
3.5.2. Engraftment Syndrome (ES)
3.5.3. Capillary Leak Syndrome (CLS)
3.5.4. Transplant-Associated Thrombotic Microangiopathies (TA-TMAs)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valcarcel, D.; Sureda, A. Graft Failure. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Carreras, E., Dufour, C., Mohty, M., Kroger, N., Eds.; Springer: Cham, Switzerland, 2019; pp. 307–313. [Google Scholar] [CrossRef]
- Olsson, R.F.; Logan, B.R.; Chaudhury, S.; Zhu, X.; Akpek, G.; Bolwell, B.J.; Bredeson, C.N.; Dvorak, C.C.; Gupta, V.; Ho, V.T.; et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia 2015, 29, 1754–1762. [Google Scholar] [CrossRef] [Green Version]
- Balashov, D.; Laberko, A.; Shcherbina, A.; Trakhtman, P.; Abramov, D.; Gutovskaya, E.; Kozlovskaya, S.; Shelikhova, L.; Novichkova, G.; Maschan, M.; et al. A Conditioning Regimen with Plerixafor Is Safe and Improves the Outcome of TCRαβ(+) and CD19(+) Cell-Depleted Stem Cell Transplantation in Patients with Wiskott-Aldrich Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Pietro, M.; Ignazio, C.; Rita De, V.; Luisa, S.; Gerrit, W.; Francesca Del, B.; Vanessa, B.; Paolo, M.; Maria Giuseppina, C.; Angela, P.; et al. Role of interferon-γ in immune-mediated graft failure after allogeneic hematopoietic stem cell transplantation. Haematologica 2019, 104, 2314–2323. [Google Scholar] [CrossRef]
- Ahmed, S.; Bashir, Q.; Bassett, R.; Poon, M.C.; Valdez, B.; Konoplev, S.; Alousi, A.M.; Andersson, B.S.; Ciurea, S.; Hosing, C.; et al. Eltrombopag for Post-Transplantation Thrombocytopenia: Results of Phase II Randomized, Double-Blind, Placebo-Controlled Trial. Transplant. Cell. Ther. 2021, 27, 430.e1–430.e7. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, Y.; Zhang, Y.Y.; Shi, M.M.; Mo, X.D.; Sun, Y.Q.; Chang, Y.J.; Xu, L.P.; Zhang, X.H.; Liu, K.Y.; et al. Prophylactic oral NAC reduced poor hematopoietic reconstitution by improving endothelial cells after haploidentical transplantation. Blood Adv. 2019, 3, 1303–1317. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Fan, Q.; Huang, F.; Zhang, Y.; Wang, Y.; Chen, X.Y.; Fan, Z.P.; Zhou, H.S.; Xiao, Y.; Xu, X.J.; et al. Mesenchymal stem cells versus mesenchymal stem cells combined with cord blood for engraftment failure after autologous hematopoietic stem cell transplantation: A pilot prospective, open-label, randomized trial. Biol. Blood Marrow Transpl. 2014, 20, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.; Thomson, K.; Russell, N.; Ewing, J.; Stewart, W.; Cook, G.; Devereux, S.; Lovell, R.; Chopra, R.; Marks, D.I.; et al. Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: A British Society of Blood and Marrow Transplantation Study. Blood 2006, 107, 1724–1730. [Google Scholar] [CrossRef]
- Lankester, A.C.; Locatelli, F.; Bader, P.; Rettinger, E.; Egeler, M.; Katewa, S.; Pulsipher, M.A.; Nierkens, S.; Schultz, K.; Handgretinger, R.; et al. Will post-transplantation cell therapies for pediatric patients become standard of care? Biol. Blood Marrow Transpl. 2015, 21, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Soiffer, R.J.; Chen, Y.-B. Pharmacologic agents to prevent and treat relapse after allogeneic hematopoietic cell transplantation. Blood Adv. 2017, 1, 2473–2482. [Google Scholar] [CrossRef] [Green Version]
- Liga, M.; Triantafyllou, E.; Tiniakou, M.; Lambropoulou, P.; Karakantza, M.; Zoumbos, N.C.; Spyridonidis, A. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol. Blood Marrow Transpl. 2013, 19, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Wahlstrom, J.T.; Horn, B.N.; Fraser-Browne, C.; Hoeweler, R.; Lu, Y.; Melton, A.; Willert, J.; Dvorak, C.C. Azacitidine Administration Following Hematopoietic Stem Cell Transplantation Is Safe and Feasible in Children with Acute Leukemia. Blood 2016, 128, 4805. [Google Scholar] [CrossRef]
- Yu, S.; Huang, F.; Fan, Z.; Xuan, L.; Nie, D.; Xu, Y.; Yang, T.; Wang, S.; Jiang, Z.; Xu, N.; et al. Haploidentical versus HLA-matched sibling transplantation for refractory acute leukemia undergoing sequential intensified conditioning followed by DLI: An analysis from two prospective data. J. Hematol. Oncol. 2020, 13, 18. [Google Scholar] [CrossRef]
- Hallett, W.H.D.; Ames, E.; Alvarez, M.; Barao, I.; Taylor, P.A.; Blazar, B.R.; Murphy, W.J. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol. Blood Marrow Transpl. 2008, 14, 1088–1099. [Google Scholar] [CrossRef] [Green Version]
