Periodontal Disease in Young Adults as a Risk Factor for Subclinical Atherosclerosis: A Clinical, Biochemical and Immunological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Examination for Periodontal Disease and Subgingival Sampling
2.3. Analysis of Biochemical and Hematological Parameters
2.4. Immunological Analysis
2.5. IMT Measurement of Carotid Arteries
2.6. Statistical Analysis
3. Results
3.1. The Demographic Characteristics of Study Participants
3.2. Comparison of Periodontal, Biochemical, and Clinical Parameters Associated with Atherosclerosis in Subjects with and without PD
3.3. Comparison of Cytokine Levels in Serum and GCF in Subjects with and without PD
3.4. Correlation between Clinical Periodontal Parameters and Cytokine Levels
3.5. Association of Periodontal, Biochemical, and Immunological Parameters with Atherosclerosis Parameters
3.6. Correlation of Periodontal Parameters with hs-CRP, Fibrinogen, and Lipid Status
3.7. Correlation of Cytokine Profile with hs-CRP, Lipid Status, and Fibrinogen
4. Discussion
4.1. Association of Cytokines with PD and Subclinical Atherosclerosis
4.2. Association of Dyslipidemia and CRP with PD Status and Atherosclerosis
4.3. Limitation of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavlic, V.; Peric, D.; Kalezic, I.S.; Madi, M.; Bhat, S.G.; Brkic, Z.; Staletovic, D. Identification of periopathogens in atheromatous plaques obtained from carotid and coronary arteries. Biomed Res. Int. 2021, 2021, 9986375. [Google Scholar] [CrossRef]
- Pucar, A.; Milasin, J.; Lekovic, V.; Vukadinovic, M.; Ristic, M.; Putnik, S.; Kenney, E.B. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J. Periodontol. 2007, 78, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Alsalleeh, F.; Alhadlaq, A.S.; Althumiri, N.A.; AlMousa, N.; BinDhim, N.F. Public Awareness of the Association between Periodontal Disease and Systemic Disease. Healthcare 2023, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Buhlin, K.; Hultin, M.; Norderyd, O.; Persson, L.; Pockley, A.G.; Rabe, P.; Klinge, B.; Gustafsson, A. Risk factors for atherosclerosis in cases with severe periodontitis. J. Clin. Periodontol. 2009, 36, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Piconi, S.; Trabattoni, D.; Luraghi, C.; Perilli, E.; Borelli, M.; Pacei, M.; Rizzardini, G.; Lattuada, A.; Bray, D.H.; Catalano, M.; et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2008, 23, 1196–1204. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef]
- Awan, Z.; Genest, J. Inflammation modulation and cardiovascular disease prevention. Eur. J. Prev. Cardiol. 2015, 22, 719–733. [Google Scholar] [CrossRef]
- Nicolosi, L.N.; Lewin, P.G.; Rudzinski, J.J.; Pompeo, M.; Guanca, F.; Rodríguez, P.; Gelpi, R.J.; Rubio, M.C. Relation between periodontal disease and arterial stiffness. J. Periodontal. Res. 2017, 52, 122–126. [Google Scholar] [CrossRef]
- Wojtkowska, A.; Zapolski, T.; Wysokińska-Miszczuk, J.; Wysokiński, A.P. The inflammation link between periodontal disease and coronary atherosclerosis in patients with acute coronary syndromes: Case-control study. BMC Oral Health 2021, 21, 5. [Google Scholar] [CrossRef]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. 2017, 3, 1–14. [Google Scholar] [CrossRef]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The role of cytokines in the development of atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef]
