Targeting Peripheral N-Methyl-D-Aspartate Receptor (NMDAR): A Novel Strategy for the Treatment of Migraine
Abstract
:1. Introduction
2. Methods
3. Ion Channels in Migraine Pathophysiology
4. Glutamate and Migraine
5. N-Methyl-D-Aspartate Receptor (NMDAR)
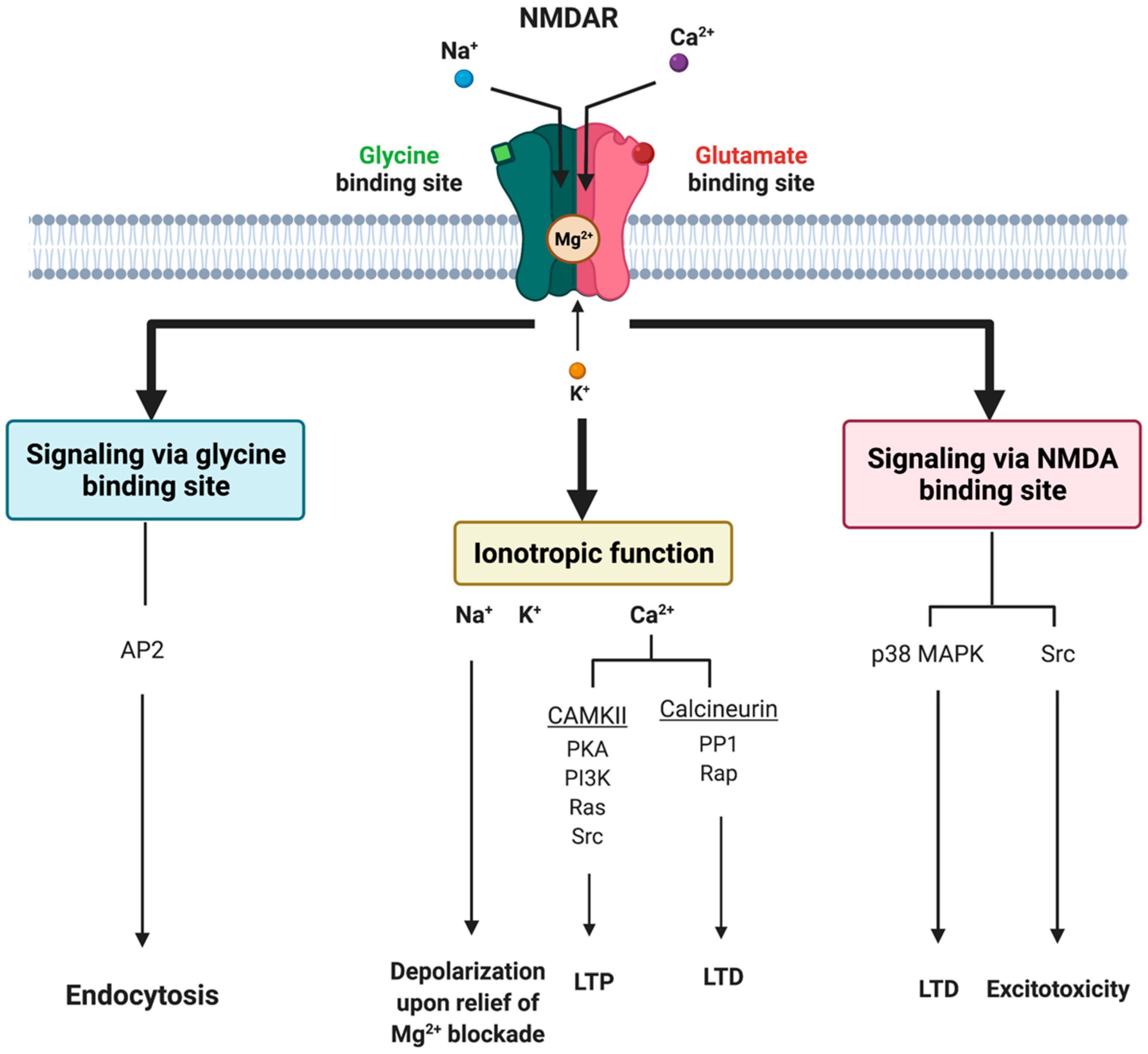
5.1. Evidence Implicating NMDAR in Migraine
5.2. NMDAR Antagonists in Preclinical Studies
- Glutamate N-Methyl-D-Aspartate Receptor (NMDAR) is expressed in nerve fibers innervating dural blood vessels. Glutamate transporter (excitatory amino acid transporter-2 (EAAT-2)) is expressed in dural blood vessels [48].