- de Lima, M.; Oran, B.; Champlin, R.E.; Papadopoulos, E.B.; Giralt, S.A.; Scott, B.L.; William, B.M.; Hetzer, J.; Laille, E.; Hubbell, B.; et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol. Blood Marrow Transpl. 2018, 24, 2017–2024. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, P.A.; Snyder, D.S.; Flowers, M.E.; Sanders, J.E.; Gooley, T.A.; Martin, P.J.; Appelbaum, F.R.; Radich, J.P. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood 2007, 109, 2791–2793. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, H.; Wassmann, B.; Bethge, W.; Dengler, J.; Bornhäuser, M.; Stadler, M.; Beelen, D.; Vucinic, V.; Burmeister, T.; Stelljes, M.; et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR–ABL1-positive acute lymphoblastic leukemia. Leukemia 2013, 27, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Gaballa, M.R.; Banerjee, P.; Milton, D.R.; Jiang, X.; Ganesh, C.; Khazal, S.; Nandivada, V.; Islam, S.; Kaplan, M.; Daher, M.; et al. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia. Blood 2022, 139, 1908–1919. [Google Scholar] [CrossRef]
- Oshikawa, G.; Kakihana, K.; Saito, M.; Aoki, J.; Najima, Y.; Kobayashi, T.; Doki, N.; Sakamaki, H.; Ohashi, K. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br. J. Haematol. 2015, 169, 756–759. [Google Scholar] [CrossRef]
- Schwartz, S.; Patel, N.; Longmire, T.; Jayaraman, P.; Jiang, X.; Lu, H.; Baker, L.; Velez, J.; Ramesh, R.; Wavreille, A.S.; et al. Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor. Immunother. Adv. 2022, 2, ltac019. [Google Scholar] [CrossRef]
- Grube, M.; Holler, E.; Weber, D.; Holler, B.; Herr, W.; Wolff, D. Risk Factors and Outcome of Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation-Results from a Single-Center Observational Study. Biol. Blood Marrow Transpl. 2016, 22, 1781–1791. [Google Scholar] [CrossRef] [Green Version]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2017, 23, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Wolff, D.; Lawitschka, A. Chronic Graft-Versus-Host Disease. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Carreras, E., Dufour, C., Mohty, M., Kroger, N., Eds.; Springer: Cham, Switzerland, 2019; pp. 331–345. [Google Scholar] [CrossRef]
- Baird, K.; Cooke, K.; Schultz, K.R. Chronic graft-versus-host disease (GVHD) in children. Pediatr. Clin. N. Am. 2010, 57, 297–322. [Google Scholar] [CrossRef] [Green Version]
- Launspach, M.; Temel, D.; Ohlendorf, E.; Zirngibl, F.; Materne, B.; Oevermann, L.; Deubzer, H.E.; Henssen, A.G.; Kunkele, A.; Hundsdorfer, P.; et al. Rituximab therapy after pediatric hematopoietic stem cell transplantation can cause prolonged B cell impairment and increases the risk for infections—A retrospective matched cohort study. Haematologica 2022, 108, 267–272. [Google Scholar] [CrossRef]
- Morata-Tarifa, C.; Macias-Sanchez, M.D.M.; Gutierrez-Pizarraya, A.; Sanchez-Pernaute, R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease-a meta-analysis. Stem Cell Res. Ther. 2020, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Vadakekolathu, J.; Rutella, S. T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation. Biomedicines 2017, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.W.; Su, X.H.; Wang, M.Y.; Han, M.Z.; Feng, X.M.; Jiang, E.L. Regulatory T Cells in GVHD Therapy. Front. Immunol. 2021, 12, 697854. [Google Scholar] [CrossRef]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Miklos, D.; Abu Zaid, M.I. Ibrutinib vs Placebo in Combination with Corticosteroids in Patients with New-Onset Chronic Graft-Versus-Host Disease (Cgvhd): Results From the Randomized, Double-Blind Phase 3 Integrate Study; EHA Library: Ultimo NSW, Australia, 2021. [Google Scholar]
- Abboud, R.; Choi, J.; Ruminski, P.; Schroeder, M.A.; Kim, S.; Abboud, C.N.; DiPersio, J.F. Insights into the role of the JAK/STAT signaling pathway in graft-versus-host disease. Ther. Adv. Hematol 2020, 11, 2040620720914489. [Google Scholar] [CrossRef]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef] [Green Version]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Cutler, C.; Lee, S.J.; Arai, S.; Rotta, M.; Zoghi, B.; Lazaryan, A.; Ramakrishnan, A.; DeFilipp, Z.; Salhotra, A.; Chai-Ho, W.; et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar Study. Blood 2021, 138, 2278–2289. [Google Scholar] [CrossRef]