- Fentoglu, O.; Bozkurt, F.Y. The bi-directional relationship between periodontal disease and hyperlipidemia. Eur. J. Dent. 2008, 2, 142–146. [Google Scholar] [CrossRef]
- Tang, K.; Lin, M.; Wu, Y.; Yan, F. Alterations of serum lipid and inflammatory cytokine profiles in patients with coronary heart disease and chronic periodontitis: A pilot study. J. Int. Med. Res. 2011, 39, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Castellani, S.; Gori, A.M.; Nieri, M.; Baldelli, G.; Abbate, R.; Pini-Prato, G.P. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J. Clin. Periodontol. 2008, 35, 465–472. [Google Scholar] [CrossRef]
- Cairo, F.; Nieri, M.; Gori, A.; Tonelli, P.; Branchi, R.; Castellani, S.; Abbate, R.; Pini-Prato, G.P. Markers of systemic inflammation in periodontal patients: Chronic versus aggressive periodontitis. An explorative cross-sectional study. Eur. J. Oral Implantol. 2010, 3, 147–153. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Ramfjord, S.P. Indices for prevalence and incidence of periodontal disease. J. Periodontol. 1959, 30, 51–59. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal disease in pregnancy (II). Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Löe, H.; Silness, J. Periodontal disease in pregnancy (I). Prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Barnett, M.L.; Ciancio, S.G.; Mather, M.L. The modified papillary bleeding index: Comparison with gingival index during the resolution of gingivitis. J. Prev. Dent. 1980, 6, 135–138. [Google Scholar]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Güler, A.; Yılmaz, E.; Demir, A.; Bayıroğlu, N.; Kalkan, A.K.; Uzun, F.; Ertürk, M. The relationship between the severity of atherosclerosis and periodontal disease index in diabetic patients. Koşuyolu Heart J. 2022, 25, 149–156. [Google Scholar] [CrossRef]
- Fatima, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival crevicular fluid (GCF): A diagnostic tool for the detection of periodontal health and diseases. Molecules 2021, 26, 1208. [Google Scholar]
- Zweig, M.H.; Campbell, G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin. Chem. 1993, 39, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Unal, I. Defining an optimal cut-point value in ROC analysis: An alternative approach. Comput. Math Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, R.; Rimmele, D.L.; Schnabel, R.B.; Heydecke, G.; Seedorf, U.; Walther, C.; Mayer, C.; Struppek, J.; Borof, K.; Behrendt, C.; et al. Cross-sectional analysis of the association of periodontitis with carotid intima media thickness and atherosclerotic plaque in the Hamburg City health study. J. Periodontal. Res. 2022, 57, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.C.P.S.C.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. Is there an association between periodontitis and atherosclerosis in adults? A systematic review. Curr. Vasc. Pharmacol. 2018, 16, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Zardawi, F.; Gul, S.; Abdulkareem, A.; Sha, A.; Yates, J. Association between periodontal disease and atherosclerotic cardiovascular diseases: Revisited. Front. Cardiovasc. Med. 2021, 7, 625579. [Google Scholar] [CrossRef]