- In rats, intravenous administration of 50 mg/kg monosodium glutamate (MSG) activated and sensitized trigeminovascular neurons associated with dural vasodilation. Co-administration of a peripherally restricted NMDAR antagonist (2R)-amino-5-phosphonovaleric (APV) attenuated MSG-induced effects [53].
- Intraperitoneal injections of 1000 mg/kg MSG caused headache-like and nausea symptoms in association with an increase in plasma calcitonin-gene related peptide (CGRP) concentration. MSG-induced headache-like symptoms were inhibited by APV and partly attenuated by olcegepant, a selective CGRP receptor antagonist [64].
- Administration of dizocilpine maleate, a NDMAR antagonist, and GYKI 52466, a non-NMDAR antagonist, resulted in a substantial showed blockade of neuronal firing and inhibition of trigeminovascular-evoked responses in the trigeminovascular complex in cats [47].
5.3. NMDAR Antagonists in Clinical Studies
- Genome-wide association studies have shown significant polymorphisms in glutamate receptor and transporter genes that are associated with migraine [82].
- Consumption of a single dose (150 mg/kg) of monosodium glutamate (MSG) caused headache, craniofacial sensitivity, and nausea in healthy participants [33].
- Repeated MSG intake (150 mg/kg) in five daily sessions for one week reduced pressure pain thresholds and caused headaches in healthy participants compared to placebo [34].
6. Perspective and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPAR | Alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor |
| CSD | Cortical spreading depression |
| KAR | Kainate receptor |
| LTP | Long-term potentiation |
| LTD | Long-term depression |
| NMDAR | N-Methyl-D-Aspartate Receptor |
| NTD | N-terminus domain |
| TG | Trigeminal ganglion |
| TVS | Trigeminovascular system |
| TMD | Transmembrane domain |
| TNC | Trigeminal nucleus caudalis |
References
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S.; et al. Migraine: Epidemiology and systems of care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Eikermann-Haerter, K.; Ayata, C. Cortical spreading depression and migraine. Curr. Neurol. Neurosci. Rep. 2010, 10, 167–173. [Google Scholar] [CrossRef]
- Holland, P.R.; Akerman, S.; Goadsby, P.J. Cortical spreading depression-associated cerebral blood flow changes induced by mechanical stimulation are modulated by AMPA and GABA receptors. Cephalalgia 2010, 30, 519–527. [Google Scholar] [CrossRef]
- Pietrobon, D. Ion channels in Migraine disorders. Curr. Opin. Physiol. 2018, 2, 98–108. [Google Scholar] [CrossRef]
- Olesen, J.; Friberg, L.; Olsen, T.S.; Iversen, H.K.; Lassen, N.A.; Andersen, A.R.; Karle, A. Timing and topography of cerebral blood flow, aura, and headache during migraine attacks. Ann. Neurol. 1990, 28, 791–798. [Google Scholar] [CrossRef]
- Olesen, J.; Larsen, B.; Lauritzen, M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann. Neurol. 1981, 9, 344–352. [Google Scholar] [CrossRef]
- Lee, M.J.; Al-Karagholi, M.A.; Reuter, U. New migraine prophylactic drugs: Current evidence and practical suggestions for non-responders to prior therapy. Cephalalgia 2023, 43, 3331024221146315. [Google Scholar] [CrossRef]
