Review of Possible Therapies in Treatment of Novichoks Poisoning and HAZMAT/CBRNE Approaches: State of the Art
Abstract
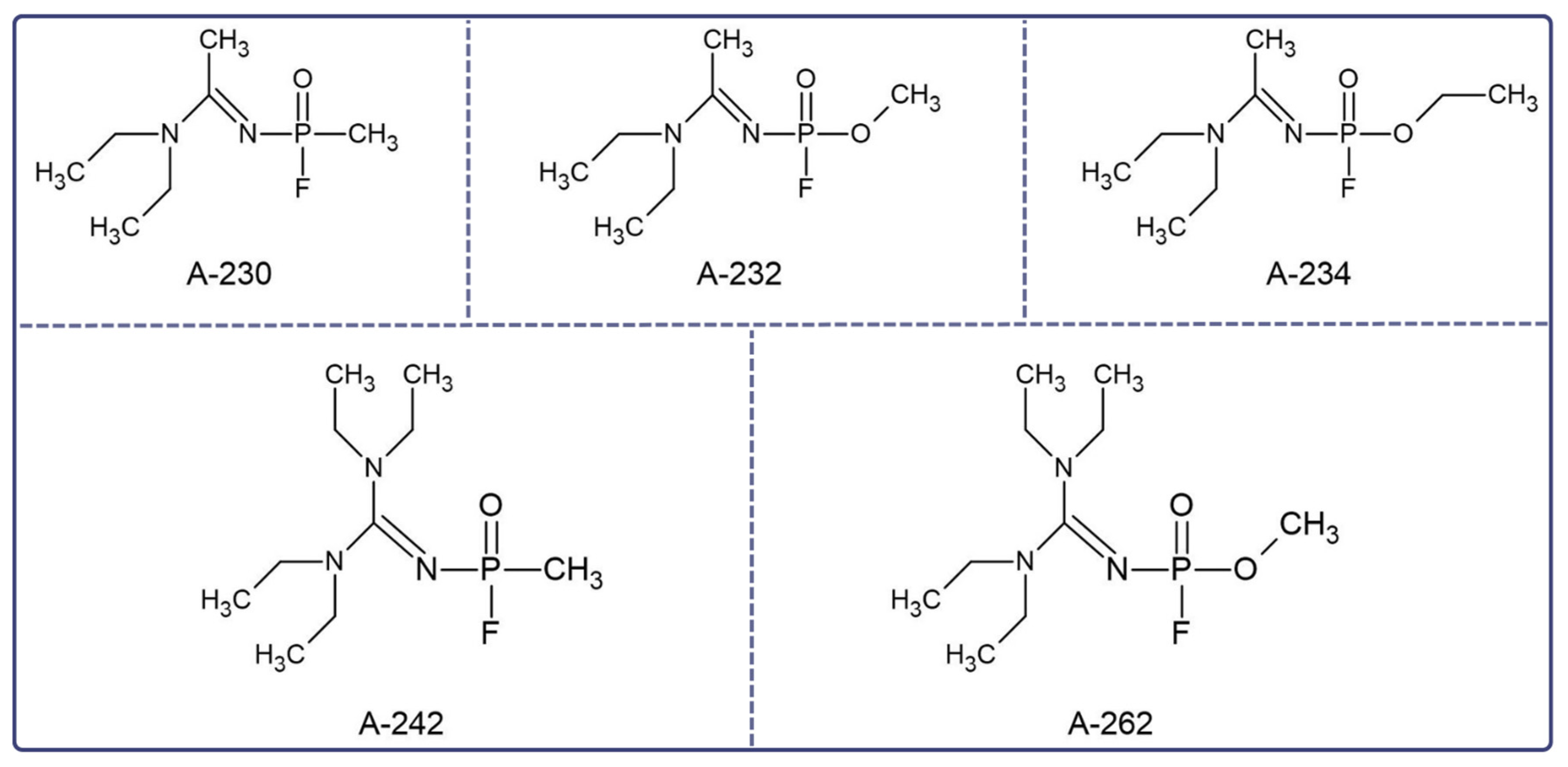
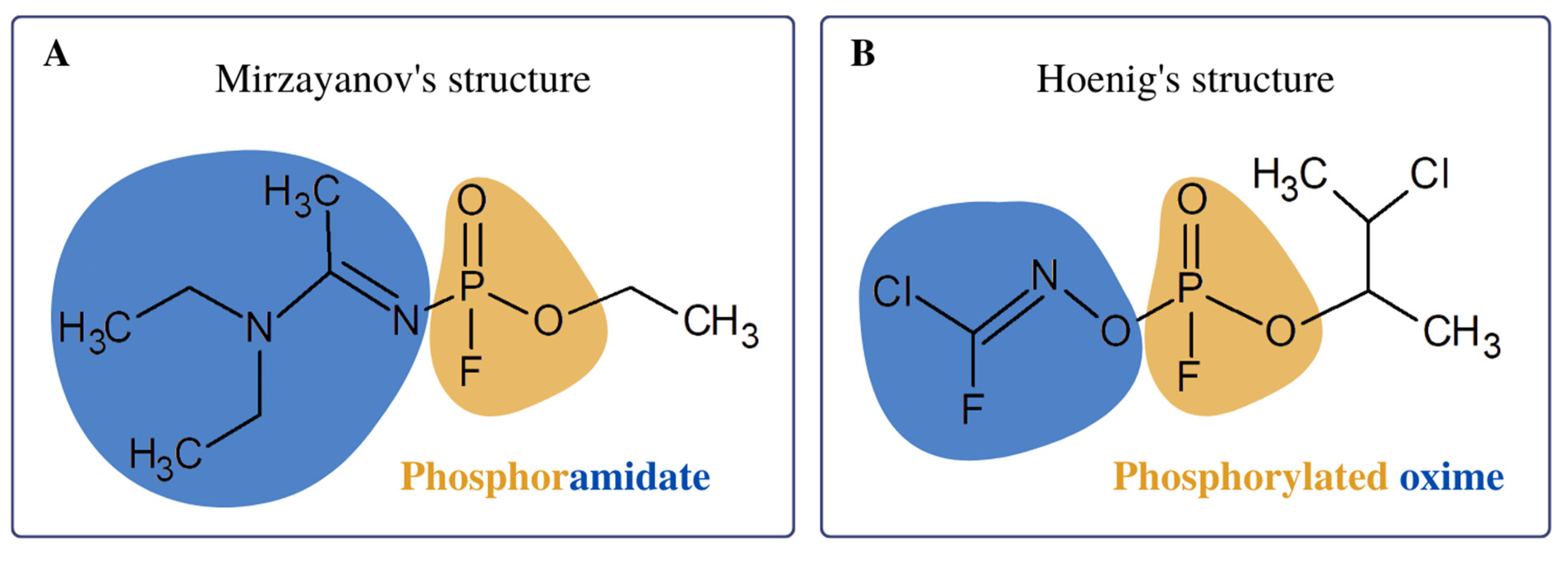
1. Introduction
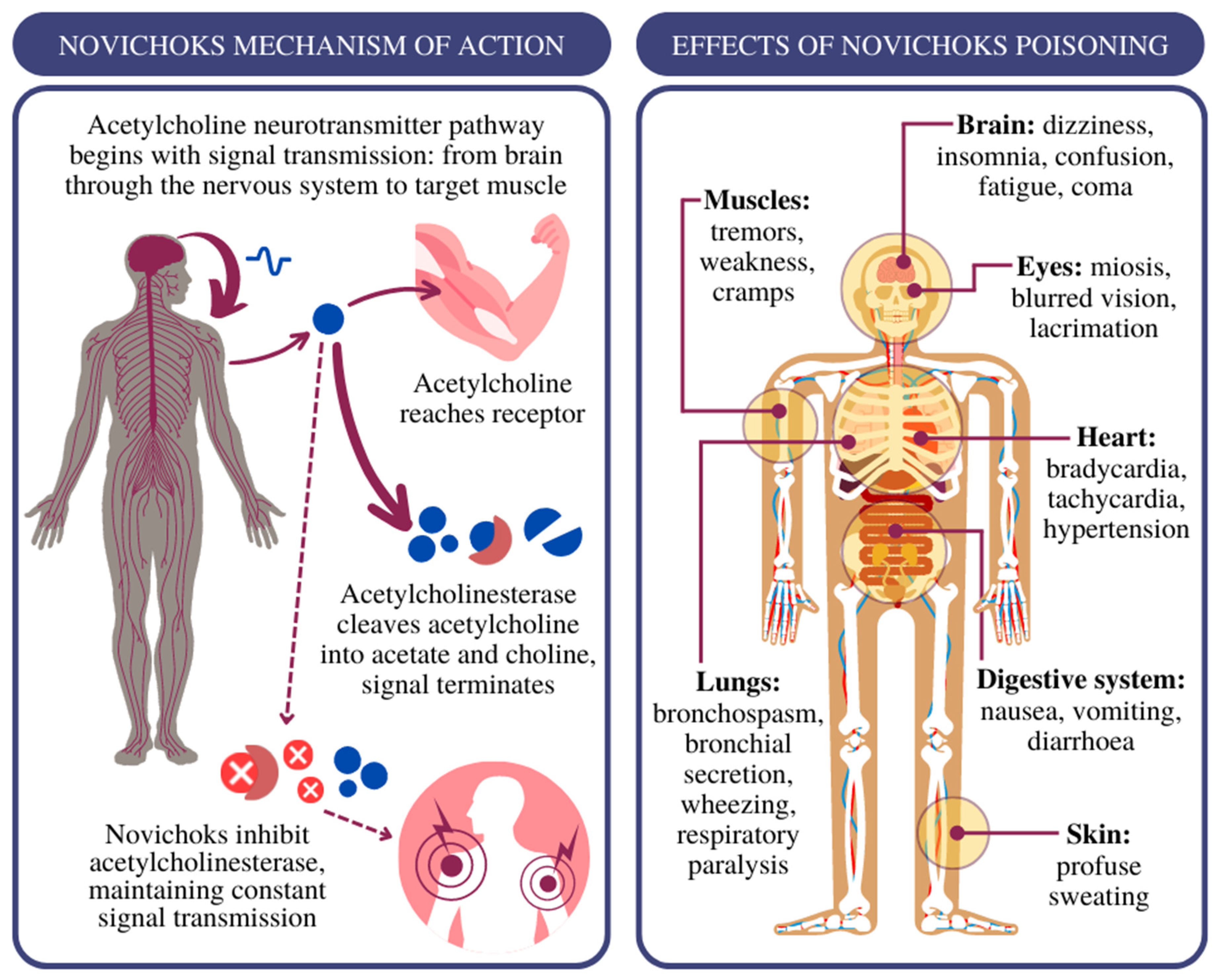
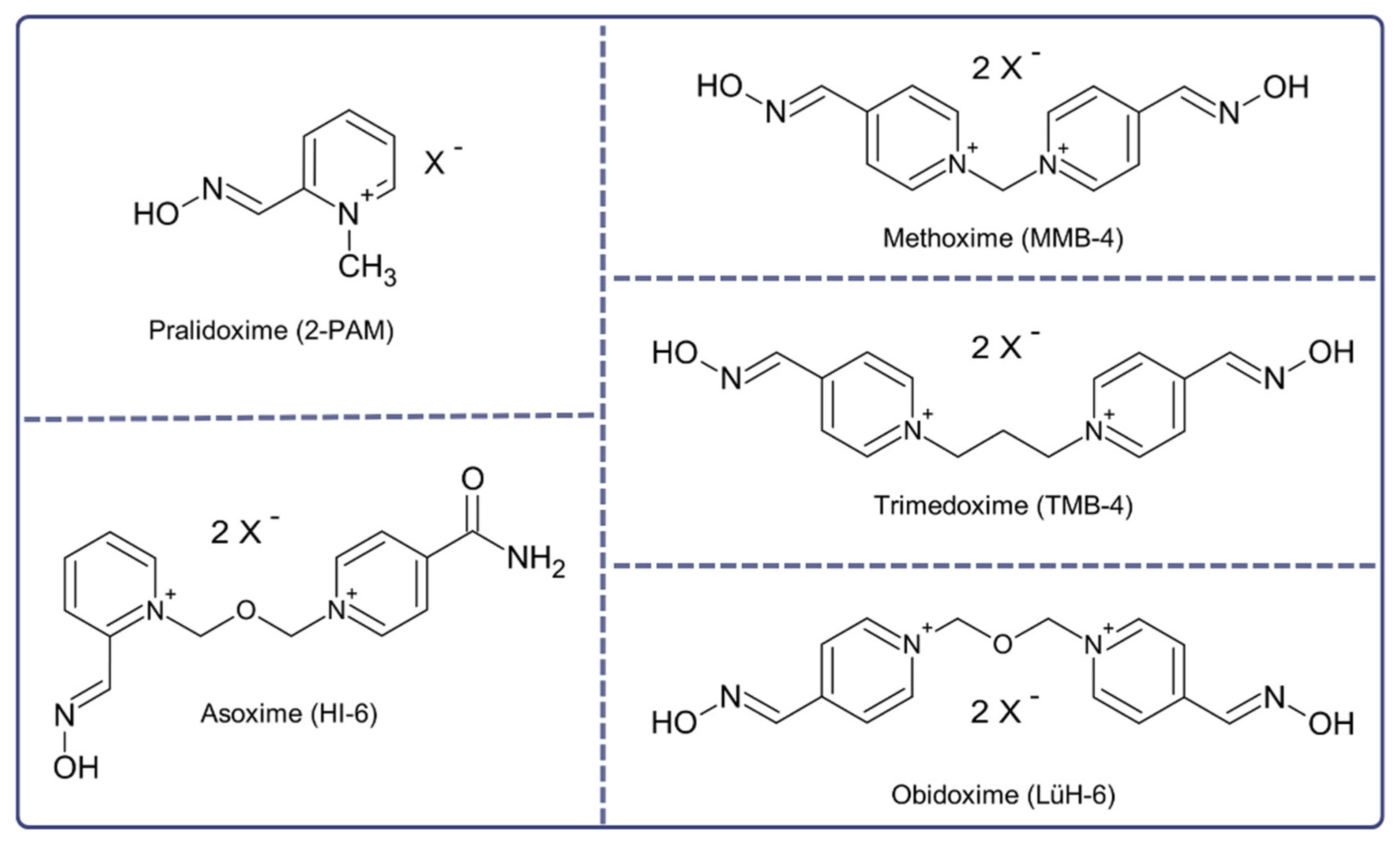
2. Treatment of Novichok Exposure: Where to Start?
3. HAZMAT/CBRNE Approaches
- Level A, a gas-tight protective suit with a self-contained breathing apparatus structure, prevents the implementation of medical procedures;
- Level B, a protective suit with thin but solid fabrics and special protective coatings, is equipped with a self-contained breathing apparatus;
- Level C, a protective suit with a filter mask or escape hood with a wide visor and a powered air respirator;
- Level D, essential protective clothing (goggles and mask with a P3 filter), provides adequate protection in daily practise [81].
3.1. Personal Protection Equipment
3.2. Supplies
3.3. Decontamination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, N.M.; Sharma, S.C. Novichok: An Overview of the World’s Deadliest Nerve Agent. Br. Stud. Dr. J. 2019, 3, 48. [Google Scholar] [CrossRef]
- Mirzayanov, V.S. State Secrets: An Insider’s Chronicle of the Russian Chemical Weapons Program; Outskirts Press, Incorporated: Denver, CO, USA, 2008; ISBN 978-1-4327-1923-4. [Google Scholar]
- Watson, A.; Opresko, D.; Young, R.A.; Hauschild, V.; King, J.; Bakshi, K. Organophosphate Nerve Agents. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2015; pp. 87–109. ISBN 978-0-12-800159-2. [Google Scholar]
- Hoenig, S.L. Compendium of Chemical Warfare Agents; Springer: New York, NY, USA, 2007; ISBN 978-0-387-34626-7. [Google Scholar]
- Ellison, D.H. Handbook of Chemical and Biological Warfare Agents; Taylor & Francis: Abingdon, UK, 2007; ISBN 978-1-4200-0329-1. [Google Scholar]
- Nepovimova, E.; Kuca, K. Novichoks. In Handbook of Toxicology of Chemical Warfare Agents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–148. ISBN 978-0-12-819090-6. [Google Scholar]
- Chai, P.R.; Hayes, B.D.; Erickson, T.B.; Boyer, E.W. Novichok Agents: A Historical, Current, and Toxicological Perspective. Toxicol. Commun. 2018, 2, 45–48. [Google Scholar] [CrossRef]