- Cecoro, G.; Annunziata, M.; Iuorio, M.T.; Nastri, L.; Guida, L. Periodontitis, low-grade inflammation and systemic health: A scoping review. Medicina 2020, 56, 272. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and chemokines in periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central regulatory role of cytokines in periodontitis and targeting options. Curr. Med. Chem. 2021, 28, 3032–3058. [Google Scholar] [CrossRef] [PubMed]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef]
- Patini, R.; Gallenzi, P.; Spagnuolo, G.; Cordaro, M.; Cantiani, M.; Amalfitano, A.; Arcovito, A.; Callà, C.; Mingrone, G.; Nocca, G. Correlation Between Metabolic Syndrome, Periodontitis and Reactive Oxygen Species Production. A Pilot Study. Open Dent. J. 2017, 11, 621–627. [Google Scholar] [CrossRef]
- Džopalić, T.; Tomić, S.; Bekić, M.; Vučević, D.; Mihajlović, D.; Eraković, M.; Čolić, M. Ex vivo study of IL-6 expression and function in immune cell subsets from human periapical lesions. Int. Endod. J. 2022, 55, 480–494. [Google Scholar] [CrossRef]
- Gencer, S.; Evans, B.R.; van der Vorst, E.P.C.; Döring, Y.; Weber, C. Inflammatory chemokines in atherosclerosis. Cells 2021, 10, 226. [Google Scholar] [CrossRef]
- Georgakis, M.K.; van der Laan, S.W.; Asare, Y.; Mekke, J.M.; Haitjema, S.; Schoneveld, A.H.; de Jager, S.C.; Nurmohamed, N.S.; Kroon, J.; Stroes, E.S.; et al. Monocyte-Chemoattractant Protein-1 Levels in Human Atherosclerotic Lesions Associate with Plaque Vulnerability. Arter. Thromb. Vasc. Biol. 2021, 41, 2038–2048. [Google Scholar] [CrossRef]
- Słotwińska, S. Review paper. Cytokines and periodontitis. Part I: Interleukin-1 and interleukin-1 receptor antagonist. Cent. Eur. J. Immunol. 2012, 37, 173–177. [Google Scholar]
- Tawfig, N. Proinflammatory cytokines and periodontal disease. J. Dent. Probl. Solut. 2016, 3, 12–17. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Reis, C.; Manzanares-Céspedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Ulm, C.; Reischl, H.; Nguyen, P.Q.; Matejka, M.; Rausch-Fan, X. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J. Periodontol. 2011, 82, 885–892. [Google Scholar] [CrossRef]
- Kusuhara, M.; Isoda, K.; Ohsuzu, F. Interleukin-1 and occlusive arterial diseases. Cardiovasc. Hematol. Agents Med. Chem. 2006, 4, 229–235. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, X.; Zheng, P.; Chen, H. Inflammatory cytokine levels in patients with periodontitis and/or coronary heart disease. Int. J. Clin. Exp. Med. 2015, 8, 2214–2220. [Google Scholar]
- Marcaccini, A.M.; Meschiari, C.A.; Sorgi, C.A.; Saraiva, M.C.; de Souza, A.M.; Faccioli, L.H.; Tanus-Santos, J.E.; Novaes, A.B.; Gerlach, R.F. Circulating interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J. Periodontol. 2009, 80, 594–602. [Google Scholar] [CrossRef]
- Kurita-Ochiai, T.; Jia, R.; Cai, Y.; Yamaguchi, Y.; Yamamoto, M. Periodontal disease-induced atherosclerosis and oxidative stress. Antioxidants 2015, 4, 577–590. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kurita-Ochiai, T.; Kobayashi, R.; Suzuki, T.; Ando, T. Activation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated atherosclerosis. Pathog. Dis. 2015, 73, ftv011. [Google Scholar] [CrossRef]
- Suh, J.S.; Kim, S.; Boström, K.I.; Wang, C.Y.; Kim, R.H.; Park, N.H. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial–mesenchymal transition in mice. Int. J. Oral Sci. 2019, 11, 21. [Google Scholar] [CrossRef]