- Vikelis, M.; Mitsikostas, D.D. The role of glutamate and its receptors in migraine. CNS Neurol. Disord. Drug Targets 2007, 6, 251–257. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Odink, J.; Bos, K.D.; Malessy, M.; Bruyn, G.W. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology 1990, 40, 1582–1586. [Google Scholar] [CrossRef]
- Lauritzen, M. Spreading depression and migraine. Pathol. Biol. 1992, 40, 332–337. [Google Scholar] [PubMed]
- Tallaksen-Greene, S.J.; Young, A.B.; Penney, J.B.; Beitz, A.J. Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci. Lett. 1992, 141, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, Z.; Coombes, N.; Waring, R.H.; Williams, A.C.; Steventon, G.B. Plasma levels of neuroexcitatory amino acids in patients with migraine or tension headache. J. Neurol. Sci. 1998, 156, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; Salter, M.W. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008, 7, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Waung, M.W.; Akerman, S.; Wakefield, M.; Keywood, C.; Goadsby, P.J. Metabotropic glutamate receptor 5: A target for migraine therapy. Ann. Clin. Transl. Neurol. 2016, 3, 560–571. [Google Scholar] [CrossRef]
- Chan, K.; MaassenVanDenBrink, A. Glutamate receptor antagonists in the management of migraine. Drugs 2014, 74, 1165–1176. [Google Scholar] [CrossRef]
- Mayberg, M.; Langer, R.S.; Zervas, N.T.; Moskowitz, M.A. Perivascular meningeal projections from cat trigeminal ganglia: Possible pathway for vascular headaches in man. Science 1981, 213, 228–230. [Google Scholar] [CrossRef]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef]
- Schulte, L.H.; Peng, K.P. Current understanding of premonitory networks in migraine: A window to attack generation. Cephalalgia 2019, 39, 1720–1727. [Google Scholar] [CrossRef]
- Hutchings, C.J.; Colussi, P.; Clark, T.G. Ion channels as therapeutic antibody targets. MAbs 2019, 11, 265–296. [Google Scholar] [CrossRef]
- Wickenden, A.; Priest, B.; Erdemli, G. Ion channel drug discovery: Challenges and future directions. Future Med. Chem. 2012, 4, 661–679. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Dussor, G. Ion channels and migraine. Headache 2014, 54, 619–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrobon, D. Calcium channels and migraine. Biochim. Biophys. Acta 2013, 1828, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D. Migraine. Lancet 1998, 351, 1043–1051. [Google Scholar] [CrossRef]
- Fabricius, M.; Jensen, L.H.; Lauritzen, M. Microdialysis of interstitial amino acids during spreading depression and anoxic depolarization in rat neocortex. Brain Res. 1993, 612, 61–69. [Google Scholar] [CrossRef]
- Molchanova, S.; Kööbi, P.; Oja, S.S.; Saransaari, P. Interstitial concentrations of amino acids in the rat striatum during global forebrain ischemia and potassium-evoked spreading depression. Neurochem. Res. 2004, 29, 1519–1527. [Google Scholar] [CrossRef]
- Lauritzen, M.; Hansen, A.J. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J. Cereb. Blood Flow Metab. 1992, 12, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Ayata, C.; Lauritzen, M. Spreading Depression, Spreading Depolarizations, and the Cerebral Vasculature. Physiol. Rev. 2015, 95, 953–993. [Google Scholar] [CrossRef] [Green Version]
- Marrannes, R.; Willems, R.; De Prins, E.; Wauquier, A. Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res. 1988, 457, 226–240. [Google Scholar] [CrossRef]