- Arora, M.; Jagasia, M.; Di Stasi, A.; Meyers, M.L.; Quaranto, C.; Schmitt, A.; Sankoh, S.; Abu Zaid, M.I.; Hill, G.R.; Weisdorf, D.J.; et al. Phase 1 Study of Axatilimab (SNDX-6352), a CSF-1R Humanized Antibody, for Chronic Graft-Versus-Host Disease after 2 or More Lines of Systemic Treatment. Blood 2020, 136, 1–2. [Google Scholar] [CrossRef]
- Fang, S.; Meng, X.; Zhang, Z.; Wang, Y.; Liu, Y.; You, C.; Yan, H. Vorinostat Modulates the Imbalance of T Cell Subsets, Suppresses Macrophage Activity, and Ameliorates Experimental Autoimmune Uveoretinitis. Neuromolecular Med. 2016, 18, 134–145. [Google Scholar] [CrossRef]
- Edelson, R.; Wu, Y.; Schneiderman, J. American council on ECP (ACE): Why now? J. Clin. Apher. 2018, 33, 464–468. [Google Scholar] [CrossRef]
- Bergeron, A.; Chevret, S.; Peffault de Latour, R.; Chagnon, K.; de Margerie-Mellon, C.; Rivière, F.; Robin, M.; Mani, J.; Lorillon, G.; Socié, G.; et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur. Respir. J. 2018, 51, 1702617. [Google Scholar] [CrossRef] [Green Version]
- Carreras, E.; Dufour, C.; Mohty, M.; Kroger, N. (Eds.) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef] [Green Version]
- Yanik, G.A.; Mineishi, S.; Levine, J.E.; Kitko, C.L.; White, E.S.; Vander Lugt, M.T.; Harris, A.C.; Braun, T.; Cooke, K.R. Soluble tumor necrosis factor receptor: Enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol. Blood Marrow Transpl. 2012, 18, 1044–1054. [Google Scholar] [CrossRef] [Green Version]
- Yanik, G.A.; Grupp, S.A.; Pulsipher, M.A.; Levine, J.E.; Schultz, K.R.; Wall, D.A.; Langholz, B.; Dvorak, C.C.; Alangaden, K.; Goyal, R.K.; et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol. Blood Marrow Transpl. 2015, 21, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Barker, A.F.; Bergeron, A.; Rom, W.N.; Hertz, M.I. Obliterative bronchiolitis. N. Engl. J. Med. 2014, 370, 1820–1828. [Google Scholar] [CrossRef]
- Yadav, H.; Peters, S.G.; Keogh, K.A.; Hogan, W.J.; Erwin, P.J.; West, C.P.; Kennedy, C.C. Azithromycin for the Treatment of Obliterative Bronchiolitis after Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transpl. 2016, 22, 2264–2269. [Google Scholar] [CrossRef]
- Glanville, A.R.; Benden, C.; Bergeron, A.; Cheng, G.-S.; Gottlieb, J.; Lease, E.D.; Perch, M.; Todd, J.L.; Williams, K.M.; Verleden, G.M. Bronchiolitis obliterans syndrome after lung or haematopoietic stem cell transplantation: Current management and future directions. ERJ Open Res. 2022, 8, 00185–02022. [Google Scholar] [CrossRef]
- Zeiser, R.; Polverelli, N.; Ram, R.; Hashmi, S.K.; Chakraverty, R.; Middeke, J.M.; Musso, M.; Giebel, S.; Uzay, A.; Langmuir, P.; et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2021, 385, 228–238. [Google Scholar] [CrossRef]
- Streiler, C.; Shaikh, F.; Davis, C.; Abhyankar, S.; Brownback, K.R. Ruxolitinib is an effective steroid sparing agent in bronchiolitis obliterans due to chronic graft-versus-host-disease. Bone Marrow Transpl. 2020, 55, 1194–1196. [Google Scholar] [CrossRef]
- Olivieri, J.; Coluzzi, S.; Attolico, I.; Olivieri, A. Tirosin kinase inhibitors in chronic graft versus host disease: From bench to bedside. Sci. World J. 2011, 11, 1908–1931. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, A.; Cimminiello, M.; Locatelli, F.; Zecca, M.; Corradini, P.; Patriarca, F.; Mordini, N.; Iacopino, P.; Donelli, A.; Selleri, C.; et al. Imatinib Is Safe and Effective In Patients with Refractory Chronic Graft Versus Host Disease: Analysis of Two Consecutive Prospective GITMO* Studies.*Gruppo Italiano Trapianto Midollo Osseo. Blood 2010, 116, 246. [Google Scholar] [CrossRef]
- Bergeron, A.; Chevret, S.; Chagnon, K.; Godet, C.; Bergot, E.; Peffault de Latour, R.; Dominique, S.; de Revel, T.; Juvin, K.; Maillard, N.; et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 1242–1249. [Google Scholar] [CrossRef]