- Korabecny, J.; Soukup, O.; Dolezal, R.; Spilovska, K.; Nepovimova, E.; Andrs, M.; Nguyen, T.; Jun, D.; Musilek, K.; Kucerova-Chlupacova, M.; et al. From Pyridinium-Based to Centrally Active Acetylcholinesterase Reactivators. MRMC 2014, 14, 215–221. [Google Scholar] [CrossRef]
- Carlsen, L. After Salisbury Nerve Agents Revisited. Mol. Inf. 2019, 38, 1800106. [Google Scholar] [CrossRef]
- Harvey, S.P.; McMahon, L.R.; Berg, F.J. Hydrolysis and Enzymatic Degradation of Novichok Nerve Agents. Heliyon 2020, 6, e03153. [Google Scholar] [CrossRef] [PubMed]
- Kloske, M.; Witkiewicz, Z. Novichoks—The A Group of Organophosphorus Chemical Warfare Agents. Chemosphere 2019, 221, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Bhakhoa, H.; Rhyman, L.; Ramasami, P. Theoretical Study of the Molecular Aspect of the Suspected Novichok Agent A234 of the Skripal Poisoning. R. Soc. Open Sci. 2019, 6, 181831. [Google Scholar] [CrossRef]
- Haslam, J.D.; Russell, P.; Hill, S.; Emmett, S.R.; Blain, P.G. Chemical, Biological, Radiological, and Nuclear Mass Casualty Medicine: A Review of Lessons from the Salisbury and Amesbury Novichok Nerve Agent Incidents. Br. J. Anaesth. 2022, 128, e200–e205. [Google Scholar] [CrossRef] [PubMed]
- Steindl, D.; Boehmerle, W.; Körner, R.; Praeger, D.; Haug, M.; Nee, J.; Schreiber, A.; Scheibe, F.; Demin, K.; Jacoby, P.; et al. Novichok Nerve Agent Poisoning. Lancet 2021, 397, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Rahmania, T.A.; Wardhani, B.W.K.; Renesteen, E.; Harahap, Y. Chemical Properties, Biological Activities and Poisoning Treatment of Novichok: A Review. PSR 2021, 8, 2. [Google Scholar] [CrossRef]
- Franca, T.; Kitagawa, D.; Cavalcante, S.; da Silva, J.; Nepovimova, E.; Kuca, K. Novichoks: The Dangerous Fourth Generation of Chemical Weapons. Int. J. Mol. Sci. 2019, 20, 1222. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Miyaguchi, H. Theoretical Evaluation of the Hydrolysis of Conventional Nerve Agents and Novichok Agents. Chem. Phys. Lett. 2021, 785, 139116. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, B.; Singh, N.; Acharya, J.R.; Musilek, K.; Kuca, K.; Ghosh, K. Development and Structural Modifications of Cholinesterase Reactivators against Chemical Warfare Agents in Last Decade: A Review. MRMC 2015, 15, 58–72. [Google Scholar] [CrossRef]
