EUS-Guided Vascular Interventions
Abstract
:1. Introduction
2. EUS-Guided Vascular Interventions
2.1. Diagnostic Applications
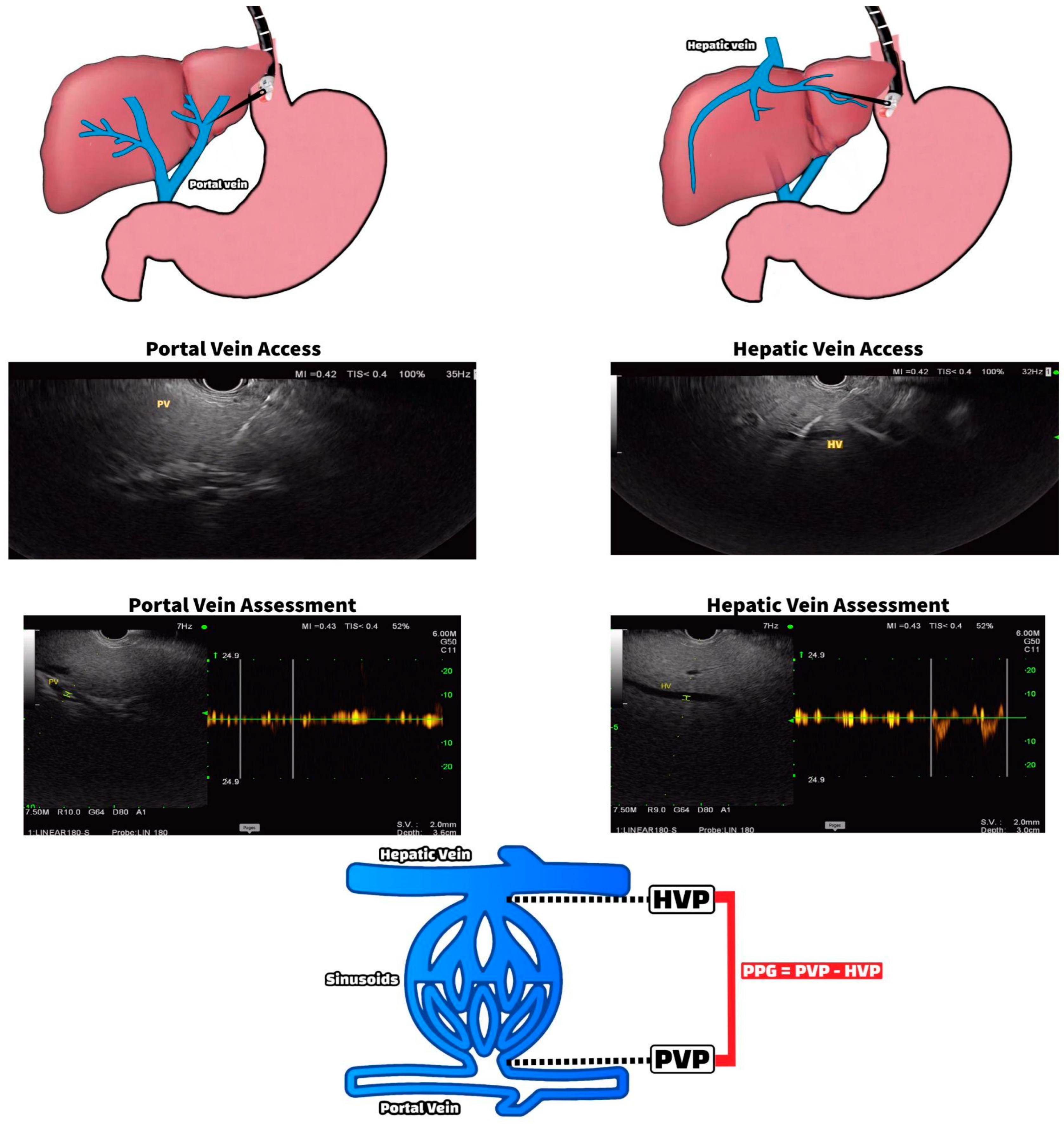
2.1.1. EUS-Guided Portal Pressure Measurement
2.1.2. EUS-Guided Portal Venous Sampling
2.2. Therapeutic Applications
2.2.1. EUS-Guided Gastric Variceal Coiling
2.2.2. EUS-Guided Arterial Pseudoaneurysm Coiling
2.3. Future Directions
2.3.1. EUS-Guided Liver Tumor Ablation
2.3.2. EUS-Guided Intrahepatic Portosystemic Shunt Placement
2.3.3. EUS-Guided Cardiac Interventions and Thrombolysis
2.4. Limitations and Complications
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- DiMagno, E.P.; DiMagno, M.J. Endoscopic Ultrasonography: From the Origins to Routine EUS. Dig. Dis. Sci. 2015, 61, 342–353. [Google Scholar] [CrossRef]
- Burmester, E.; Tiede, U. Longitudinal Endoscopic Ultrasound–Anatomical Guiding Structures in the Upper Abdomen (Cranial–Right). Video J. Encycl. GI Endosc. 2013, 1, 501–504. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Trebicka, J. Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy. JHEP Rep. 2021, 3, 100316. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Abraldes, J.G.; Berzigotti, A.; García-Pagan, J.C. The clinical use of HVPG measurements in chronic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Pomier-Layrargues, G.M.D.; Kusielewicz, D.; Willems, B.; Villeneuve, J.; Marleau, D.; Cǒté, J.; Huet, P. Presinusoidal portal hypertension in non-alcoholic cirrhosis. Hepatology 1985, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Bazarbashi, A.N.; Ryou, M. Portal pressure measurement: Have we come full circle? Gastrointest. Endosc. 2021, 93, 573–576. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, C.; Zhang, S.; Huang, S.; Shen, S.; Xu, G.; Zhang, F.; Xiao, J.; Zhang, M.; Zhuge, Y.; et al. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest. Endosc. 2020, 93, 565–572. [Google Scholar] [CrossRef]
- Huang, J.Y.; Samarasena, J.B.; Tsujino, T.; Chang, K.J. EUS-guided portal pressure gradient measurement with a novel 25-gauge needle device versus standard transjugular approach: A comparison animal study. Gastrointest. Endosc. 2016, 84, 358–362. [Google Scholar] [CrossRef]
- Huang, J.Y.; Samarasena, J.B.; Tsujino, T.; Lee, J.; Hu, K.-Q.; McLaren, C.E.; Chen, W.-P.; Chang, K.J. EUS-guided portal pressure gradient measurement with a simple novel device: A human pilot study. Gastrointest. Endosc. 2016, 85, 996–1001. [Google Scholar] [CrossRef] [Green Version]
