Epicardial Adipose Tissue: A Piece of The Puzzle in Pediatric Hypertension
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Assessment
2.3. Imaging Acquisition
2.4. Image Analysis
2.4.1. Assessment of the EAT
2.4.2. Reproducibility
2.5. Statistical Analysis
3. Results
3.1. Study Population, Clinical Assessment and Body Composition
3.2. Parameters of Cardiac Morphology and Function
3.3. Intra-Observer Reproducibility
3.4. Associations of EAT Volume with Anthropometric, Morphologic and Functional Parameters
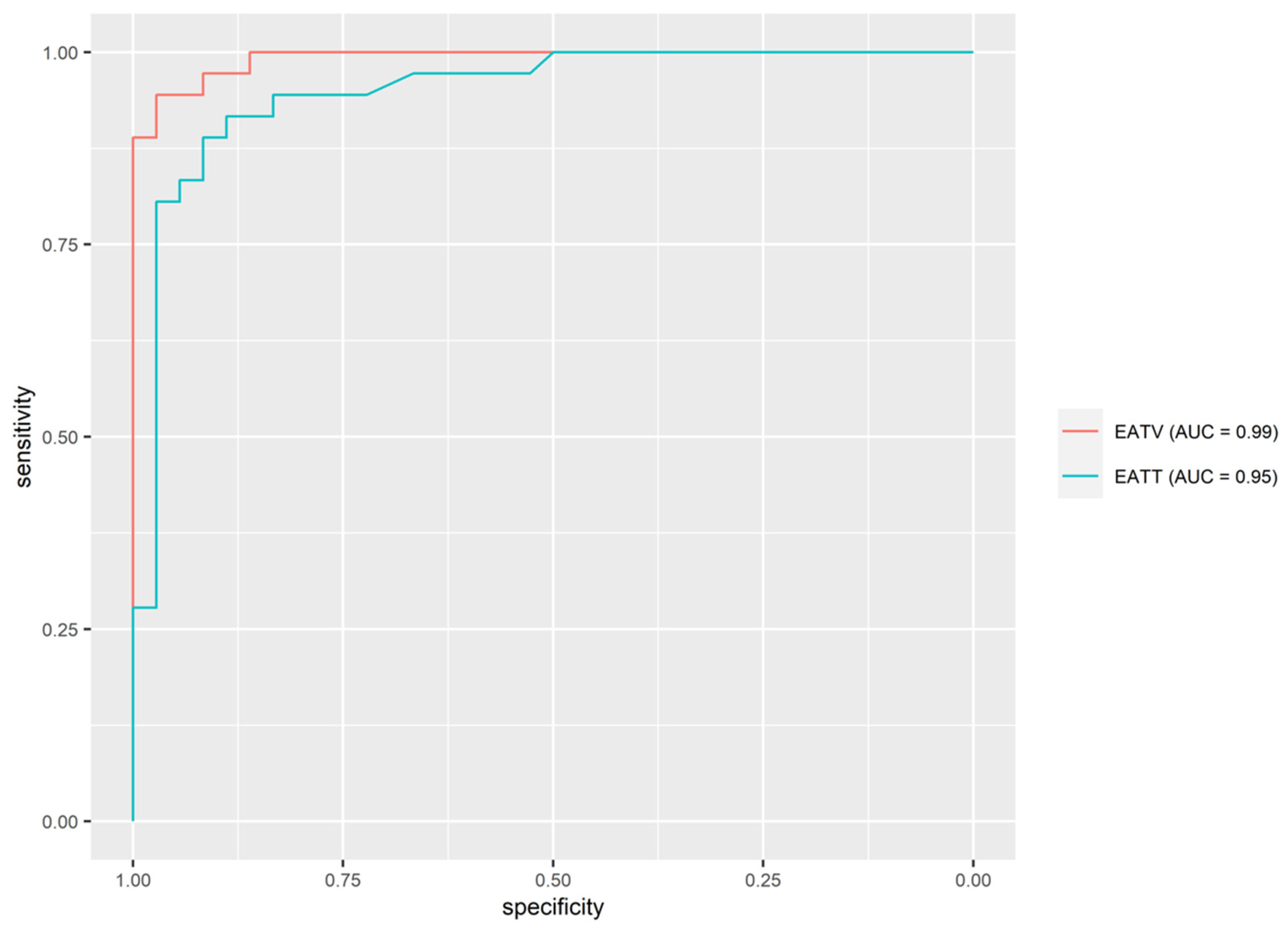
3.5. Predicting Hypertension on the Basis of EAT Volume and EAT Thickness
3.6. Predicting EAT Volume on the Basis of EAT Thickness
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashraf, M.; Irshad, M.; Parry, N.A. Pediatric hypertension: An updated review. Clin. Hypertens. 2020, 26, 22. [Google Scholar] [CrossRef] [PubMed]
- Theodore, R.F.; Broadbent, J.; Nagin, D.; Ambler, A.; Hogan, S.; Ramrakha, S.; Cutfield, W.; Williams, M.J.; Harrington, H.; Moffitt, T.E.; et al. Childhood to Early-Midlife Systolic Blood Pressure Trajectories: Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension 2015, 66, 1108–1115. [Google Scholar] [CrossRef]
- Marčun Varda, N.; Hanzelj, N. Overweight of Slovenian school children. Acta Med. Biotech. 2015, 8, 46–54. [Google Scholar] [CrossRef]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Urbina, E.M.; Mendizábal, B.; Becker, R.C.; Daniels, S.R.; Falkner, B.E.; Hamdani, G.; Hanevold, C.; Hooper, S.R.; Ingelfinger, J.R.; Lanade, M.; et al. Association of Blood Pressure Level With Left Ventricular Mass in Adolescents. Hypertension 2019, 74, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Urbina, E.M. Cardiac and Vascular Target Organ Damage in Pediatric Hypertension. Front. Pediatr. 2018, 6, 148. [Google Scholar] [CrossRef]