- Nepovimova, E.; Kuca, K. Chemical Warfare Agent NOVICHOK—Mini-Review of Available Data. Food Chem. Toxicol. 2018, 121, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hrvat, N.M.; Kovarik, Z. Counteracting Poisoning with Chemical Warfare Nerve Agents. Arch. Ind. Hyg. Toxicol. 2020, 71, 266–284. [Google Scholar] [CrossRef]
- Leikin, J.B.; Thomas, R.G.; Walter, F.G.; Klein, R.; Meislin, H.W. A Review of Nerve Agent Exposure for the Critical Care Physician. Crit. Care Med. 2002, 30, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Saber, H. Recent Advances in the Treatment of Organophosphorous Poisonings. Iran. J. Med. Sci. 2012, 37, 74–91. [Google Scholar]
- Timperley, C.M.; Forman, J.E.; Abdollahi, M.; Al-Amri, A.S.; Baulig, A.; Benachour, D.; Borrett, V.; Cariño, F.A.; Geist, M.; Gonzalez, D.; et al. Advice on Assistance and Protection Provided by the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons: Part 1. On Medical Care and Treatment of Injuries from Nerve Agents. Toxicology 2019, 415, 56–69. [Google Scholar] [CrossRef]
- Bird, S.B. Diphenhydramine as a Protective Agent in a Rat Model of Acute, Lethal Organophosphate Poisoning. Acad. Emerg. Med. 2002, 9, 1369–1372. [Google Scholar] [CrossRef]
- Arendse, R.; Irusen, E. An Atropine and Glycopyrrolate Combination Reduces Mortality in Organophosphate Poisoning. Hum. Exp. Toxicol. 2009, 28, 715–720. [Google Scholar] [CrossRef]
- Bhandarkar, A.A. Efficacy of Atropine Alone and with Glycopyrrolate Combination in Organophosphate Poisoning. Value Health 2014, 17, A750–A751. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Aaron, C.K. Organophosphate and Carbamate Poisoning. Emerg. Med. Clin. N. Am. 2015, 33, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Martellini, M.; Trapp, R. (Eds.) 21st Century Prometheus: Managing CBRN Safety and Security Affected by Cutting-Edge Technologies; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-28284-4. [Google Scholar]
- Cornelissen, A.S.; Klaassen, S.D.; van Groningen, T.; Bohnert, S.; Joosen, M.J.A. Comparative Physiology and Efficacy of Atropine and Scopolamine in Sarin Nerve Agent Poisoning. Toxicol. Appl. Pharmacol. 2020, 396, 114994. [Google Scholar] [CrossRef] [PubMed]