- Samarasena, J.B.; Huang, J.Y.; Tsujino, T.; Thieu, D.; Yu, A.; Hu, K.Q.; Lee, J.; Chang, K.J. EUS-guided portal pressure gradient measurement with a simple novel device: A human pilot study. VideoGIE 2018, 3, 361–363. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.Y.; Kolb, J.; Shah, S.; Chahine, A.; Hashimoto, R.; Patel, A.; Tsujino, T.; Huang, J.; Hu, K.; Chang, K.; et al. Endoscopic ultrasound-guided portal pressure gradient with liver biopsy: 6 years of endo-hepatology in practice. J. Gastroenterol. Hepatol. 2022, 37, 1373–1379. [Google Scholar] [CrossRef]
- Yeo, D.; Bastian, A.; Strauss, H.; Saxena, P.; Grimison, P.; Rasko, J.E.J. Exploring the Clinical Utility of Pancreatic Cancer Circulating Tumor Cells. Int. J. Mol. Sci. 2022, 23, 1671. [Google Scholar] [CrossRef]
- Bissolati, M.; Sandri, M.T.; Burtulo, G.; Zorzino, L.; Balzano, G.; Braga, M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumor Biol. 2015, 36, 991–996. [Google Scholar] [CrossRef]
- Tien, Y.W.; Kuo, H.C.; Ho, B.I.; Chang, M.C.; Chang, Y.T.; Cheng, M.F.; Chen, H.L.; Liang, T.Y.; Wang, C.F.; Huang, C.Y. A High Circulating Tumor Cell Count in Portal Vein Predicts Liver Metastasis from Periampullary or Pancreatic Cancer: A High Portal Venous CTC Count Predicts Liver Metastases. Medicine 2016, 95, e3407. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Chapman, C.G.; Xu, P.; Koons, A.; Konda, V.J.; Siddiqui, U.D.; Waxman, I. Acquisition of Portal Venous Circulating Tumor Cells from Patients with Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology 2015, 149, 1794–1803.e4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Su, H.; Wang, H.; Xu, C.; Zhou, S.; Zhao, J.; Shen, S.; Xu, G.; Wang, L.; Zou, X.; et al. Endoscopic Ultrasound-Guided Acquisition of Portal Venous Circulating Tumor Cells as a Potential Diagnostic and Prognostic Tool for Pancreatic Cancer. Cancer Manag. Res. 2021, 13, 7649–7661. [Google Scholar] [CrossRef]
- Thiruvengadam, S.S.; Sedarat, A. The Role of Endoscopic Ultrasound (EUS) in the Management of Gastric Varices. Curr. Gastroenterol. Rep. 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Bazarbashi, A.N.; Ryou, M. Gastric variceal bleeding. Curr. Opin. Gastroenterol. 2019, 35, 524–534. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, A. Gastric varices: Profile, classification, and management. Am. J. Gastroenterol. 1989, 84, 1244–1249. [Google Scholar]
- Iwase, H.; Maeda, O.; Shimada, M.; Tsuzuki, T.; Peek, R.M., Jr.; Nishio, Y.; Ando, T.; Ina, K.; Kusugami, K. Endoscopic ablation with cyanoacrylate glue for isolated gastric variceal bleeding. Gastrointest. Endosc. 2001, 53, 585–592. [Google Scholar] [CrossRef]