- D’Andrea, G.; Cananzi, A.R.; Joseph, R.; Morra, M.; Zamberlan, F.; Milone, F.F.; Grunfeld, S.; Welch, K. Platelet glycine, glutamate and aspartate in primary headache. Cephalalgia 1991, 11, 197–200. [Google Scholar] [CrossRef]
- Cananzi, A.R.; D’Andrea, G.; Perini, F.; Zamberlan, F.; Welch, K. Platelet and plasma levels of glutamate and glutamine in migraine with and without aura. Cephalalgia 1995, 15, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Spaccapelo, L.; Pinetti, D.; Tacchi, R.; Bertolini, A. Effective prophylactic treatments of migraine lower plasma glutamate levels. Cephalalgia 2009, 29, 423–429. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; Cairns, B.; Ernberg, M.; Svensson, P. Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalalgia 2010, 30, 68–76. [Google Scholar] [CrossRef]
- Shimada, A.; Cairns, B.E.; Vad, N.; Ulriksen, K.; Pedersen, A.M.L.; Svensson, P.; Baad-Hansen, L. Headache and mechanical sensitization of human pericranial muscles after repeated intake of monosodium glutamate (MSG). J. Headache Pain 2013, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.; Kong, X.; Han, J.; Zhao, G. N-methyl-D-aspartate receptor antagonists for migraine: A potential therapeutic approach. Med. Hypotheses 2009, 72, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef]
- Hoffmann, J.; Storer, R.J.; Park, J.W.; Goadsby, P.J. N-Methyl-d-aspartate receptor open-channel blockers memantine and magnesium modulate nociceptive trigeminovascular neurotransmission in rats. Eur. J. Neurosci. 2019, 50, 2847–2859. [Google Scholar] [CrossRef]
- Kleckner, N.W.; Dingledine, R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988, 241, 835–837. [Google Scholar] [CrossRef]
- Ciabarra, A.M.; Sullivan, J.M.; Gahn, L.G.; Pecht, G.; Heinemann, S.; Sevarino, K. Cloning and characterization of chi-1: A developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J. Neurosci. 1995, 15, 6498–6508. [Google Scholar] [CrossRef]
- Nishi, M.; Hinds, H.; Lu, H.P.; Kawata, M.; Hayashi, Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J. Neurosci. 2001, 21, Rc185. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Kamiya, Y.; Matsuda, S.; Yuzaki, M. Cloning and characterization of a novel NMDA receptor subunit NR3B: A dominant subunit that reduces calcium permeability. Brain Res. Mol. Brain Res. 2002, 100, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Low, C.M.; Wee, K.S. New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: Localization, structure, and function. Mol. Pharmacol. 2010, 78, 1–11. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rajani, V.; Sengar, A.S.; Salter, M.W. Tripartite signalling by NMDA receptors. Mol. Brain 2020, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Sucher, N.J.; Akbarian, S.; Chi, C.L.; Leclerc, C.L.; Awobuluyi, M.; Deitcher, D.; Wu, M.K.; Yuan, J.P.; Jones, E.G.; A Lipton, S. Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J. Neurosci. 1995, 15, 6509–6520. [Google Scholar] [CrossRef] [Green Version]
- Storer, R.J.; Goadsby, P.J. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience 1999, 90, 1371–1376. [Google Scholar] [CrossRef]