- Sidell, F.; Newmark, J.; McDonough, J. Nerve Agent Bioscavenger: Development of a New Approach to Protect against 243 Organophosphorus Exposure. In Textbooks of Military Medicine, Medical Aspects of Chemical Warfare; Lenhart, M.K., Tuorinsky, S.D., Eds.; Department of the Army, United States of America: Washington, DC, USA, 2008; pp. 155–219. [Google Scholar]
- Masson, P. Evolution of and Perspectives on Therapeutic Approaches to Nerve Agent Poisoning. Toxicol. Lett. 2011, 206, 5–13. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.H.; Zoeffel, L.D.; McMonagle, J.; Copeland, T.L.; Smith, C.D.; Shih, T.-M. Anticonvulsant Treatment of Nerve Agent Seizures: Anticholinergics versus Diazepam in Soman-Intoxicated Guinea Pigs. Epilepsy Res. 2000, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.R.; Prasad, P.N.; Ghimire, R. Management of Organophosphorus Poisoning. J. Nepal Health Res. Counc. 2016, 14, 131–138. [Google Scholar]
- Balali-Mood, M.; Balali-Mood, K. Neurotoxic Disorders of Organophosphorus Compounds and Their Managements. Arch. Iran. Med. 2008, 11, 65–89. [Google Scholar]
- Shih, T.-M.; Rowland, T.C.; McDonough, J.H. Anticonvulsants for Nerve Agent-Induced Seizures: The Influence of the Therapeutic Dose of Atropine. J. Pharmacol. Exp. Ther. 2007, 320, 154–161. [Google Scholar] [CrossRef]
- Wu, X.; Kuruba, R.; Reddy, D.S. Midazolam-Resistant Seizures and Brain Injury after Acute Intoxication of Diisopropylfluorophosphate, an Organophosphate Pesticide and Surrogate for Nerve Agents. J. Pharmacol. Exp. Ther. 2018, 367, 302–321. [Google Scholar] [CrossRef]
- Reddy, S.D.; Reddy, D.S. Midazolam as an Anticonvulsant Antidote for Organophosphate Intoxication-A Pharmacotherapeutic Appraisal. Epilepsia 2015, 56, 813–821. [Google Scholar] [CrossRef]
- Tintinalli, J.E.; Stapczynski, J.S.; Ma, O.J.; Yealy, D.M.; Meckler, G.D.; Cline, D.M. (Eds.) Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2020; ISBN 978-1-260-01993-3. [Google Scholar]
- Eisenkraft, A.; Falk, A. The Possible Role of Intravenous Lipid Emulsion in the Treatment of Chemical Warfare Agent Poisoning. Toxicol. Rep. 2016, 3, 202–210. [Google Scholar] [CrossRef]