- Cheng, L.-F.; Wang, Z.-Q.; Li, C.-Z.; Cai, F.-C.; Huang, Q.-Y.; Linghu, E.-Q.; Li, W.; Chai, G.-J.; Sun, G.-H.; Mao, Y.-P.; et al. Treatment of gastric varices by endoscopic sclerotherapy using butyl cyanoacrylate: 10 years’ experience of 635 cases. Chin. Med. J. 2007, 120, 2081–2085. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.C.; Neumann, H.; Olano, C.; Malfertheiner, P.; Mönkemüller, K. Efficacy, complications and clinical outcomes of endoscopic sclerotherapy with N-butyl-2-cyanoacrylate for bleeding gastric varices. Dig. Dis. 2008, 26, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Robles-Medranda, C.; Valero, M.; Nebel, J.A.; de Britto, S.R., Jr.; Puga-Tejada, M.; Ospina, J.; Muñoz-Jurado, G.; Pitanga-Lukashok, H. Endoscopic-ultrasound-guided coil and cyanoacrylate embolization for gastric varices and the roles of endoscopic Doppler and endosonographic varicealography in vascular targeting. Dig. Endosc. 2019, 31, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.M.; Weilert, F.; Fredrick, R.T.; Kane, S.D.; Shah, J.N.; Hamerski, C.M.; Binmoeller, K.F. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: A large U.S. experience over 6 years (with video). Gastrointest. Endosc. 2016, 83, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Bazarbashi, A.N.; Wang, T.J.; Jirapinyo, P.; Thompson, C.C.; Ryou, M. Endoscopic Ultrasound-Guided Coil Embolization with Absorbable Gelatin Sponge Appears Superior to Traditional Cyanoacrylate Injection for the Treatment of Gastric Varices. Clin. Transl. Gastroenterol. 2020, 11, e00175. [Google Scholar] [CrossRef]
- Maharshi, S.; Sharma, S.S.; Sharma, D.; Sapra, B.; Nijhawan, S. Endoscopic ultrasound-guided thrombin injection, a management approach for visceral artery pseudoaneurysms. Endosc. Int. Open 2020, 8, E407–E412. [Google Scholar] [CrossRef] [Green Version]
- Bokun, T.; Grgurevic, I.; Kujundzic, M.; Banic, M. EUS-Guided Vascular Procedures: A Literature Review. Gastroenterol. Res. Pract. 2013, 2013, 865945. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.; Kc, H.; Goel, A.; Aggarwal, R.; Sharma, M. Endoscopic ultrasound-guided coil and glue for treatment of splenic artery pseudo-aneurysm: New kid on the block! Endosc. Int. Open 2018, 6, E821–E825. [Google Scholar] [CrossRef] [Green Version]
- Gamanagatti, S.; Thingujam, U.; Garg, P.; Nongthombam, S.; Dash, N.R. Endoscopic ultrasound guided thrombin injection of angiographically occult pancreatitis associated visceral artery pseudoaneurysms: Case series. World J. Gastrointest. Endosc. 2015, 7, 1107–1113. [Google Scholar] [CrossRef]