- Williams, K.M.; Cheng, G.S.; Pusic, I.; Jagasia, M.; Burns, L.; Ho, V.T.; Pidala, J.; Palmer, J.; Johnston, L.; Mayer, S.; et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transpl. 2016, 22, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Athale, J.; Gormley, N.J.; Reger, R.; Alsaaty, S.; Reda, D.; Worthy, T.; Saxena, A.; Tian, X.; Childs, R.W.; Suffredini, A.F. Effect of Cyclosporine Inhalation Solution (CIS) on Lung Function and Inflammatory Biomarkers in Patients with Hematopoietic Stem Cell Transplant (HSCT) Associated Bronchiolitis Obliterans Syndrome (BOS). Blood 2019, 134, 4552. [Google Scholar] [CrossRef]
- Raghu, G.; Meyer, K.C. Cryptogenic organising pneumonia: Current understanding of an enigmatic lung disease. Eur. Respir. Rev. 2021, 30, 210094. [Google Scholar] [CrossRef]
- Cooke, K.R.; Yanik, G.A. Lung Injury Following Hematopoietic Cell Transplantation. In Thomas’ Hematopoietic Cell Transplantation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1156–1172. [Google Scholar] [CrossRef]
- Varelias, A.; Gartlan, K.H.; Kreijveld, E.; Olver, S.D.; Lor, M.; Kuns, R.D.; Lineburg, K.E.; Teal, B.E.; Raffelt, N.C.; Cheong, M.; et al. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 2015, 125, 2435–2444. [Google Scholar] [CrossRef]
- Rathi, N.K.; Tanner, A.R.; Dinh, A.; Dong, W.; Feng, L.; Ensor, J.; Wallace, S.K.; Haque, S.A.; Rondon, G.; Price, K.J.; et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transpl. 2015, 50, 420–426. [Google Scholar] [CrossRef] [Green Version]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transpl. 2018, 53, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Schechter, T.; Perez-Albuerne, E.; Lin, T.F.; Irwin, M.S.; Essa, M.; Desai, A.V.; Frangoul, H.; Yanik, G.; Dupuis, L.L.; Jacobsohn, D.; et al. Veno-occlusive disease after high-dose busulfan–melphalan in neuroblastoma. Bone Marrow Transpl. 2020, 55, 531–537. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, M.H.; Lee, H.; Kim, H.S.; Kim, K.; Kim, W.S.; Jung, C.W.; Im, Y.H.; Yoon, S.S.; Kang, W.K.; et al. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2002, 29, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, J.; Sender, L.; Secola, R.; Killen, R.; Millerick, M.; Murphy, L.; Cairo, M.S. Phase II trial of heparin prophylaxis for veno-occlusive disease of the liver in children undergoing bone marrow transplantation. Bone Marrow Transpl. 1996, 18, 185–191. [Google Scholar]
- Corbacioglu, S.; Cesaro, S.; Faraci, M.; Valteau-Couanet, D.; Gruhn, B.; Rovelli, A.; Boelens, J.J.; Hewitt, A.; Schrum, J.; Schulz, A.S.; et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: An open-label, phase 3, randomised controlled trial. Lancet 2012, 379, 1301–1309. [Google Scholar] [CrossRef]
- Richardson, P.G.; Riches, M.L.; Kernan, N.A.; Brochstein, J.A.; Mineishi, S.; Termuhlen, A.M.; Arai, S.; Grupp, S.A.; Guinan, E.C.; Martin, P.L.; et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 2016, 127, 1656–1665. [Google Scholar] [CrossRef] [Green Version]
- Grupp, S.A.; Corbacioglu, S.; Kang, H.J.; Teshima, T.; Zanette, M.; Lopez, P.; Amber, V.; Pagliuca, A.; Richardson, P.G. A Phase 3, Randomized, Adaptive Study of Defibrotide (DF) Vs Best Supportive Care (BSC) for the Prevention of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome (VOD/SOS) in Patients (pts) Undergoing Hematopoietic Cell Transplantation (HCT): Preliminary Results. Blood 2021, 138, 749. [Google Scholar] [CrossRef]
- Morris, J.D.; Harris, R.E.; Hashmi, R.; Sambrano, J.E.; Gruppo, R.A.; Becker, A.T.; Morris, C.L. Antithrombin-III for the treatment of chemotherapy-induced organ dysfunction following bone marrow transplantation. Bone Marrow Transpl. 1997, 20, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Gluckman, E.; Jolivet, I.; Scrobohaci, M.L.; Devergie, A.; Traineau, R.; Bourdeau-Espérou, H.; Lehn, P.; Faure, P.; Drouet, L. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br. J. Haematol. 1990, 74, 277–281. [Google Scholar] [CrossRef]
- Morio, S.; Oh, H.; Kogure, K.; Ishii, H.; Ishii, A.; Nakaseko, C.; Ikegami, T.; Kawano, E.; Matsuura, Y.; Nishimura, M.; et al. A trial use of prostaglandin E1 for prevention of hepatic veno-occlusive disease after allogeneic bone marrow transplantation. Rinsho Ketsueki 1994, 35, 846–852. [Google Scholar]
- Cornell, R.F.; Hari, P.; Drobyski, W.R. Engraftment Syndrome after Autologous Stem Cell Transplantation: An Update Unifying the Definition and Management Approach. Biol. Blood Marrow Transpl. 2015, 21, 2061–2068. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Sun, Z.; Liu, H.; Zhu, X.; Zhou, Y.; Fu, B.; Zheng, X.; Song, K.; Tang, B.; Wu, Y.; et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat. Commun. 2021, 12, 4137. [Google Scholar] [CrossRef]