- Shin, H.E.; Han, S.J.; Lee, K.S.; Park, J.-W. Polymorphism of the Glutamate Transporter Protein EAAT2 and Migraine Transformation into Chronic Daily Headache. J. Clin. Neurol. 2011, 7, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.D.; Mann, M.K.; Kumar, U.; Svensson, P.; Arendt-Nielsen, L.; Hu, J.; Sessle, B.; Cairns, B. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience 2007, 146, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Ro, J.Y. Differential regulation of glutamate receptors in trigeminal ganglia following masseter inflammation. Neurosci. Lett. 2007, 421, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Ivanusic, J.J.; Beaini, D.; Hatch, R.J.; Staikopoulosl, V.; Sesslel, B.; Jenningsl, E.; Ivanusic, J.J.; Beaini, D.; Hatch, R.J.; Staikopoulos, V.; et al. Peripheral N-methyl-d-aspartate receptors contribute to mechanical hypersensitivity in a rat model of inflammatory temporomandibular joint pain. Eur. J. Pain 2011, 15, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Montoya, J.; Buendia, I.; Martin, Y.B.; Egea, J.; Negredo, P.; Avendaño, C. Sensory Input-Dependent Changes in Glutamatergic Neurotransmission- Related Genes and Proteins in the Adult Rat Trigeminal Ganglion. Front. Mol. Neurosci. 2016, 9, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, M.; Cairns, B.E. Monosodium glutamate alters the response properties of rat trigeminovascular neurons through activation of peripheral NMDA receptors. Neuroscience 2016, 334, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Toro, C.; Koroleva, K.; Ermakova, E.; Gafurov, O.; Abushik, P.; Tavi, P.; Sitdikova, G.; Giniatullin, R. Testing the Role of Glutamate NMDA Receptors in Peripheral Trigeminal Nociception Implicated in Migraine Pain. Int. J. Mol. Sci. 2022, 23, 1529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Mokha, S.S. Opioids modulate N-methyl-D-aspartic acid (NMDA)-evoked responses of trigeminothalamic neurons. J. Neurophysiol. 1996, 76, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Richter, J.A.; Hurley, J.H. Release of glutamate and CGRP from trigeminal ganglion neurons: Role of calcium channels and 5-HT1 receptor signaling. Mol. Pain 2008, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Mészáros, M.; Phan, T.H.M.; Vigh, J.P.; Porkoláb, G.; Kocsis, A.; Páli, E.K.; Polgár, T.F.; Walter, F.R.; Bolognin, S.; Schwamborn, J.C.; et al. Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood-Brain Barrier Model and Entry to Human Brain Organoids. Cells 2023, 12, 503. [Google Scholar] [CrossRef]
- Paoletti, P.; Ascher, P.; Neyton, J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J. Neurosci. 1997, 17, 5711–5725. [Google Scholar] [CrossRef] [Green Version]
- Faerber, L.; Drechsler, S.; Ladenburger, S.; Gschaidmeier, H.; Fischer, W. The neuronal 5-HT3 receptor network after 20 years of research—Evolving concepts in management of pain and inflammation. Eur. J. Pharmacol. 2007, 560, 1–8. [Google Scholar] [CrossRef]
- Shatillo, A.; Salo, R.A.; Giniatullin, R.; Gröhn, O.H. Involvement of NMDA receptor subtypes in cortical spreading depression in rats assessed by fMRI. Neuropharmacology 2015, 93, 164–170. [Google Scholar] [CrossRef]