- Villa, E.; Melitas, C.; Ibrahim Naga, Y.M.; Pandhi, M.; Shah, K.; Boulay, B. Endoscopic ultrasound-guided embolization of refractory splenic pseudoaneurysm. VideoGIE 2022, 7, 331–333. [Google Scholar] [CrossRef]
- Sharma, M.; Jindal, S.; Somani, P.; Basnet, B.K.; Bansal, R. EUS-guided coiling of hepatic artery pseudoaneurysm in 2 stages. Videogie 2017, 2, 262–263. [Google Scholar] [CrossRef]
- Romero-Castro, R.; Rios-Martin, J.J.; Jimenez-Garcia, V.A.; Pellicer-Bautista, F.; Hergueta-Delgado, P. EUS-FNA of 2 right atrial masses. Videogie 2019, 4, 323–324. [Google Scholar] [CrossRef] [Green Version]
- Somani, P.; Talele, R.; Sharma, M. Endoscopic Ultrasound-Guided Thrombolysis of Pulmonary Artery Thrombus and Mesenteric Vein Thrombus. Am. J. Gastroenterol. 2019, 114, 379. [Google Scholar] [CrossRef]
- Tekola, B.D.; Arner, D.M.; Behm, B.W. Coil Migration after Transarterial Coil Embolization of a Splenic Artery Pseudoaneurysm. Case Rep. Gastroenterol. 2013, 7, 487–491. [Google Scholar] [CrossRef]
- Wallace, M.B.; Sabbagh, L.C. EUS 2008 Working Group document: Evaluation of EUS-guided tumor ablation. Gastrointest. Endosc. 2009, 69, S59–S63. [Google Scholar] [CrossRef]
- Okasha, H.H.; Farouk, M.; El Hendawy, R.I.; Mahmoud, R.M.; El-Meligui, A.; Atalla, H.; Hashim, A.M.; Pawlak, K.M. Practical approach to linear EUS examination of the liver. Endosc. Ultrasound 2021, 10, 161–167. [Google Scholar] [CrossRef]
- Chua, T.; Faigel, D.O. Endoscopic Ultrasound-Guided Ablation of Liver Tumors. Gastrointest. Endosc. Clin. N. Am. 2019, 29, 369–379. [Google Scholar] [CrossRef]
- Chang, K.J.; Irisawa, A. EUS 2008 Working Group document: Evaluation of EUS-guided injection therapy for tumors. Gastrointest. Endosc. 2009, 69, S54–S58. [Google Scholar] [CrossRef]
- Boyer, T.D.; Haskal, Z.J.; American Association for the Study of Liver Diseases. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005, 41, 386–400. [Google Scholar] [CrossRef]
- Colombato, L. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension. J. Clin. Gastroenterol. 2007, 41, S344–S351. [Google Scholar] [CrossRef]
- Buscaglia, J.M.; Dray, X.; Shin, E.J.; Magno, P.; Chmura, K.M.; Surti, V.C.; Dillon, T.E.; Ducharme, R.W.; Donatelli, G.; Thuluvath, P.J.; et al. A new alternative for a transjugular intrahepatic portosystemic shunt: EUS-guided creation of an intrahepatic portosystemic shunt (with video). Gastrointest. Endosc. 2009, 69, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Binmoeller, K.F.; Shah, J.N. Sa1428 EUS-Guided Transgastric Intrahepatic Portosystemic Shunt Using the Axios Stent. Gastrointest. Endosc. 2011, 73, AB167. [Google Scholar] [CrossRef]