- Lucchini, G.; Willasch, A.M.; Daniel, J.; Soerensen, J.; Jarisch, A.; Bakhtiar, S.; Rettinger, E.; Brandt, J.; Klingebiel, T.; Bader, P. Epidemiology, risk factors, and prognosis of capillary leak syndrome in pediatric recipients of stem cell transplants: A retrospective single-center cohort study. Pediatr. Transpl. 2016, 20, 1132–1136. [Google Scholar] [CrossRef]
- Yabe, H.; Yabe, M.; Koike, T.; Shimizu, T.; Morimoto, T.; Kato, S. Rapid improvement of life-threatening capillary leak syndrome after stem cell transplantation by bevacizumab. Blood 2010, 115, 2723–2724. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: A review of pathophysiology, diagnosis, and treatment. J. Blood Med. 2016, 7, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Epperla, N.; Li, A.; Logan, B.; Fretham, C.; Chhabra, S.; Aljurf, M.; Chee, L.; Copelan, E.; Freytes, C.O.; Hematti, P.; et al. Incidence, Risk Factors for and Outcomes of Transplant-Associated Thrombotic Microangiopathy. Br. J. Haematol. 2020, 189, 1171–1181. [Google Scholar] [CrossRef]
- Jodele, S.; Zhang, K.; Zou, F.; Laskin, B.; Dandoy, C.E.; Myers, K.C.; Lane, A.; Meller, J.; Medvedovic, M.; Chen, J.; et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 2016, 127, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Higham, C.S.; Shimano, K.A.; Melton, A.; Kharbanda, S.; Chu, J.; Dara, J.; Winestone, L.E.; Hermiston, M.L.; Huang, J.N.; Dvorak, C.C. A pilot trial of prophylactic defibrotide to prevent serious thrombotic microangiopathy in high-risk pediatric patients. Pediatr. Blood Cancer 2022, 69, e29641. [Google Scholar] [CrossRef]
- Jodele, S.; Dandoy, C.E.; Lane, A.; Laskin, B.L.; Teusink-Cross, A.; Myers, K.C.; Wallace, G.; Nelson, A.; Bleesing, J.; Chima, R.S.; et al. Complement blockade for TA-TMA: Lessons learned from a large pediatric cohort treated with eculizumab. Blood 2020, 135, 1049–1057. [Google Scholar] [CrossRef]
- Syed, Y.Y. Ravulizumab: A Review in Atypical Haemolytic Uraemic Syndrome. Drugs 2021, 81, 587–594. [Google Scholar] [CrossRef]
- Khaled, S.K.; Claes, K.; Goh, Y.T.; Kwong, Y.L.; Leung, N.; Mendrek, W.; Nakamura, R.; Sathar, J.; Ng, E.; Nangia, N.; et al. Narsoplimab, a Mannan-Binding Lectin-Associated Serine Protease-2 Inhibitor, for the Treatment of Adult Hematopoietic Stem-Cell Transplantation-Associated Thrombotic Microangiopathy. J. Clin. Oncol. 2022, 40, 2447–2457. [Google Scholar] [CrossRef]
- Lankester, A.C.; Albert, M.H.; Booth, C.; Gennery, A.R.; Güngör, T.; Hönig, M.; Morris, E.C.; Moshous, D.; Neven, B.; Schulz, A.; et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transpl. 2021, 56, 2052–2062. [Google Scholar] [CrossRef]
- Olsson, R.; Remberger, M.; Schaffer, M.; Berggren, D.M.; Svahn, B.M.; Mattsson, J.; Ringden, O. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transpl. 2013, 48, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Lee, J.-H.; Lee, J.-H.; Park, H.-S.; Choi, E.-J.; Kang, Y.-A.; Kang, H.; Woo, J.M.; Lee, Y.-S.; Jeon, M.; et al. Incidence, Management, and Prognosis of Graft Failure and Autologous Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. J. Korean Med. Sci. 2021, 36, e151. [Google Scholar] [CrossRef]
- Pasvolsky, O.; Shimony, S.; Yeshurun, M.; Shargian, L.; Wolach, O.; Raanani, P.; Gafter-Gvili, A.; Gurion, R. Maintenance therapy after allogeneic hematopoietic transplant for acute myeloid leukemia: A systematic review and meta-analysis. Acta Oncol. 2021, 60, 1335–1341. [Google Scholar] [CrossRef]
- Barone, A.; Casey, D.; McKee, A.E.; Reaman, G. Cancer drugs approved for use in children: Impact of legislative initiatives and future opportunities. Pediatr. Blood Cancer 2019, 66, e27809. [Google Scholar] [CrossRef]
- Neel, D.V.; Shulman, D.S.; DuBois, S.G. Timing of first-in-child trials of FDA-approved oncology drugs. Eur. J. Cancer 2019, 112, 49–56. [Google Scholar] [CrossRef]
- Blackmon, A.; Aldoss, I.; Ball, B.J. FLT3 Inhibitors as Maintenance Therapy after Allogeneic Stem-Cell Transplantation. Blood Lymphat. Cancer 2022, 12, 137–147. [Google Scholar] [CrossRef]