- Worek, F.; Thiermann, H.; Wille, T. Oximes in Organophosphate Poisoning: 60 Years of Hope and Despair. Chem. Biol. Interact. 2016, 259, 93–98. [Google Scholar] [CrossRef]
- Lorke, D.E.; Kalasz, H.; Petroianu, G.A.; Tekes, K. Entry of Oximes into the Brain: A Review. Curr. Med. Chem. 2008, 15, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Nepovimova, E.; Malinak, D.; Kucera, T.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. Progress in Acetylcholinesterase Reactivators and in the Treatment of Organophosphorus Intoxication: A Patent Review (2006–2016). Expert Opin. Ther. Pat. 2017, 27, 971–985. [Google Scholar] [CrossRef]
- DeClementi, C. Prevention and Treatment of Poisoning. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1361–1379. ISBN 978-0-12-385926-6. [Google Scholar]
- Nelson, L.; Goldfrank, L.R. (Eds.) Goldfrank’s Toxicologic Emergencies, 9th ed.; McGraw-Hill Medical: New York, NY, USA, 2011; ISBN 978-0-07-160593-9. [Google Scholar]
- Moshiri, M.; Darchini-Maragheh, E.; Balali-Mood, M. Advances in Toxicology and Medical Treatment of Chemical Warfare Nerve Agents. DARU J. Pharm. Sci. 2012, 20, 81. [Google Scholar] [CrossRef]
- Newmark, J. Therapy for Acute Nerve Agent Poisoning: An Update. Neurol. Clin. Pract. 2019, 9, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Eyer, P. The Role of Oximes in the Management of Organophosphorus Pesticide Poisoning. Toxicol. Rev. 2003, 22, 165–190. [Google Scholar] [CrossRef]
- Cuya, T.; da Silva Gonçalves, A.; da Silva, J.A.V.; Ramalho, T.C.; Kuca, K.; França, T.C.C. The Role of the Oximes HI-6 and HS-6 inside Human Acetylcholinesterase Inhibited with Nerve Agents: A Computational Study. J. Biomol. Struct. Dyn. 2018, 36, 3444–3452. [Google Scholar] [CrossRef]
- Kranawetvogl, T.; Steinritz, D.; Thiermann, H.; John, H. A Novel High-performance Liquid Chromatography with Diode Array Detector Method for the Simultaneous Quantification of the Enzyme-reactivating Oximes Obidoxime, Pralidoxime, and HI-6 in Human Plasma. Drug Test. Anal. 2020, 12, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Machamer, J.B.; Apland, J.P.; Winner, B.M.; Wolfe, S.E.; Pagarigan, K.T.; Bounader, K.M.; Kasten, S.A.; Adler, M.; McNutt, P.M. Functional Basis for Dose-Dependent Antagonism of Rat and Rabbit Neuromuscular Transmission by the Bis-Pyridinium Oxime MMB4. Arch. Toxicol. 