- Geginat, J.; Paroni, M.; Maglie, S.; Alfen, J.S.; Kastirr, I.; Gruarin, P.; De Simone, M.; Pagani, M.; Abrignani, S. Plasticity of human CD4 T cell subsets. Front. Immunol. 2014, 5, 630. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Okui, T.; Aoki-Nonaka, Y.; Nakajima, T.; Yamazaki, K. The role of distinct T cell subsets in periodontitis-studies from humans and rodent models. Curr. Oral Health Rep. 2014, 1, 114–123. [Google Scholar] [CrossRef]
- Stadler, A.F.; Angst, P.D.; Arce, R.M.; Gomes, S.C.; Oppermann, R.V.; Susin, C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: A meta-analysis. J. Clin. Periodontol. 2016, 43, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Chen, L.L.; Tan, J.Y.; Shi, D.H.; Ke, T.; Lei, L.H. Th17 and Th1 lymphocytes are correlated with chronic periodontitis. Immunol. Investig. 2016, 45, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Whitman, S.C.; Ravisankar, P.; Daugherty, A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(-/-) mice through release of interferon-gamma. Circ. Res. 2002, 90, E34–E38. [Google Scholar] [CrossRef] [PubMed]
- Lockshin, B.; Balagula, Y.; Merola, J.F. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J. Am. Acad Dermatol. 2018, 79, 345–352. [Google Scholar] [CrossRef]
- Basiak, M.; Kosowski, M.; Hachula, M.; Okopien, B. Plasma concentrations of cytokines in patients with combined hyperlipidemia and atherosclerotic plaque before treatment initiation-a pilot study. Medicina 2022, 58, 624. [Google Scholar] [CrossRef] [PubMed]
- Manhart, S.S.; Reinhardt, R.A.; Payne, J.B.; Seymour, G.J.; Gemmell, E.; Dyer, J.K.; Petro, T.M. Gingival cell IL-2 and IL-4 in early-onset periodontitis. J. Periodontol. 1994, 65, 807–813. [Google Scholar] [CrossRef]
- Tokoro, Y.; Matsuki, Y.; Yamamoto, T.; Suzuki, T.; Hara, K. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin. Exp. Immunol. 1997, 107, 166–174. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Taubman, M.A. The protective nature of host responses in periodontal diseases. Periodontol 2000 1994, 5, 112–141. [Google Scholar] [CrossRef]
- Takeichi, O.; Haber, J.; Kawai, T.; Smith, D.J.; Moro, I.; Taubman, M.A. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J. Dent. Res. 2000, 79, 1548–1555. [Google Scholar] [CrossRef]
- Ukai, T.; Mori, Y.; Onoyama, M.; Hara, Y. Immunohistological study of interferon-γ- and interleukin-4-bearing cells in human periodontitis gingiva. Arch. Oral Biol. 2001, 46, 901–908. [Google Scholar] [CrossRef]
- Malcolm, J.; Awang, R.A.R.; Oliver-Bell, J.; Butcher, J.; Campbell, L.; Planell, A.A.; Lappin, D.; Fukada, S.; Nile, C.; Liew, F.; et al. IL-33 Exacerbates Periodontal Disease through Induction of RANKL. J. Dent. Res. 2015, 94, 968–975. [Google Scholar] [CrossRef]
- Saǧlam, M.; Köseoǧlu, S.; Aral, C.A.; Savran, L.; Pekbaǧrıyanık, T.; Çetinkaya, A. Increased levels of interleukin-33 in gingival crevicular fluids of patients with chronic periodontitis. Odontology 2017, 105, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gegen, T.; Zhu, Y.; Sun, Q.; Hou, B. Role of interleukin-33 in the clinical pathogenesis of chronic apical periodontitis. J. Int. Med. Res. 2019, 47, 3332–3343. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000 2015, 69, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, A.; Binz, C.; Sandrock, I.; Rampoldi, F.; Lienenklaus, S.; Blank, E.; Winkel, A.; Demera, A.; Hovav, A.-H.; Stiesch, M.; et al. Interleukin-17 is disease promoting in early stages and protective in late stages of experimental periodontitis. PLoS ONE. 2022, 17, e0265486. [Google Scholar] [CrossRef]