| Disease of Interest (If Specified) | Ages | Phase | Investigational Agent(s) | Intervention | Trial Number | Estimated Enrollment (Number of Participants) | Status | |
|---|---|---|---|---|---|---|---|---|
| Graft failure | ||||||||
| Sickle cell disease and β-thalassemia | 4 Years and Older | Phase 2 | Alemtuzumab, low dose radiation, oral cyclophosphamide, pentostatin, and sirolimus | N/A | NCT02105766 | 162 | Active, recruiting | |
| All hematological diseases | 3 Years to 70 Years | Phase 2 | Cyclophosphamide (CP) | Post-transplant preventive CP at 50 mg/kg/day at D + 3 and D + 4 | NCT05126186 | 35 | Active, recruiting | |
| Chronic granulomatous disease | 1 Month to 24 Years | Phase 2 | Plerixafor/G-CSF | Both agents are administered before transplant | NCT03547830 | 17 | Active, recruiting | |
| Non-malignant diseases, leukemia, and lymphoma | 1 Year and Older | Phase 2 | Emapalumab | Given IV from day 0 every 3–4 days for 15 doses or engraftment evidence at 6 mg/kg (first dose) and 3 mg/kg (subsequent doses) | NCT04731298 | 250 | Terminated | |
| Leukemia and patients with poor graft function criteria | 6 Years and Older | Phase 2 | Eltrombopag | Eltrombopag OD at 50 mg/day starting not earlier than D60 post-HSCT. In the absence of response, dose can be increased up to 150 mg/day in blocks of 50 mg | NCT03948529 | 25 | Active, recruiting | |
| Acute leukemia patients | 15 Years to 60 Years | Phase 2 | *N-acetyl-L-cysteine | N-acetyl-L-cysteine given orally at dosages of 400 mg three times per day from 14 days pre-allotransplant to 2 months after allotransplant | NCT03236220 | 35 | Completed | |
| Prevention of relapse including early interventions after detection of MRD positivity following HSCT in ALL and AML | ||||||||
| Cellular therapy | ||||||||
| ALL, AML | 10 Years to 65 Years | Phase 1 | Donor γδT cell infusion | One infusion of 0.5 × 106–8 × 107 γδT/kg | NCT04439721 | 5 | Active, recruiting | |
| AML, MDS, JMML | 1 Year and Older | Phase 1 | Cytokine-induced memory-like natural killer (CIML-NK) cells | CIML-NK given IV on day 0 with IL-2, three doses of fludarabine from day −5 to −3 and two doses CP on day −4 and −4 | NCT04024761 | 50 | Active, recruiting | |
| CD19 positive B-ALL/B-CLL/NHL | All | Phase 1 | CD19/CD28 CAR T cells derived from donor | Maximum of six doses, 4 to 6 weeks apart, of CAR-T | NCT02050347 | 40 | Active, recruiting | |
| Leukemia, lymphoma, and myeloproliferative diseases | Up to 21 Years | Phase 2 | * TCRαβ-depleted progenitor cell graft with additional memory T cell DLI and blinatumomab | CD45RA-depleted DLI two weeks after engraftment of TCRα/β+ and CD19+-depleted, for CD19 pos; Blinatumomab will be given at least one-week post-DLI | NCT03849651 | 140 | Active, recruiting | |
| ALL, AML, JMML, MDS | Up to 29 Years | Phase 2 | Azacitidine and DLI | Up to seven cycles of low dose azacitidine (40 mg/m2 IV/SC daily × 4 days) at six weekly intervals and additional cycles according to risk levels | NCT02458235 | 17 | Completed | |
| Acute leukemia | 14 Years to 65 Years | Phase 2/3 | DLI | DLI administered at a median dose of 1.0 (range 0.7–1.4) × 108 mononuclear cells/kg once by day +60 and further based on MRD and GVHD status | NCT02673008 | 206 | Completed | |
| Hypomethylating agents (HMA) | ||||||||
| AML, MDS, JMML, MPAL | 1 Year to 21 Years | Phase 1 | Vorinostat/azacitidine | Two cycles of standard post-transplant azacitidine followed by vorinostat orally | NCT03843528 | 15 | Active, recruiting | |
| AML, MDS | 1 Year to 75 Years | Phase 2 | Low dose of azacitidine | Azacitidine at 32 mg/m2 SC for 5 days every 28 days. At 60–120 days post-T-cell depletion of allo-HSCT | NCT01995578 | 32 | Active, not recruiting | |
| All hematological diseases | 10 Years to 70 Years | Phase 3 | Decitabine and acetylcysteine | Decitabine (20 mg/m2/d) on days −10 to −8 and acetylcysteine from day −10 to +365 | NCT04945096 | 100 | Active, not yet recruiting | |
| Targeted therapy | ||||||||
| AML | 12 Years to 99 Years | Phase 1/2 | Sabatolimab | Sabatolimab will be given every 4 weeks in combination with azacitidine | NCT04623216 | 59 | Active, recruiting | |
| AML FLT3 ITD | 1 Month to 21 Years | Phase 1/2 | * Quizartinib | Quizartinib once daily starting on day 6 and continuing through to day 28 | NCT03793478 | 65 | Active, recruiting | |