2020, 94, 3877–3891. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.Z.; Ding, X.J.; Ding, Y. Clinical Observation and Comparison of the Effectiveness of Several Oxime Cholinesterase Reactivators. Scand. J. Work Environ. Health 1985, 11 (Suppl. 4), 46–48. [Google Scholar] [PubMed]
- Gorecki, L.; Soukup, O.; Kucera, T.; Malinak, D.; Jun, D.; Kuca, K.; Musilek, K.; Korabecny, J. Oxime K203: A Drug Candidate for the Treatment of Tabun Intoxication. Arch. Toxicol. 2019, 93, 673–691. [Google Scholar] [CrossRef]
- Santos, M.C.; Botelho, F.D.; Gonçalves, A.S.; Kitagawa, D.A.S.; Borges, C.V.N.; Carvalho-Silva, T.; Bernardo, L.B.; Ferreira, C.N.; Rodrigues, R.B.; Ferreira Neto, D.C.; et al. Are the Current Commercially Available Oximes Capable of Reactivating Acetylcholinesterase Inhibited by the Nerve Agents of the A-Series? Arch. Toxicol. 2022, 96, 2559–2572. [Google Scholar] [CrossRef]
- Chambers, J.E.; Meek, E.C. Central Neuroprotection Demonstrated by Novel Oxime Countermeasures to Nerve Agent Surrogates. Ann. N. Y. Acad. Sci. 2020, 1479, 5–12. [Google Scholar] [CrossRef]
- Nerve Agents. J. R. Army Med. Corps 2002, 148, 344–357. [CrossRef]
- Greathouse, B.; Zahra, F.; Brady, M.F. Acetylcholinesterase Inhibitors Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Prado, M.B.; Adiao, K.J. Acetylcholinesterase Inhibitors in Myasthenic Crisis: A Systematic Review of Observational Studies. Neurocrit. Care 2021, 35, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A. Acetylcholinesterase Inhibitors and Gulf War Illnesses. Proc. Natl. Acad. Sci. USA 2008, 105, 4295–4300. [Google Scholar] [CrossRef] [PubMed]
- Seeger, T.; Eichhorn, M.; Lindner, M.; Niessen, K.V.; Tattersall, J.E.H.; Timperley, C.M.; Bird, M.; Green, A.C.; Thiermann, H.; Worek, F. Restoration of Soman-Blocked Neuromuscular Transmission in Human and Rat Muscle by the Bispyridinium Non-Oxime MB327 in Vitro. Toxicology 2012, 294, 80–84. [Google Scholar] [CrossRef]