- Kotla, S.; Singh, N.K.; Heckle, M.R.; Tigyi, G.J.; Rao, G.N. The transcription factor CREB enhances interleukin-17A production and inflammation in a mouse model of atherosclerosis. Sci. Signal. 2013, 6, ra83. [Google Scholar] [CrossRef]
- Lim, H.; Kim, Y.U.; Sun, H.; Lee, J.H.; Reynolds, J.M.; Hanabuchi, S.; Wu, H.; Teng, B.B.; Chung, Y. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 2014, 40, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [Google Scholar] [CrossRef]
- Abbas, A.; Gregersen, I.; Holm, S.; Daissormont, I.; Bjerkeli, V.; Krohg-Sørensen, K.; Skagen, K.R.; Dahl, T.B.; Russell, D.; Almås, T.; et al. Interleukin 23 levels are increased in carotid atherosclerosis: Possible role for the interleukin 23/interleukin 17 axis. Stroke 2015, 46, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Fraser, D.; Caton, J.; Benoit, D.S.W. Periodontal wound healing and regeneration: Insights for engineering new therapeutic approaches. Front. Dent. Med. 2022, 3, 815810. [Google Scholar] [CrossRef]
- Skaleric, U.; Kramar, B.; Petelin, M.; Pavlica, Z.; Wahl, S.M. Changes in TGF-beta 1 levels in gingiva, crevicular fluid and serum associated with periodontal inflammation in humans and dogs. Eur. J. Oral Sci. 1997, 105, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Berberich, A.J.; Hegele, R. A modern approach to dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, diabetes and atherosclerosis: Role of inflammation and ROS-Redox-Sensitive factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Sheha, D.; El-Korashi, L.; AbdAllah, A.M.; El Begermy, M.M.; Elzoghby, D.M.; Elmahdi, A. Lipid profile and IL-17A in allergic rhinitis: Correlation with disease severity and quality of life. J. Asthma. Allergy. 2021, 14, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal inflammation and systemic diseases: An overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Sun, X.J.; Meng, H.X.; Shi, D.; Xu, L.; Zhang, L.; Chen, Z.B.; Feng, X.H.; Lu, R.F.; Ren, X.Y. Elevation of C-reactive protein and interleukin-6 in plasma of patients with aggressive periodontitis. J. Periodontal. Res. 2009, 44, 311–316. [Google Scholar] [CrossRef]
- Toprak, A.; Kandavar, R.; Toprak, D.; Chen, W.; Srinivasan, S.; Xu, J.H.; Anwar, A.; Berenson, G.S. C-reactive protein is an independent predictor for carotid artery intima-media thickness progression in asymptomatic younger adults (from the Bogalusa Heart Study). BMC Cardiovasc. Disord. 2011, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Elbelkimy, M.; ELkhayat, N.; ElSadek, A.; Mansour, A.; Aboutaleb, M. Predictive value of C-reactive protein and carotid intimal medial thickness in acute ischemic stroke. Egypt J. Neurol. Psychiatry Neurosurg. 2019, 55, 72. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Abraham, S.; Premnath, A.; Arunima, P.R.; Kassim, R.M. Critical appraisal of bidirectional relationship between periodontitis and hyperlipidemia. J. Int. Soc. Prev. Community Dent. 2019, 9, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, R.; Pigossi, S.C.; Finoti, L.S.; Orrico, S.R.P.; Cirelli, J.A.; Barros, S.P.; Offenbacher, S.; Scarel-Caminaga, R.M. Serum lipid levels in patients with periodontal disease: A meta-analysis and meta-regression. J. Clin. Periodontol. 2017, 44, 1192–1207. [Google Scholar] [CrossRef]