| B-ALL | Up to 25 Years | Phase 2 | αβ T cell- and B cell-depleted allogeneic hematopoietic cell transplantation (HCT) followed by blinatumomab | Twenty-eight-day continuous infusion of blinatumomab starting on day 100 post-transplant | NCT04746209 | 25 | Active, recruiting | |
| Leukemia, lymphoma and myeloproliferative diseases | Up to 21 Years | Phase 2 | TCRαβ- and CD45RA-depleted haploidentical donor progenitor cell transplantation followed by post-HSCT Blinatumomab | Continuous IV blinatumomab infusion at least 2 weeks post-engraftment for CD19-positive patients | NCT02790515 | 52 | Active, recruiting | |
| B-ALL | 6 Months to 21 Years | Phase 2 | * Blinatumomab | Blinatumomab over a 28-day cycle. MRD-positive patients before HSCT start between day +60–+100 and for patients, who become MRD positive post-HSCT between day +60–+360 | NCT04785547 | 32 | Active, recruiting | |
| B-ALL | 1 Year and Older | Phase 2 | Blinatumomab | Blinatumomab for 6 weeks (4 weeks followed by a 2-week treatment-free period) for up to four cycles | NCT04044560 | 8 | Terminated | |
| Chronic GVHD | ||||||||
| Cellular therapy | ||||||||
| All | Phase 1 | Donor regulatory T cells | CD25hi regulatory T cells from CD8 and/or CD19 pre-depleted leukapheresis products will be given in three dose levels | NCT03683498 | 16 | Completed | ||
| 14 Years to 70 Years | Phase 1/2 | Umbilical cord mesenchymal stem cells | N/A | NCT05152160 | 10 | Active, recruiting | ||
| All | Phase 2 | Donor regulatory T cells | 2 × 106 cells/kg dose of regulatory T cell-enriched infusion | NCT05095649 | 15 | Active, recruiting | ||
| Targeted therapy | ||||||||
| Up to 18 Years | Phase 1 | Ruxolitinib | Ruxolitinib BID daily | NCT05121142 | 28 | Active, recruiting | ||
| 1 Year to 21 Years | Phase 1/2 | Ibrutinib | Ibrutinib orally once daily | NCT03790332 | 59 | Active, not recruiting | ||
| ALL, AML, CML, MDS, mature B-cell malignancies | 3 Years to 39 Years | Phase 1/2 | Vorinostat | Vorinostat at 30, 45, or 60 mg/m2 BID orally from day −10 days, until day +30 post-transplant.Haploidentical BMT recipients: same intervention but starting from day +5 | NCT03842696 | 49 | Active, recruiting | |
| 6 Years and Older | Phase 1/2 | Axatilimab (SNDX-6352) | SNDX-6352 IV will be given at a dose of 0.15–3 mg/kg | NCT03604692 | 40 | Active, not recruiting | ||
| 12 Years and Older | Phase1/2 | TQ05105 | TQ05105 10 mg given orally, twice daily in 28 day-cycle | NCT04944043 | 97 | Active, recruiting | ||
| 28 Days to 18 Years | Phase 2 | Ruxolitinib | Ruxolitinib 5 mg BID | NCT03774082 | 46 | Active, not recruiting | ||
| ALL, AML, CML, MDS, myelofibrosis | Up to 80 Years | Phase 2 | Itacitinib | CP IV QD on days 3 and 4, itacitinib PO QD on days 5–100, and tacrolimus IV or PO on days 6–65 | NCT05364762 | 50 | Active, not yet recruiting | |
| 12 Years and Older | Phase 2 | Belumosudil | Belumosudil 200 mg once or twice daily according to randomization | NCT03640481 | 175 | Active, recruiting | ||
| 12 Years and Older | Phase 2 | Belumosudil | Belumosudil orally, OD, or BID (if taking CYP3A4 inhibitors or proton pump inhibitors) | NCT05567406 | 12 | Active, not yet recruiting | ||
| 2 Years and Older | Phase 2 | Axatilimab (SNDX- 6352) | Axatilimab 0.3–3 mg/kg IV every 2 weeks for up to 2 years | NCT04710576 | 210 | Active, not recruiting | ||
| Up to 21 Years | Phase 2 | Mycophenolate mofetil (MMF) and imatinib | MMF 15–20 mg/kg BID and imatinib QD 260 mg/m2/d | NCT01898377 | 9 | Terminated | ||
| 12 Years and Older | Phase 3 | Ibrutinib in combination with corticosteroids | Ibrutinib 420 mg is given orally OD starting on day 1 until cGVHD progression; in addition, 1 mg/kg/d prednisone OD until unacceptable toxicity or until participant is successfully tapered from the prednisone | NCT02959944 | 193 | Completed | ||
| Chemotherapeutics | ||||||||
| AML, MDS | 16 Years to 70 Years | Phase 2 | Cyclophosphamide | ATG, 4.5 mg/kg IV on days −2, −1, and +1 and CP at 50 mg/kg IV daily on days +3 and +4 vs. ATG alone | NCT04202835 | 80 | Active, recruiting | |
| ALL, AML, CML, MDS, CLL, Lymphoma | 5 Years to 75 Years | Phase 2 | Tacrolimus, high dose cyclophosphamide, and MMF | CP on days 3–4, mycophenolate mofetil TID on day 5 and stopping on day 35 if no severe GVHD is present. Tacrolimus IV continuously on days 5–180 with a taper beginning on day 90 in the absence of disease progression or unacceptable toxicity | NCT03128359 | 38 | Active, not recruiting | |