- Salerno, M.; Sharif, B.; Arheden, H.; Kumar, A.; Axel, L.; Li, D.; Neubauer, S. Recent Advances in Cardiovascular Magnetic Resonance: Techniques and Applications. Circ. Cardiovasc. Imaging 2017, 10, e003951. [Google Scholar] [CrossRef]
- Leo, L.A.; Paiocchi, V.L.; Schlossbauer, S.A.; Ho, S.Y.; Faletra, F.F. The Intrusive Nature of Epicardial Adipose Tissue as Revealed by Cardiac Magnetic Resonance. J. Cardiovasc. Echogr. 2019, 29, 45–51. [Google Scholar] [CrossRef]
- Douglass, E.; Greif, S.; Frishman, W.H. Epicardial Fat: Pathophysiology and Clinical Significance. Cardiol. Rev. 2017, 25, 230–235. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, A.; Hamilton, D.J.; Deng, T. Epicardial Fat in the Maintenance of Cardiovascular Health. Methodist DeBakey Cardiovasc. J. 2017, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef]
- McLean, D.S.; Stillman, A.E. Epicardial adipose tissue as a cardiovascular risk marker. Clin. Lipidol. 2009, 4, 55–62. [Google Scholar] [CrossRef]
- Conceição, G.; Martins, D.; Miranda, I.M.; Leite-Moreira, A.F.; Vitorino, R.; Falcão-Pires, I. Unraveling the Role of Epicardial Adipose Tissue in Coronary Artery Disease: Partners in Crime? Int. J. Mol. Sci. 2020, 21, 8866. [Google Scholar] [CrossRef]
- Austys, D.; Jablonskienė, V.; Valevičienė, N.; Stukas, R.; Dobrovolskij, A.; Dobrovolskij, V. Epicardial Adipose Tissue Accumulation and Essential Hypertension in Non-Obese Adults. Medicina 2019, 55, 456. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, H.H.; Fouda, E.R.; Kamal, N.M.; Bassiouny, M.M.; Fathi, W.M. Evaluation oF Epicardial Fat and Carotid Intima-Media Thickness in Obese Children. Iran. J. Pediatr. 2016, 26, e2968. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.; Koca, B.; Ture, M.; Guzel, B. Epicardial adiposity in children with obesity and metabolic syndrome. Iran. J. Pediatr. 2014, 24, 411–417. [Google Scholar]