- Yeun, Y.R.; Kwak, Y.S.; Kim, H.Y. Association serum lipid levels with periodontal disease in Korean adults over the age of 50: The Korea National health and nutrition examination survey, 2016–2018. Exerc. Sci. 2022, 31, 312–318. [Google Scholar] [CrossRef]
- Iacopino, A.M.; Cutler, C.W. Pathophysiological relationships between periodontitis and systemic disease: Recent concepts involving serum lipids. J. Periodontol. 2000, 71, 1375–1384. [Google Scholar] [CrossRef]
- Mai, J.; Nanayakkara, G.; Lopez-Pastrana, J.; Li, X.; Li, Y.-F.; Wang, X.; Song, A.; Virtue, A.; Shao, Y.; Shan, H.; et al. Interleukin-17A Promotes Aortic Endothelial Cell Activation via Transcriptionally and Post-translationally Activating p38 Mitogen-activated Protein Kinase (MAPK) Pathway. J. Biol. Chem. 2016, 291, 4939–4954. [Google Scholar] [CrossRef]
- Meehan, E.V.; Wang, K. Interleukin-17 family cytokines in metabolic disorders and cancer. Genes 2022, 13, 1643. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.W.; Shinedling, E.A.; Nunn, M.; Jotwani, R.; Kim, B.O.; Nares, S.; Iacopino, A.M. Association between periodontitis and hyperlipidemia: Cause or effect? J. Periodontol. 1999, 70, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Rodean, I.P.; Lazar, L.; Halatiu, V.B.; Biris, C.; Benedek, I.; Benedek, T. Periodontal disease is associated with increased vulnerability of coronary atheromatous plaques in patients undergoing coronary computed tomography angiography-results from the Atherodent study. J. Clin. Med. 2021, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
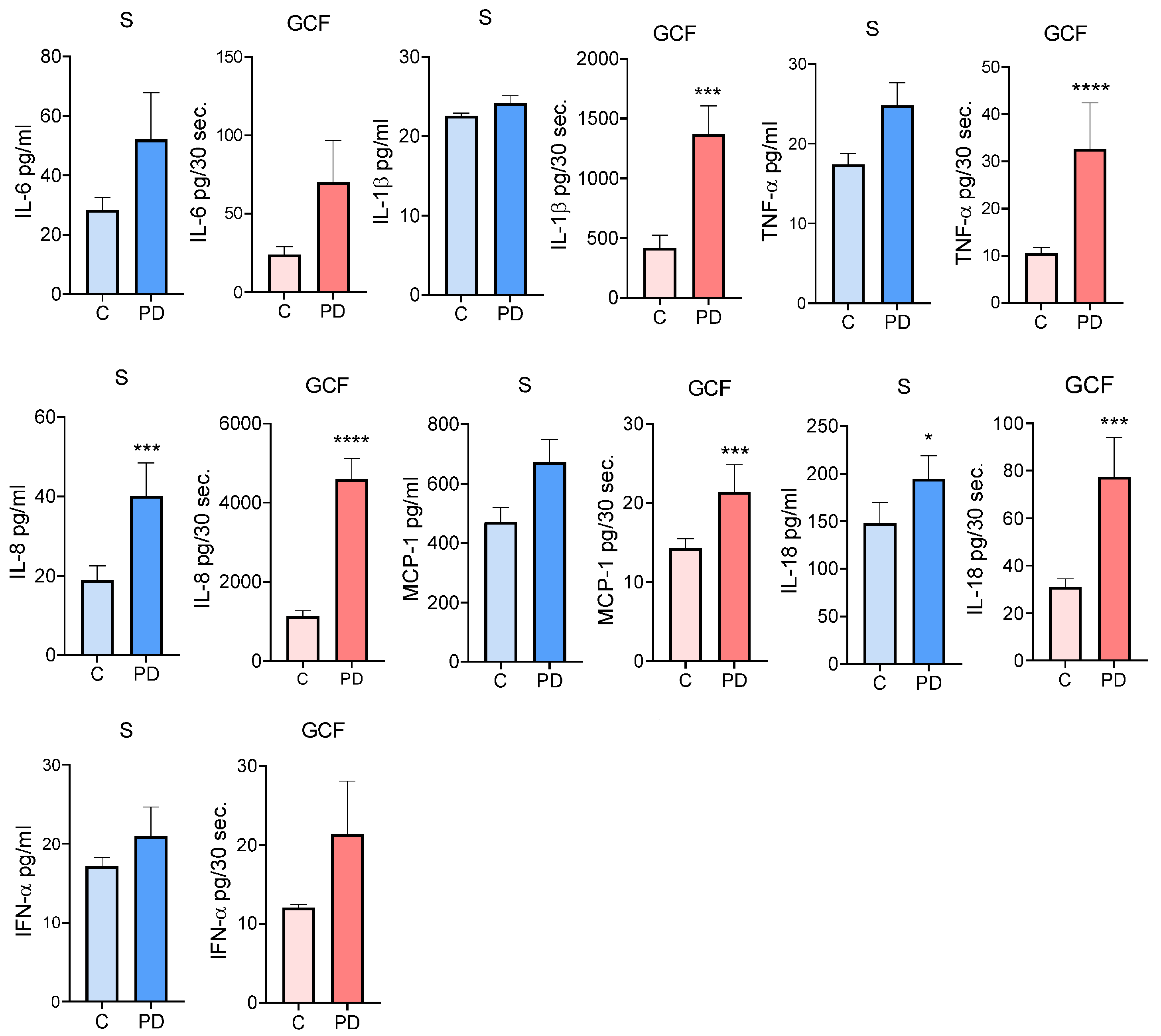


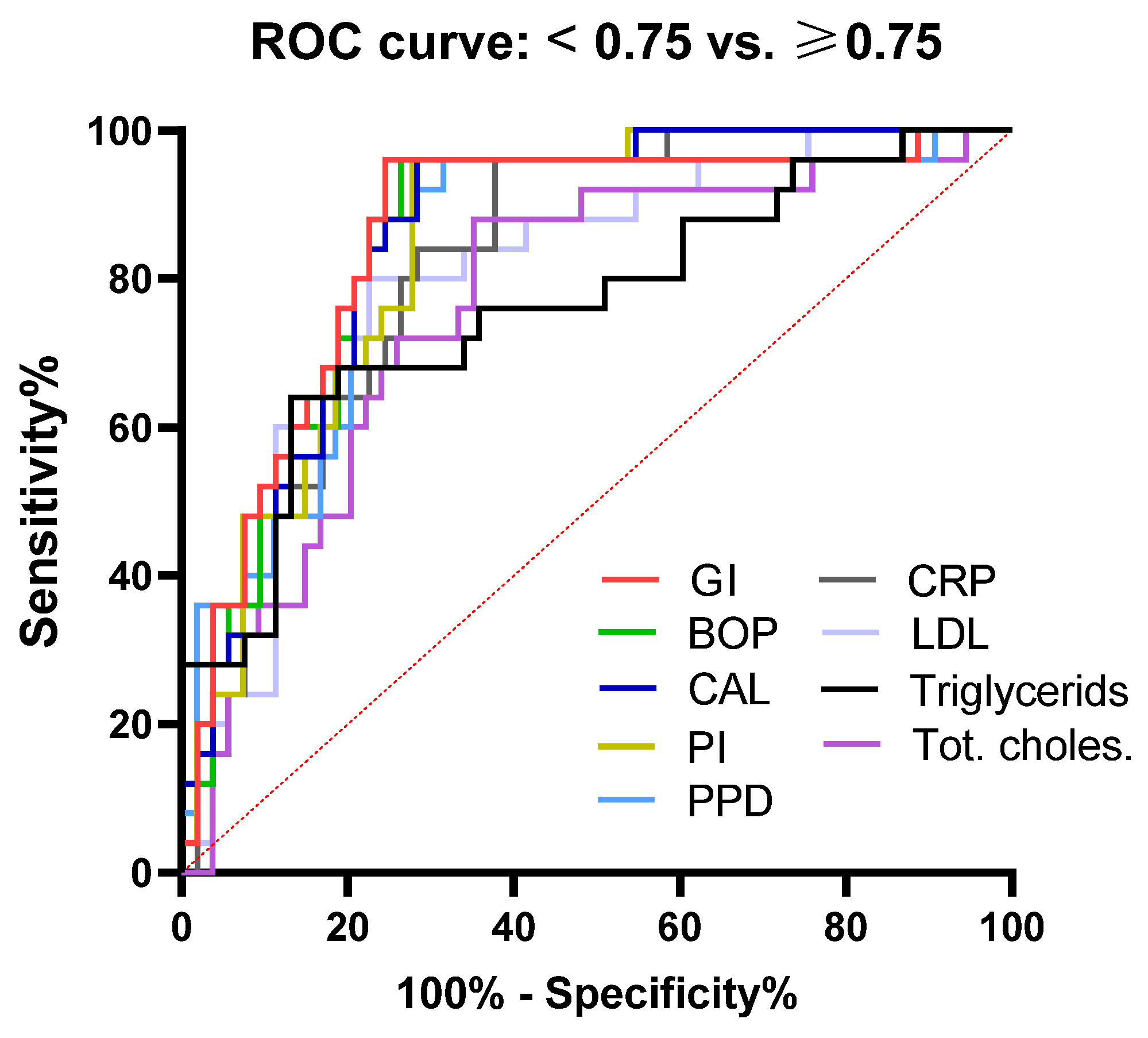
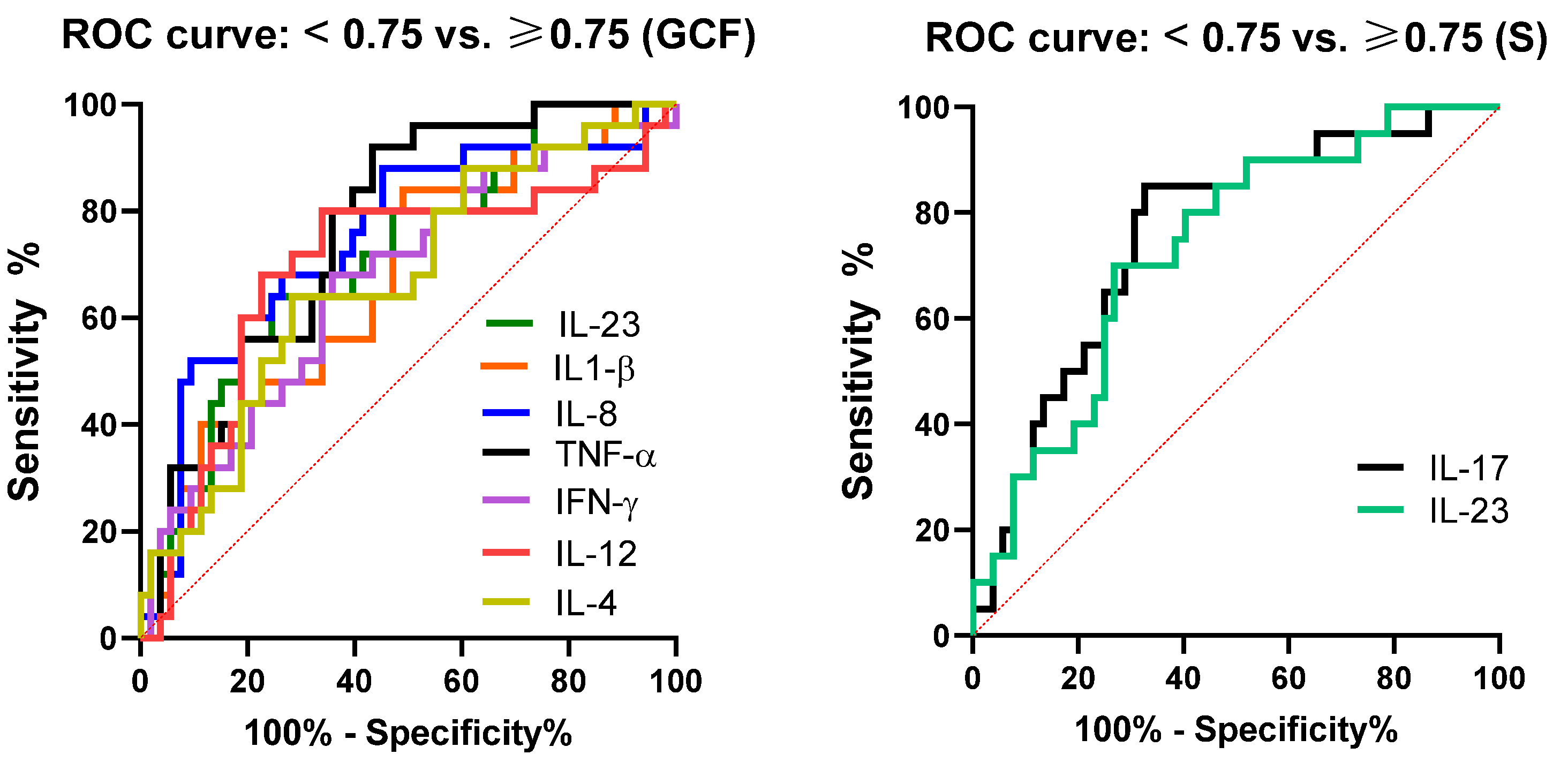
| Characteristics | All Subjects n = 78 | Subjects with PD n = 39 | Subjects without PD n = 39 | p * |
|---|---|---|---|---|
| Age ( ± SD) | 35.92 ± 3.36 | 36.18 ± 3.49 | 35.56 ± 3.04 | >0.05 |
| Gender (%) | ||||
| 1. Male 2. Female | 39.7 60.3 | 41.0 59.0 | 38.5 61.5 | >0.05 |
| BMI ( ± SD) | 24.27 ± 2.53 | 25.04 ± 2.66 | 22.72 ± 2.43 | >0.05 |
| Education (%) | ||||
| 1. High school 2. University | 47.4 52.6 | 61.5 38.5 | 12.8 87.2 | <0.001 |
| Current smokers | ||||
| 1. Yes (%) 2. No | 24.4 75.6 | 20.5 79.5 | 28.2 71.8 | >0.05 |
| Parameters | Subjects with PD n = 39 | Subjects without PD n = 39 | p * |
|---|---|---|---|
| PI | 1.46 ± 0.39 | 0.24 ± 0.15 | <0.0001 |
| GI | 1.18 ± 0.33 | 0.08 ± 0.09 | <0.0001 |
| BOP | 1.19 ± 0.35 | 0.09 ± 0.12 | <0.0001 |
| PPD | 4.27 ± 0.62 | 1.99 ± 0.23 | <0.0001 |
| CAL | 3.84 ± 0.90 | 0.17 ± 0.20 | <0.0001 |
| hs-CRP(mg/L) | 1.24 ± 0.99 | 0.43 ± 0.40 | <0.0001 |
| Fibrinogen (µmol/L) | 8.60 ± 2.80 | 7.57 ± 2.28 | <0.05 |
| Leukocytes (109/L) | 6.31 ± 1.44 | 5.91 ± 1.59 | 0.1974 |
| Triglycerides (mmol/L) | 1.85 ± 1.40 | 1.08 ± 0.45 | <0.01 |
| Total cholesterol (mmol/L) | 5.68 ± 1.07 | 5.15 ± 0.88 | <0.05 |
| HDL-cholesterol (mmol/L) | 1.47 ± 0.40 | 1.58 ± 0.33 | 0.3691 |
| LDL-cholesterol (mmol/L) | 4.22 ± 1.15 | 3.54 ± 0.88 | <0.01 |
| SBP (mmHg) | 119.49 ± 13.42 | 114.36 ± 9.68 | <0.05 |
| DBP (mmHg) | 77.69 ± 9.02 | 73.72 ± 8.00 | <0.05 |
| IMTL (mm) | 0.75 ± 0.13 | 0.58 ± 0.08 | <0.0001 |
| IMTR (mm) | 0.78 ± 0.14 | 0.57 ± 0.08 | <0.0001 |
| Parameter | AUC | 95% CI | p * | Cut-Off * | Sensitivity | Specificity (%) |
|---|---|---|---|---|---|---|
| IL-8 (GCF) | 0.87 | 0.79–0.96 | <0.0001 | 1624 | 84.82 | 76.92 |
| TNF-α (GCF) | 0.79 | 0.69–0.89 | <0.0001 | 9.6 | 76.92 | 66.67 |