| Other | ||||||||
| All | Phase 2 | Extracorporeal photopheresis | Six cycles of extracorporeal photopheresis every 2 weeks | NCT03083574 | 100 | Active, recruiting | ||
| up to 65 Years | Phase 2 | Hydrogen-rich water | Hydrogen-rich water 4 mL/kg orally TID one day | NCT02918188 | 21 | Active, recruiting | ||
| Noninfectious pulmonary complications | ||||||||
| 5 Years to 25 Years | Phase 2 | Ruxolitinib | Ruxolitinib orally twice daily for 24 weeks plus standard fluticasone/montelukast and steroids | NCT04908735 | 40 | Active, recruiting | ||
| 6 Years to 99 Years | Phase 2 | Fluticasone propionate, azithromycin, and montelukast sodium (FAM) | Fluticasone propionate inhaled PO BID, azithromycin PO 3 days a week, and montelukast sodium PO QD for 6 months | NCT01307462 | 36 | Completed | ||
| 10 Years to 80 Years | Phase 2 | Cyclosporine inhalation solution | Cyclosporine inhalation solution (CIS) 150 mg three times weekly during weeks 1–5. Dose escalated to 300 mg three times weekly from weeks 6–8 until week 19 | NCT01287078 | 25 | Completed | ||
| 6 Years to 17 Years | Phase 3 | Fluticasone propionate and salmeterol | Inhaled fluticasone propionate 50 or 125 μg and 25 μg salmeterol, BID from randomization and until 6 months | NCT04655508 | 243 | Active, recruiting | ||
| Complications of endothelial origin | ||||||||
| VOD/SOS | ||||||||
| up to 65 Years | Phase 2 | Antithrombin-III | AT-III at units required (IU)/kg = 50 + [(desired-baseline AT-III level) × weight (kg)/1.4] | NCT01886248 | 32 | Active, not recruiting | ||
| All | Phase 2/3 | Lipoprostaglandin E1 | Dose of 1.5 mcg/kg/day, continuous infusion | NCT02338440 | 30 | Active, not recruiting | ||
| 1 Month and Older | Phase 3 | Defibrotide | Defibrotide 25 mg/kg/day IV in addition to best supportive care, day before the first day of the conditioning for a recommended minimum of 21 days | NCT02851407 | 372 | Completed | ||
| TA-TMA | ||||||||
| All | Phase 2 | Eculizumab | Eculizumab IV for 24 weeks | NCT03518203 | 23 | Active, recruiting | ||
| up to 30 Years | Phase 2 | Defibrotide | Defibrotide 6.25 mg/kg administered intravenously for 28–35 days | NCT03384693 | 25 | Completed | ||
| All | Phase 3 | N-Acetylcysteine | 50 mg/kg orally | NCT03252925 | 170 | Completed | ||
| 12 Years and Older | Phase 3 | Ravulizumab | Weight-based doses of ravulizumab will be administered intravenously as loading dose regimen followed by maintenance dosing every 8 weeks plus best supportive care (BSC) vs. placebo | NCT04543591 | 184 | Active, not recruiting | ||
| 28 Days to 17 Years | Phase 3 | Ravulizumab | Weight-based doses of ravulizumab administered IV as a loading dose regimen followed by maintenance every 4 or 8 weeks plus best supportive care | NCT04557735 | 40 | Active, recruiting | ||
| 6 Months to 18 Years | Phase 3 | Nomacopan | NA | NCT04784455 | 50 | Active, recruiting | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilan, U.; Brivio, E.; Algeri, M.; Balduzzi, A.; Gonzalez-Vincent, M.; Locatelli, F.; Zwaan, C.M.; Baruchel, A.; Lindemans, C.; Bautista, F. The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children. J. Clin. Med. 2023, 12, 2149. https://doi.org/10.3390/jcm12062149
Ilan U, Brivio E, Algeri M, Balduzzi A, Gonzalez-Vincent M, Locatelli F, Zwaan CM, Baruchel A, Lindemans C, Bautista F. The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children. Journal of Clinical Medicine. 2023; 12(6):2149. https://doi.org/10.3390/jcm12062149
Chicago/Turabian StyleIlan, Uri, Erica Brivio, Mattia Algeri, Adriana Balduzzi, Marta Gonzalez-Vincent, Franco Locatelli, Christian Michel Zwaan, Andre Baruchel, Caroline Lindemans, and Francisco Bautista. 2023. "The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children" Journal of Clinical Medicine 12, no. 6: 2149. https://doi.org/10.3390/jcm12062149
APA StyleIlan, U., Brivio, E., Algeri, M., Balduzzi, A., Gonzalez-Vincent, M., Locatelli, F., Zwaan, C. M., Baruchel, A., Lindemans, C., & Bautista, F. (2023). The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children. Journal of Clinical Medicine, 12(6), 2149. https://doi.org/10.3390/jcm12062149