- van Hoek, E.; Koopman, L.P.; Feskens, E.J.M.; Janse, A.J. Assessment of epicardial adipose tissue in young obese children. Child Adolesc. Obes. 2019, 2, 96–107. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- Soergel, M.; Kirschstein, M.; Busch, C.; Danne, T.; Gellermann, J.; Holl, R.; Krull, F.; Reichert, H.; Reusz, G.S.; Rascher, W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: A multicenter trial including 1141 subjects. J. Pediatr. 1997, 130, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.F.; Leo, L.A.; Paiocchi, V.L.; Schlossbauer, S.A.; Borruso, M.G.; Pedrazzini, G.; Moccetti, T.; Ho, S.Y. Imaging-based tricuspid valve anatomy by computed tomography, magnetic resonance imaging, two and three-dimensional echocardiography: Correlation with anatomic specimen. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Chang, S.; Lee, W.; Kwag, B.; Chung, Y.H.; Kang, I.S. Maximal pericoronary adipose tissue thickness is associated with hypertension in nonobese patients with acute or chronic illness. Korean J. Intern. Med. 2017, 32, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Liu, L.; Li, X.; Huang, X.; Yang, W.; Sun, S.; Ma, Y.; Yu, Y.; Luo, J.; Cao, J. Association between epicardial adipose tissue and blood pressure: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2547–2556. [Google Scholar] [CrossRef]
- Erdogan, G.; Belen, E.; Sungur, M.A.; Sungur, A.; Yaylak, B.; Güngör, B.; Akyüz, S.; Satilmis, S. Assessment of epicardial adipose tissue thickness in patients with resistant hypertension. Blood Press. Monit. 2016, 21, 16–20. [Google Scholar] [CrossRef]
- Matloch, Z.; Kotulak, T.; Haluzik, M. The role of epicardial adipose tissue in heart disease. Physiol. Res. 2016, 65, 23–32. [Google Scholar] [CrossRef]