| IL1-β(GCF) | 0.76 | 0.65–0.87 | <0.0001 | 403.6 | 71.79 | 71.9 |
| IL-8 (S) | 0.75 | 0.63–0.86 | 0.0002 | 14.75 | 76.47 | 66.67 |
| IL-17 (GCF) | 0.74 | 0.64–0.86 | 0.0002 | 66.05 | 74.36 | 71.79 |
| MCP-1 (GCF) | 0.74 | 0.62–0.85 | 0.0003 | 12.5 | 71.79 | 69.23 |
| IL-18 (GCF) | 0.72 | 0.61–0.84 | 0.0007 | 30.00 | 71.79 | 71.79 |
| IL-17 (S) | 0.68 | 0.55–0.81 | 0.0080 | 57.25 | 64.71 | 69.23 |
| IL-18 (S) | 0.65 | 0.52–0.77 | 0.0270 | 121.3 | 64.1 | 58.97 |
| IL-12 (S) | 0.64 | 0.51–0.77 | 0.0364 | 7.6 | 55.88 | 60.53 |
| PI | GI | BOP | PPD | CAL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with PD | |||||||||||
| IL-1β | GCF | - | - | - | - | r = 0.36 | p < 0.05 | r = 0.34 | p < 0.05 | - | - |
| IL-6 | GCF | - | - | - | - | - | - | r = 0.33 | p < 0.05 | - | - |
| TNF-α | S | r = 0.56 | p < 0.001 | r = 0.33 | p < 0.05 | - | - | r = 0.43 | p = 0.01 | r = 0.36 | p < 0.05 |
| TNF-α | GCF | r = 0.34 | p < 0.05 | - | - | - | - | r = 0.46 | p < 0.01 | r = 0.34 | p < 0.05 |
| IL-8 | GCF | r = 0.44 | p < 0.01 | r = 0.34 | p < 0.05 | - | - | r = 0.36 | p < 0.05 | r = 0.34 | p < 0.05 |
| MCP-1 | GCF | r = 0.33 | p < 0.05 | - | - | r= 0.35 | p < 0.05 | r = 0.42 | p < 0.01 | r = 0.33 | p < 0.05 |
| IL-18 | S | - | - | r = 0.34 | p < 0.05 | r = 0.36 | p < 0.05 | r = 0.33 | p < 0.05 | ||
| IL-18 | GCF | r = 0.35 | p < 0.05 | - | - | r= 0.33 | p < 0.05 | r = 0.33 | p < 0.05 | - | - |
| IFN-α | S | r = 0.35 | p < 0.05 | - | - | - | - | - | - | - | - |
| IL-12 | S | - | - | - | - | - | - | r = 0.37 | p < 0.05 | - | - |
| IL-12 | GCF | - | - | r = 0.46 | p < 0.01 | r= 0.54 | p < 0.01 | - | - | - | - |
| IL-23 | GCF | r = 0.38 | p < 0.05 | r = 0.49 | p < 0.01 | r= 0.56 | p < 0.001 | r = 0.36 | p < 0.05 | - | - |
| IL-17A | GCF | r= 0.34 | p < 0.05 | r = 0.36 | p < 0.05 | r = 0.33 | p < 0.05 | ||||
| Il-4 | S | - | - | r = -0.38 | p < 0.05 | r= -0.36 | p < 0.05 | - | - | - | - |
| IL-33 | GCF | - | - | r = 0.41 | p < 0.05 | r= 0.47 | p < 0.01 | - | - | - | - |
| TGF-β | S | - | - | r = 0.38 | p < 0.05 | r= 0.36 | p < 0.05 | - | - | - | - |
| Control subjects | |||||||||||
| IL-6 | GCF | - | - | - | - | - | - | r = 0.34 | p < 0.05 | - | - |
| TNF-α | S | r = 0.36 | p < 0.05 | - | - | - | - | - | - | - | - |
| IL-18 | S | - | - | r = −0.33 | p < 0.05 | r = -0.33 | p < 0.05 | - | - | - | - |
| IFN-γ | S | - | - | - | - | - | - | - | - | r = 0.32 | p < 0.05 |
| IL-12 | GCF | - | - | - | - | - | - | r = 0.43 | p < 0.01 | - | - |
| IL-4 | GCF | - | - | - | - | - | - | r = -0.35 | p < 0.05 | - | - |
| IL-33 | S | - | - | - | - | - | - | r = 0.32 | p < 0.05 | - | - |
| IL-33 | GCF | - | - | - | - | - | - | r = 0.37 | p < 0.05 | r = 0.32 | p < 0.05 |
| Parameter | AUC | 95% CI | p * | Cut-Off * | Sensitivity | Specificity (%) |
|---|---|---|---|---|---|---|
| GI | 0.85 | 0.76 to 0.95 | <0.0001 | 0.790 | 84.00 | 77.36 |
| BOP | 0.85 | 0.75 to 0.94 | <0.0001 | 0.770 | 84.00 | 77.36 |
| CAL | 0.85 | 0.77 to 0.94 | <0.0001 | 2.960 | 84.00 | 75.47 |
| PI | 0.85 | 0.76 to 0.93 | <0.0001 | 0.910 | 84.00 | 72.22 |
| PPD | 0.83 | 0.74 to 0.93 | <0.0001 | 3.555 | 84.00 | 72.22 |
| hs-CRP | 0.82 | 0.73 to 0.91 | <0.0001 | 0.505 | 80.00 | 71.70 |
| LDL | 0.80 | 0.70 to 0.90 | <0.0001 | 3.875 | 80.00 | 75.47 |
| Triglycerides | 0.76 | 0.64 to 0.88 | 0.0002 | 1.345 | 68.00 | 75.47 |
| Tot. cholest. | 0.76 | 0.65 to 0.89 | 0.0002 | 5.495 | 72.00 | 74.07 |