- Lauritzen, M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994, 117 Pt 1, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeters, M.; Gunthorpe, M.J.; Strijbos, P.J.; Goldsmith, P.; Upton, N.; James, M.F. Effects of pan- and subtype-selective N-methyl-D-aspartate receptor antagonists on cortical spreading depression in the rat: Therapeutic potential for migraine. J. Pharmacol. Exp. Ther. 2007, 321, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, L.C.; Mody, I. Protective effect of ifenprodil against spreading depression in the mouse entorhinal cortex. J. Neurophysiol. 2004, 92, 2610–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benbow, T.; Teja, F.; Sheikhi, A.; Exposto, F.G.; Svensson, P.; Cairns, B.E. Peripheral N-methyl-D-aspartate receptor activation contributes to monosodium glutamate-induced headache but not nausea behaviours in rats. Sci. Rep. 2022, 12, 13894. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Flippen, C.; Romero Reyes, M.; Brennan, K.C. Memantine for prevention of migraine: A retrospective study of 60 cases. J. Headache Pain 2007, 8, 248–250. [Google Scholar] [CrossRef] [Green Version]
- Dolati, S.; Rikhtegar, R.; Mehdizadeh, A.; Yousefi, M. The Role of Magnesium in Pathophysiology and Migraine Treatment. Biol. Trace Elem. Res. 2020, 196, 375–383. [Google Scholar] [CrossRef]
- Baratloo, A.; Mirbaha, S.; Delavar Kasmaei, H.; Payandemehr, P.; Elmaraezy, A.; Negida, A. Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J. Pain 2017, 30, 176–182. [Google Scholar] [CrossRef]
- Taubert, K. Magnesium in migraine. Results of a multicenter pilot study. Fortschr. Med. 1994, 112, 328–330. [Google Scholar]
- Shin, H.J.; Na, H.S.; Do, S.H. Magnesium and Pain. Nutrients 2020, 12, 2184. [Google Scholar] [CrossRef]
- Nicolodi, M.; Sicuteri, F. Exploration of NMDA receptors in migraine: Therapeutic and theoretic implications. Int. J. Clin. Pharmacol. Res. 1995, 15, 181–189. [Google Scholar]
- Kaube, H.; Herzog, J.; Kaufer, T.; Dichgans, M.; Diener, H.C. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology 2000, 55, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Afridi, S.K.; Giffin, N.J.; Kaube, H.; Goadsby, P. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. Neurology 2013, 80, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, C.; Mazuera, S.; Lipton, R.B.; Ashina, S. Intravenous ketamine for subacute treatment of refractory chronic migraine: A case series. J. Headache Pain 2016, 17, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etchison, A.R.; Bos, L.; Ray, M.; McAllister, K.B.; Mohammed, M.; Park, B.; Phan, A.V.; Heitz, C. Low-dose Ketamine Does Not Improve Migraine in the Emergency Department: A Randomized Placebo-controlled Trial. West J. Emerg. Med. 2018, 19, 952–960. [Google Scholar] [CrossRef]
- Bigal, M.; Rapoport, A.; Sheftell, F.; Tepper, D.; Tepper, S. Memantine in the preventive treatment of refractory migraine. Headache 2008, 48, 1337–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noruzzadeh, R.; Modabbernia, A.; Aghamollaii, V.; Ghaffarpour, M.; Harirchian, M.H.; Salahi, S.; Nikbakht, N.; Noruzi, N.; Tafakhori, A. Memantine for Prophylactic Treatment of Migraine Without Aura: A Randomized Double-Blind Placebo-Controlled Study. Headache 2016, 56, 95–103. [Google Scholar] [CrossRef]