- Rado, S.D.; Lorbeer, R.; Gatidis, S.; Machann, J.; Storz, C.; Nikolaou, K.; Rathmann, W.; Hoffmann, U.; Peters, A.; Bamberg, F.; et al. MRI-based assessment and characterization of epicardial and paracardial fat depots in the context of impaired glucose metabolism and subclinical left-ventricular alterations. Br. J. Radiol. 2019, 92, 20180562. [Google Scholar] [CrossRef]
- Busse, A.; Rajagopal, R.; Yücel, S.; Beller, E.; Öner, A.; Streckenbach, F.; Cantré, D.; Ince, H.; Weber, M.-A.; Meinel, F.G. Cardiac MRI—Update 2020. Der Radiol. 2020, 60, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Binkley, C.M.; Suever, J.D.; Umasankar, N.; Haggerty, C.M.; Rich, J.; Wehner, G.J.; Hamlet, S.M.; Powell, D.K.; Radulescu, A.; et al. Cardiac remodeling and dysfunction in childhood obesity: A cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2016, 18, 28. [Google Scholar] [CrossRef]
- Sousa, J.A.; Mendonça, M.I.; Serrão, M.; Borges, S.; Henriques, E.; Freitas, S.; Tentem, M.; Santos, M.; Freitas, P.; Ferreira, A.; et al. Epicardial Adipose Tissue: The Genetics Behind an Emerging Cardiovascular Risk Marker. Clin. Med. Insights Cardiol. 2021, 15, 11795468211029244. [Google Scholar] [CrossRef] [PubMed]
- Talman, A.H.; Psaltis, P.J.; Cameron, J.D.; Meredith, I.T.; Seneviratne, S.K.; Wong, D.T.L. Epicardial adipose tissue: Far more than a fat depot. Cardiovasc. Diagn. Ther. 2014, 4, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Basu, R.; Oudit, G.Y. ACE2/Ang 1-7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte 2016, 5, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Corradi, D.; Maestri, R.; Callegari, S.; Pastori, P.; Goldoni, M.; Luong, T.V.; Bordi, C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004, 13, 313–316. [Google Scholar] [CrossRef]
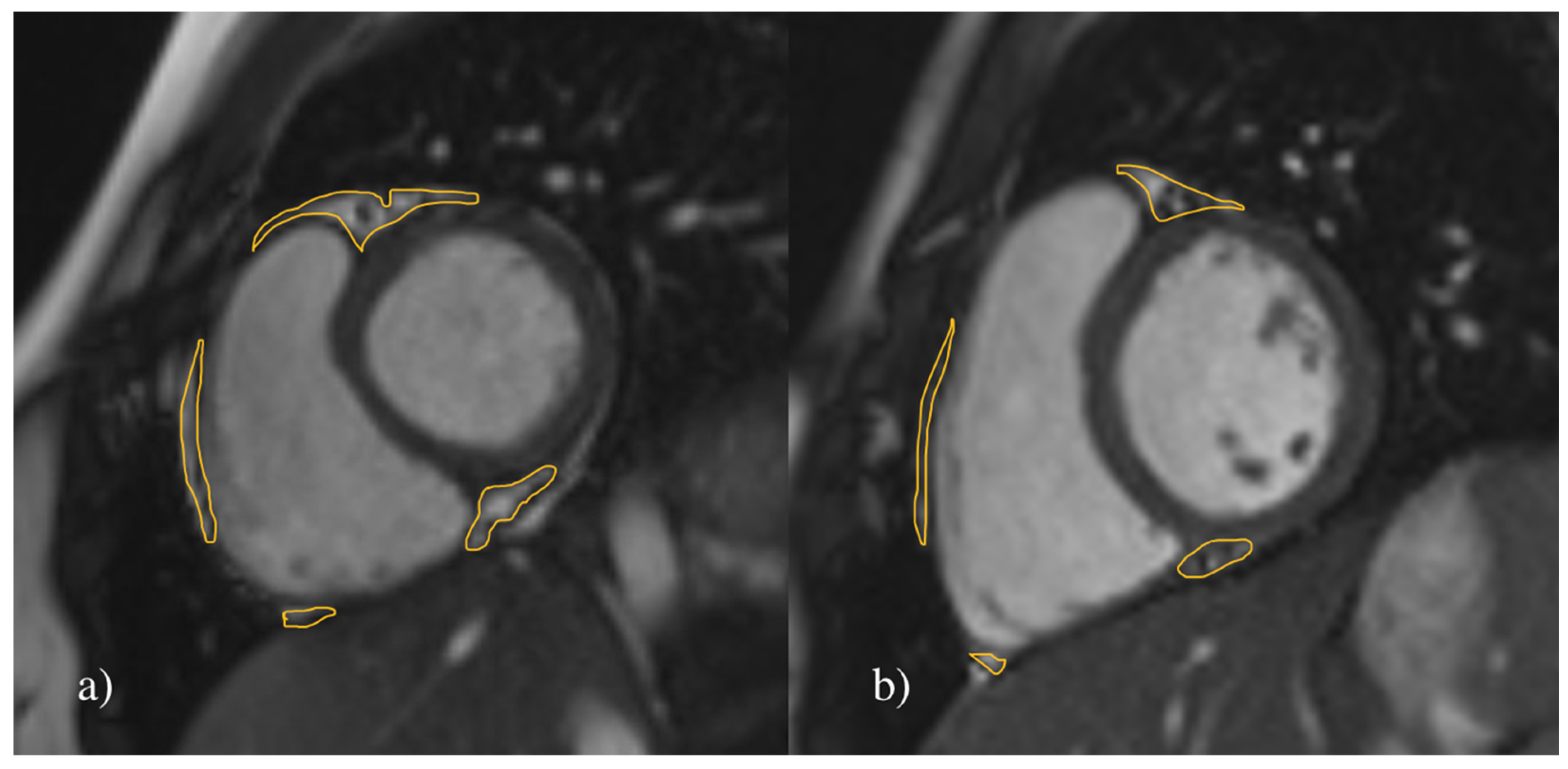

| All (N = 72) | Hypertensive (N = 36) | Normotensive (N = 36) | |||||
|---|---|---|---|---|---|---|---|
| Variable | MV ± SD | M (IQR) | MV ± SD | M (IQR) | MV ± SD | M (IQR) | t/U Value (p-Value) |