| Parameter | AUC | 95% CI | p * | Cut-Off * | Sensitivity | Specificity (%) |
|---|---|---|---|---|---|---|
| TNF-α (GCF) | 0.76 | 0.65 to 0.87 | 0.0002 | 11.25 | 76.00 | 64.15 |
| IL-17 (S) | 0.76 | 0.63 to 0.88 | 0.0008 | 70.60 | 75.00 | 69.23 |
| IL-8 (GCF) | 0.74 | 0.62 to 0.87 | 0.0005 | 2243.00 | 68.00 | 73.58 |
| IL-23 (S) | 0.72 | 0.60 to 0.85 | 0.0028 | 35.85 | 70.00 | 73.07 |
| IL-23 (GCF) | 0.70 | 0.57 to 0.82 | 0.0054 | 11.65 | 64.00 | 69.81 |
| IFN-γ (GCF) | 0.67 | 0.54 to 0.80 | 0.0158 | 494.9 | 68.00 | 64.15 |
| IL-12 (GCF) | 0.69 | 0.55 to 0.82 | 0.0079 | 10.95 | 68.00 | 71.7 |
| IL-4 (GCF) | 0.69 | 0.56 to 0.81 | 0.0080 | 5.65 | 64.00 | 71.7 |
| IL1-β (GCF) | 0.66 | 0.54 to 0.80 | 0.0162 | 403.6 | 64.00 | 56.6 |
| PI | GI | BOP | PPD | CAL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with PD | |||||||||||
| Triglycerides | - | - | - | - | - | - | r = 0.35 | p < 0.05 | r = 0.36 | p < 0.05 | |
| LDL cholest. | - | - | - | - | - | - | r = 0.33 | p < 0.05 | r = 0.32 | p < 0.05 | |
| Subjects without PD | |||||||||||
| Triglycerides | GCF | - | - | r = 0.37 | p < 0.05 | - | - | - | - | - | - |
| Hs-CRP | Triglycerides | Total Cholesterol | LDL Cholesterol | Fibrinogen | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects with PD | |||||||||||
| IL-6 | GCF | r = 0.33 | p < 0.05 | - | - | - | - | - | - | - | - |
| MCP-1 | GCF | r = 0.37 | p < 0.05 | - | - | - | - | r = 0.33 | p < 0.05 | - | - |
| IL-12 | GCF | r = 0.36 | p < 0.05 | r = 0.32 | p < 0.05 | - | - | - | - | r = 0.35 | p < 0.05 |
| IL-18 | GCF | - | - | r = 0.35 | p < 0.05 | r= 0.33 | p < 0.05 | r = 0.44 | p < 0.01 | - | - |
| IL-8 | GCF | r = 0.44 | p < 0.01 | r = 0.34 | p < 0.05 | - | - | r = 0.36 | p < 0.05 | r = 0.34 | p < 0.05 |
| IL-17A | S | r = 0.33 | p < 0.05 | - | - | - | - | r = 0.43 | p < 0.01 | - | - |
| Subjects without PD | |||||||||||
| IL-17A | GCF | r = 0.37 | p < 0.05 | - | - | - | - | - | - | - | - |
| IL-4 | S | - | - | - | - | - | - | - | - | r= 0.35 | p < 0.05 |
| IL-33 | S | - | - | - | - | r = −0.35 | p < 0.05 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicmil, S.; Cicmil, A.; Pavlic, V.; Krunić, J.; Sladoje Puhalo, D.; Bokonjić, D.; Čolić, M. Periodontal Disease in Young Adults as a Risk Factor for Subclinical Atherosclerosis: A Clinical, Biochemical and Immunological Study. J. Clin. Med. 2023, 12, 2197. https://doi.org/10.3390/jcm12062197
Cicmil S, Cicmil A, Pavlic V, Krunić J, Sladoje Puhalo D, Bokonjić D, Čolić M. Periodontal Disease in Young Adults as a Risk Factor for Subclinical Atherosclerosis: A Clinical, Biochemical and Immunological Study. Journal of Clinical Medicine. 2023; 12(6):2197. https://doi.org/10.3390/jcm12062197
Chicago/Turabian StyleCicmil, Smiljka, Ana Cicmil, Verica Pavlic, Jelena Krunić, Dragana Sladoje Puhalo, Dejan Bokonjić, and Miodrag Čolić. 2023. "Periodontal Disease in Young Adults as a Risk Factor for Subclinical Atherosclerosis: A Clinical, Biochemical and Immunological Study" Journal of Clinical Medicine 12, no. 6: 2197. https://doi.org/10.3390/jcm12062197
APA StyleCicmil, S., Cicmil, A., Pavlic, V., Krunić, J., Sladoje Puhalo, D., Bokonjić, D., & Čolić, M. (2023). Periodontal Disease in Young Adults as a Risk Factor for Subclinical Atherosclerosis: A Clinical, Biochemical and Immunological Study. Journal of Clinical Medicine, 12(6), 2197. https://doi.org/10.3390/jcm12062197