- Turner, S.R.; Chad, J.E.; Price, M.; Timperley, C.M.; Bird, M.; Green, A.C.; Tattersall, J.E.H. Protection against Nerve Agent Poisoning by a Noncompetitive Nicotinic Antagonist. Toxicol. Lett. 2011, 206, 105–111. [Google Scholar] [CrossRef]
- Katz, F.S.; Pecic, S.; Tran, T.H.; Trakht, I.; Schneider, L.; Zhu, Z.; Ton-That, L.; Luzac, M.; Zlatanic, V.; Damera, S.; et al. Discovery of New Classes of Compounds That Reactivate Acetylcholinesterase Inhibited by Organophosphates. ChemBioChem 2015, 16, 2205–2215. [Google Scholar] [CrossRef]
- de Koning, M.C.; Horn, G.; Worek, F.; van Grol, M. Discovery of a Potent Non-Oxime Reactivator of Nerve Agent Inhibited Human Acetylcholinesterase. Eur. J. Med. Chem. 2018, 157, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Horn, G.; de Koning, M.C.; van Grol, M.; Thiermann, H.; Worek, F. Interactions between Acetylcholinesterase, Toxic Organophosphorus Compounds and a Short Series of Structurally Related Non-Oxime Reactivators: Analysis of Reactivation and Inhibition Kinetics in Vitro. Toxicol. Lett. 2018, 299, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of Acute Organophosphorus Pesticide Poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Pajoumand, A.; Shadnia, S.; Rezaie, A.; Abdi, M.; Abdollahi, M. Benefits of Magnesium Sulfate in the Management of Acute Human Poisoning by Organophosphorus Insecticides. Hum. Exp. Toxicol. 2004, 23, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Brvar, M.; Chan, M.Y.; Dawson, A.H.; Ribchester, R.R.; Eddleston, M. Magnesium Sulfate and Calcium Channel Blocking Drugs as Antidotes for Acute Organophosphorus Insecticide Poisoning—A Systematic Review and Meta-Analysis. Clin. Toxicol. 2018, 56, 725–736. [Google Scholar] [CrossRef]
- Jamshidi, F.; Yazdanbakhsh, A.; Jamalian, M.; Khademhosseini, P.; Ahmadi, K.; Sistani, A.; Jokar, A. Therapeutic Effect of Adding Magnesium Sulfate in Treatment of Organophosphorus Poisoning. Open Access Maced. J. Med. Sci. 2018, 6, 2051–2056. [Google Scholar] [CrossRef]
- Myhrer, T.; Enger, S.; Aas, P. Anticonvulsant Efficacy of Drugs with Cholinergic and/or Glutamatergic Antagonism Microinfused into Area Tempestas of Rats Exposed to Soman. Neurochem. Res. 2008, 33, 348–354. [Google Scholar] [CrossRef]