| Age (years) | 15.3 ± 2 | 15 (3) | 15.2 ± 1.7 | 15 (2.2) | 15.3 ± 2.2 | 15 (3.2) | U = 649 (0.995) |
| Height (cm) | 174.4 ± 11 | 172.5 (16.2) | 176.8 ± 11.1 | 175 (10.5) | 172 ± 10.5 | 170 (15) | t = −1.877 (0.065) |
| Weight (kg) | 65.9 ± 10.9 | 66.5 (12.5) | 67.8 ± 9.8 | 70 (15) | 64 ± 11.7 | 60.5 (16.2) | t = −1.476 (0.145) |
| BMI (kg/m2) | 21.5 ± 2 | 21.9 (2.8) | 21.6 ± 1.6 | 21.9 (2.4) | 21.5 ± 2.3 | 21.7 (3.9) | t = −0.197 (0.844) |
| BSA (m2) | 1.8 ± 0.2 | 1.8 (0.3) | 1.8 ± 0.2 | 1.9 (0.2) | 1.8 ± 0.2 | 1.7 (0.3) | t = −1.74 (0.086) |
| Systolic BP (mm Hg) | 132.2 ± 15 | 130.5 (23) | 144.6 ± 10 | 145 (9.8) | 119.8 ± 6.5 | 122 (9.2) | U = 9.5 (<0.001) ** |
| Diastolic BP (mm Hg) | 82.7 ± 7.9 | 83.5 (8.2) | 87 ± 7.8 | 87 (7.2) | 78.4 ± 5.3 | 79 (9) | U = 178 (<0.001) ** |
| HR (beats/min) | 72.4 ± 13.9 | 71 (17) | 74.2 ± 15.7 | 69.5 (21.5) | 70.6 ± 11.7 | 72 (14.5) | t = −1.098 (0.276) |
| All (N = 72) | Hypertensive (N = 36) | Normotensive (N = 36) | |||||
|---|---|---|---|---|---|---|---|
| Variable | MV ± SD | M (IQR) | MV ± SD | M (IQR) | MV ± SD | M (IQR) | p Value |
| HR (beats/min) | 72.4 ± 13.9 | 71 (17) | 74.2 ± 15.7 | 69.5 (21.5) | 70.6 ± 11.7 | 72 (14.5) | t = −1.098 (0.276) |
| EATT (cm) | 0.6 ± 0.3 | 0.6 (0.4) | 0.8 ± 0.3 | 0.8 (0.3) | 0.5 ± 0.1 | 0.4 (0.1) | U = 65.5 (<0.001) ** |
| EATV (cm3) | 13.7 ± 3.3 | 13.5 (5.5) | 16.5 ± 1.9 | 16.4 (2.2) | 10.9 ± 1.5 | 10.9 (2.5) | t = −13.815 (<0.001) ** |
| LV-EF (%) | 57.7 ± 4 | 57.8 (5.4) | 58.5 ± 4 | 58.7 (4.2) | 56.8 ± 3.9 | 56.8 (6.3) | t = −1.727 (0.089) |
| MM average (g/m2) | 68 ± 14.2 | 66.2 (18.4) | 68.6 ± 14.9 | 68.7 (21.2) | 67.4 ± 13.6 | 65.5 (14.1) | t = −0.351 (0.726) |
| EDV (mL/m2) | 90.9 ± 13 | 90.1 (19.1) | 90 ± 14.8 | 89.8 (20.2) | 91.9 ± 11.1 | 91.3 (16.4) | t = 0.622 (0.536) |
| ESV (mL/m2) | 39.7 ± 8.2 | 38.4 (11.6) | 38.9 ± 8.8 | 36.8 (11.5) | 40.6 ± 7.6 | 39.6 (11) | U = 749.5 (0.255) |