- Schulman, A.R.; Ryou, M.; Aihara, H.; Abidi, W.; Chiang, A.; Jirapinyo, P.; Sakr, A.; Ajeje, E.; Ryan, M.B.; Thompson, C.C. EUS-guided intrahepatic portosystemic shunt with direct portal pressure measurements: A novel alternative to transjugular intrahepatic portosystemic shunting. Gastrointest. Endosc. 2017, 85, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritscher-Ravens, A.; Ganbari, A.; Mosse, C.A.; Swain, P.; Koehler, P.; Patel, K. Transesophageal endoscopic ultra-sound-guided access to the heart. Endoscopy 2007, 39, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Stobinski, M.; Galasso, D.; Amato, A.; Familiari, P.; Costamagna, G. EUS-guided drainage of a pericardial cyst: Closer to the heart (with video). Gastrointest. Endosc. 2009, 70, 1273–1274. [Google Scholar] [CrossRef]
- Mehta, N.; Joseph, A.; Harb, S.; Kapadia, S.; Bhatt, A. EUS–guided biopsy of an intraventricular mass in a patient with ventricular tachycardia. Videogie 2022, 7, 322–323. [Google Scholar] [CrossRef]
- Sharma, M.; Somani, P.; Jindal, S. EUS-Guided Continuous Catheter Thrombolysis of Portal Venous System: 1614. Am. J. Gastroenterol. 2017, 112, S877–S879. [Google Scholar] [CrossRef]
- Sharma, M.; Somani, P.; Jindal, S. EUS-Guided Continuous Catheter Thrombolysis of Portal Venous System. Am. J. Gastroenterol. 2019, 89, AB605. [Google Scholar] [CrossRef]





| EUS-Guided Vascular Interventions | |
|---|---|
| Category | Intervention |
| Diagnostic | Portal pressure measurement |
| Portal venous sampling | |
| Therapeutic | Gastric variceal coiling |
| Arterial pseudoaneurysm coiling | |
| Future directions | Liver tumor ablation |
| Intrahepatic portosystemic shunt placement | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliss, M.; Patel, D.; Madi, M.Y.; Bazarbashi, A.N. EUS-Guided Vascular Interventions. J. Clin. Med. 2023, 12, 2165. https://doi.org/10.3390/jcm12062165
Baliss M, Patel D, Madi MY, Bazarbashi AN. EUS-Guided Vascular Interventions. Journal of Clinical Medicine. 2023; 12(6):2165. https://doi.org/10.3390/jcm12062165
Chicago/Turabian StyleBaliss, Michelle, Devan Patel, Mahmoud Y. Madi, and Ahmad Najdat Bazarbashi. 2023. "EUS-Guided Vascular Interventions" Journal of Clinical Medicine 12, no. 6: 2165. https://doi.org/10.3390/jcm12062165
APA StyleBaliss, M., Patel, D., Madi, M. Y., & Bazarbashi, A. N. (2023). EUS-Guided Vascular Interventions. Journal of Clinical Medicine, 12(6), 2165. https://doi.org/10.3390/jcm12062165