- Gomez-Mancilla, B.; Brand, R.; Jurgens, T.P.; Göbel, H.; Sommer, C.; Straube, A.; Evers, S.; Sommer, M.; Campos, V.; O Kalkman, H.; et al. Randomized, multicenter trial to assess the efficacy, safety and tolerability of a single dose of a novel AMPA receptor antagonist BGG492 for the treatment of acute migraine attacks. Cephalalgia 2014, 34, 103–113. [Google Scholar] [CrossRef]
- Oskoui, M.; Pringsheim, T.; Holler-Managan, Y.; Potrebic, S.; Billinghurst, L.; Gloss, D.; Hershey, A.D.; Licking, N.; Sowell, M.; Victorio, M.C.; et al. Practice guideline update summary: Acute treatment of migraine in children and adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019, 93, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Ailani, J.; Burch, R.C.; Robbins, M.S. The American Headache Society Consensus Statement: Update on integrating new migraine treatments into clinical practice. Headache J. Head Face Pain 2021, 61, 1021–1039. [Google Scholar] [CrossRef]
- Antonaci, F.; Dumitrache, C.; De Cillis, I.; Allena, M. A review of current European treatment guidelines for migraine. J. Headache Pain 2010, 11, 13–19. [Google Scholar] [CrossRef] [Green Version]
- AHS-First-Contact-PreventativeTreatment; American Headache Society: Mount Royal, NJ, USA, 2021.
- Hautakangas, H.; Winsvold, B.S.; Ruotsalainen, S.E.; Bjornsdottir, G.; Harder, A.V.E.; Kogelman, L.J.A.; Thomas, L.F.; Noordam, R.; Benner, C.; Gormley, P.; et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 2022, 54, 152–160. [Google Scholar] [CrossRef] [PubMed]
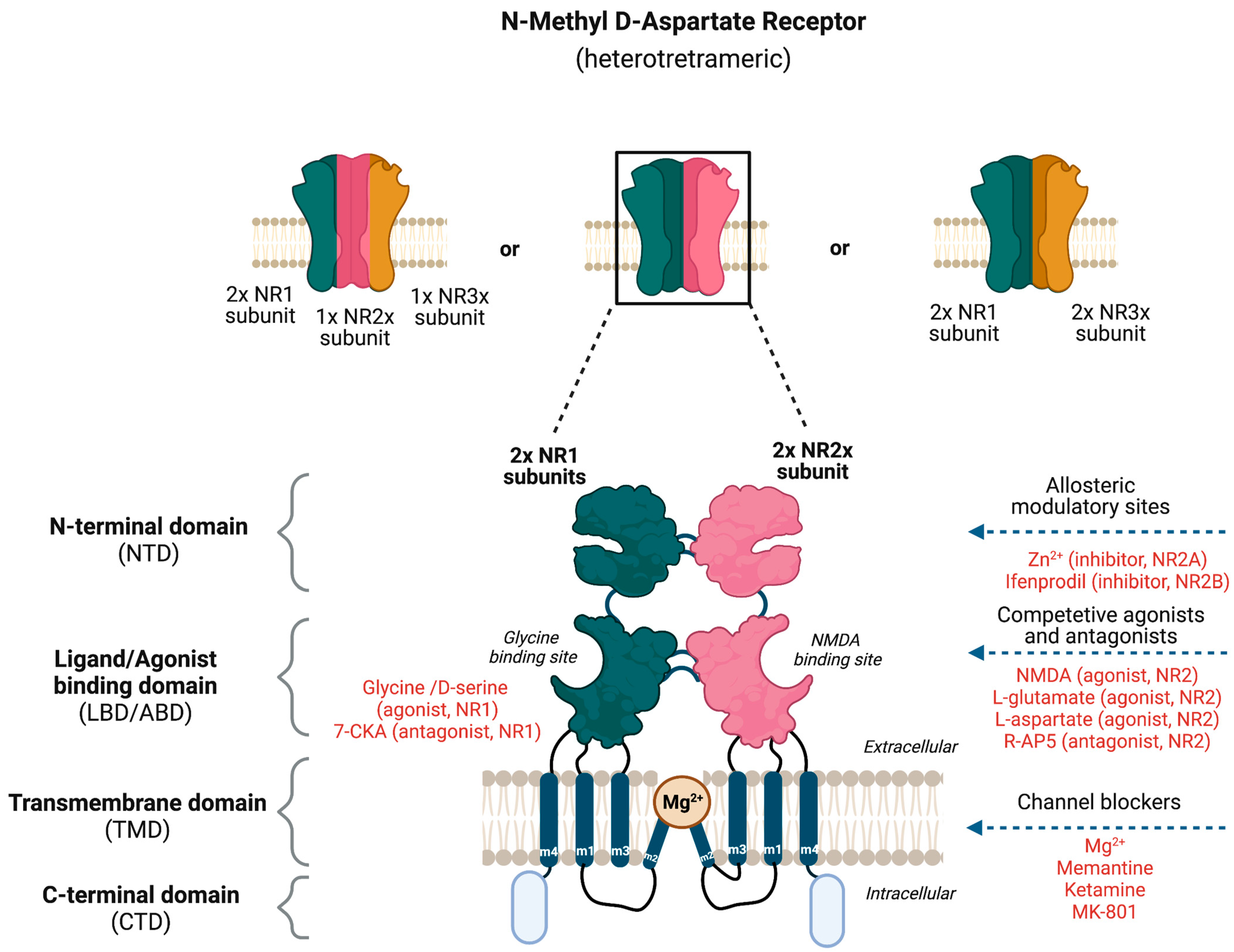
| NMDAR Subtype | Predominant Localization | References |
|---|---|---|
| NR1 | Present throughout CNS. | [9] |
| NR2A | Thalamus; prominently in the lateral thalamic nuclei. | [9] |
| NR2B | Thalamus. | [9] |
| NR2C | High expression in cerebellum, low elsewhere. | [9] |
| NR2D | High expression early during development, low expression in adults. | [9] |
| NR3A | Spinal cord, thalamus, hypothalamus, brainstem, hippocampus (CA1), amygdala, and certain parts of cortex cerebri. | [40,46] |
| NR3B | In adults: expressed in somatic motor neurons in the brainstem and spinal cord, cerebellum, and hippocampus. The distribution of NR3B seems to be as ubiquitous as NR1. | [40,41,42,47] |
| NMDAR Antagonist | Author and Year | Study Design | Number of Participants | Dose (Administration Form) | Type of migraine (With or Without Aura) | Findings |
|---|---|---|---|---|---|---|
| Magnesium | Baratloo A, et. al. (2017) [67] | Prospective quasi-experimental study | Total n = 70 Completed study n = 70 | 2 g Magnesium Sulfate (intravenous) | Individuals with migraine (Aura not reported) | Intravenous magnesium sulfate (2g) might be superior to intravenous caffeine (60 mg) for the short-term management of migraine headaches. |
| Magnesium | K. Taubert, et. al. (1994) [68] | Double-blinded, cross-over multicenter pilot study | Total n = 43 Completed study n = 43 | 600 mg/day Trimagnesium Dieitrate (oral) | Individuals with migraine (Aura not reported) | Significant reduction in the incidence of migraine attacks was observed. |
| Ketamine | Nicolodi et. al. (1995) [70] | Randomized double- blinded, cross-over study | Total n = 34 Completed study n = 34 | 80 µg/kg Ketamine hydrochloride (subcutaneous) | Individuals with migraine (Aura not reported) | Compared to the placebo group, Individuals in the ketamine group experienced a marked relief of pain both as an acute and prophylactic treatment. |