| SV (mL/m2) | 51.3 ± 8.6 | 51.2 (10.6) | 51.8 ± 9.8 | 51.6 (1.,2) | 50.7 ± 7.4 | 51 (8) | t = −0.528 (0.599) |
| Peak Filling Rate (mL/s/m2) | 297.6 ± 89.8 | 290.1 (72.7) | 303.8 ± 88.5 | 290.3 (77.2) | 291.3 ± 91.9 | 289.5 (57.5) | U = 601 (0.603) |
| Peak Filling Time (ms) | 421 ± 35.7 | 424.7 (36.7) | 418.9 ± 32.5 | 422.9 (38.1) | 423 ± 38.9 | 427.3 (36.6) | t = 0.485 (0.629) |
| Myocardial thickness LV (mm) | 7.9 ± 1.3 | 7.8 (1.7) | 8.1 ± 1.4 | 8.1 (1.7) | 7.7 ± 1.1 | 7.7 (1.5) | t = −1.563 (0.123) |
| Predictor | AUC (95% CI) | Threshold | Sen | Spec | PPV | NPV |
|---|---|---|---|---|---|---|
| EATV | 0.992 (0.981, 0.992) | 13.545 | 94.4% | 97.2% | 97.1% | 94.6% |
| EATT | 0.949 (0.899, 0.949) | 0.545 | 91.7% | 88.9% | 89.2% | 91.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweighofer, N.; Rupreht, M.; Marčun Varda, N.; Caf, P.; Povalej Bržan, P.; Kanič, V. Epicardial Adipose Tissue: A Piece of The Puzzle in Pediatric Hypertension. J. Clin. Med. 2023, 12, 2192. https://doi.org/10.3390/jcm12062192
Schweighofer N, Rupreht M, Marčun Varda N, Caf P, Povalej Bržan P, Kanič V. Epicardial Adipose Tissue: A Piece of The Puzzle in Pediatric Hypertension. Journal of Clinical Medicine. 2023; 12(6):2192. https://doi.org/10.3390/jcm12062192
Chicago/Turabian StyleSchweighofer, Nina, Mitja Rupreht, Nataša Marčun Varda, Primož Caf, Petra Povalej Bržan, and Vojko Kanič. 2023. "Epicardial Adipose Tissue: A Piece of The Puzzle in Pediatric Hypertension" Journal of Clinical Medicine 12, no. 6: 2192. https://doi.org/10.3390/jcm12062192
APA StyleSchweighofer, N., Rupreht, M., Marčun Varda, N., Caf, P., Povalej Bržan, P., & Kanič, V. (2023). Epicardial Adipose Tissue: A Piece of The Puzzle in Pediatric Hypertension. Journal of Clinical Medicine, 12(6), 2192. https://doi.org/10.3390/jcm12062192