| Ketamine | Kaube et. al. (2000) [71] | Open label study | Total n = 11 Completed study n = 11 | 25 mg (Intranasal) | Familial hemiplegic migraine | 5 out of 11 participants experienced a reduction in severity and duration of migraine attacks after ketamine administration, whereas 6 out of 11 participants had no beneficial effect. |
| Ketamine | Afridi et. al. (2013) [72] | Randomized double-blinded placebo-controlled trial | Total n = 30 Completed study n = 18 | 25 mg (Intranasal) | Individuals with migraine (With aura) | Intranasal ketamine is effective at reducing aura severity in individuals with prolonged aura. |
| Ketamine | Lauritsen et. al. (2016) [73] | Retrospective study | Total n = 6 Completed study n = 6 | 0.1 mg/kg (Intravenous) | Refractory migraine | 6 out of 6 participants obtained the target pain relief endpoint after infusion of ketamine. The mean ketamine infusion rate at the time of pain relief was 0,34 mg/kg/hour. |
| Ketamine | Etchinson et. al. (2018) [74] | Randomized double-blinded placebo-controlled trial | Total n = 34 Completed study n = 34 | 0.2 mg/kg (Intravenous) | Individuals with migraine (With and without aura) | The difference in NRS pain scores was neither statistically nor clinically significant between ketamine group (n = 16) and placebo group (n = 18). |
| Memantine | Charles et. al. (2007) [65] | Retrospective study | Total n = 60 Completed study n = 54 | 5 mg to 20 mg/day (Peroral) | Individuals with migraine (With and without aura) | 67% reported a greater than 50% reduction in estimated monthly headache frequency. |
| Memantine | Bigal et. al. (2008) [75] | Prospective, open label study | Total n = 38 Completed study n = 23 | 10 mg to 20 mg/day (Peroral) | Refractory migraine | A significant reduction in headache frequency, severity, and MIDAS score was observed after 3 months of treatment. |
| Memantine | Noruzzadeh et. al. (2016) [76] | Randomized double-blinded placebo-controlled trial | Total n = 52 Completed study n = 52 | 10 mg/day (Peroral) | Individuals with migraine (Without aura) | Individuals in the memantine group (n = 25) showed significantly greater reduction in monthly headache attack frequency and headache severity than the placebo group (n = 27). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalatharan, V.; Al-Karagholi, M.A.-M. Targeting Peripheral N-Methyl-D-Aspartate Receptor (NMDAR): A Novel Strategy for the Treatment of Migraine. J. Clin. Med. 2023, 12, 2156. https://doi.org/10.3390/jcm12062156
Kalatharan V, Al-Karagholi MA-M. Targeting Peripheral N-Methyl-D-Aspartate Receptor (NMDAR): A Novel Strategy for the Treatment of Migraine. Journal of Clinical Medicine. 2023; 12(6):2156. https://doi.org/10.3390/jcm12062156
Chicago/Turabian StyleKalatharan, Veberka, and Mohammad Al-Mahdi Al-Karagholi. 2023. "Targeting Peripheral N-Methyl-D-Aspartate Receptor (NMDAR): A Novel Strategy for the Treatment of Migraine" Journal of Clinical Medicine 12, no. 6: 2156. https://doi.org/10.3390/jcm12062156
APA StyleKalatharan, V., & Al-Karagholi, M. A.-M. (2023). Targeting Peripheral N-Methyl-D-Aspartate Receptor (NMDAR): A Novel Strategy for the Treatment of Migraine. Journal of Clinical Medicine, 12(6), 2156. https://doi.org/10.3390/jcm12062156