- Hirbec, H.; Gaviria, M.; Vignon, J. Gacyclidine: A New Neuroprotective Agent Acting at the N-Methyl-D-Aspartate Receptor. CNS Drug Rev. 2001, 7, 172–198. [Google Scholar] [CrossRef]
- Coleman, B.R.; Ratcliffe, R.H.; Oguntayo, S.A.; Shi, X.; Doctor, B.P.; Gordon, R.K.; Nambiar, M.P. [+]-Huperzine A Treatment Protects against N-Methyl-D-Aspartate-Induced Seizure/Status Epilepticus in Rats. Chem. Biol. Interact. 2008, 175, 387–395. [Google Scholar] [CrossRef]
- Garcia, G.E.; Vernon, A.; Moorad-Doctor, D.; Ratcliffe, R.H. (-)Huperzine A, Replacement for Pyridostigmine Bromide as Nerve Agent Pretreatment, Measured in Guinea Pig Plasma by a New Ultrahigh-pressure Liquid Chromatography (UHPLC)-MS Method. FASEB J. 2009, 23, 751–755. [Google Scholar] [CrossRef]
- Dhote, F.; Carpentier, P.; Barbier, L.; Peinnequin, A.; Baille, V.; Pernot, F.; Testylier, G.; Beaup, C.; Foquin, A.; Dorandeu, F. Combinations of Ketamine and Atropine Are Neuroprotective and Reduce Neuroinflammation after a Toxic Status Epilepticus in Mice. Toxicol. Appl. Pharmacol. 2012, 259, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Lyagin, I.; Efremenko, E. Theoretical Evaluation of Suspected Enzymatic Hydrolysis of Novichok Agents. Catal. Commun. 2019, 120, 91–94. [Google Scholar] [CrossRef]
- Ross, M.; Broomfield, C.; Cerasoli, D.; Doctor, B.; Lenz, D.; Maxwell, D.; Saxena, A. Chapter 7: Nerve Agent Bioscavenger: Development of a New Approach to Protect against Organophosphorus Exposure. In Textbooks of Military Medicine, Medical Aspects of Chemical Warfare; Lenhart, M.K., Tuorinsky, S.D., Eds.; Department of the Army, United States of America: Washington, DC, USA, 2008; pp. 243–259. [Google Scholar]
- Myers, T.M. Human Plasma-Derived Butyrylcholinesterase Is Behaviorally Safe and Effective in Cynomolgus Macaques (Macaca Fascicularis) Challenged with Soman. Chem.-Biol. Interact. 2019, 308, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Pazooki, S.; Solhi, H.; Vishteh, H.R.K.; Shadnia, S.; Beigi, M.J.B. Effectiveness of Fresh Frozen Plasma as Supplementary Treatment in Organophosphate Poisoning. Med. J. Malays. 2011, 66, 342–345. [Google Scholar]
- Gaydess, A.; Duysen, E.; Li, Y.; Gilman, V.; Kabanov, A.; Lockridge, O.; Bronich, T. Visualization of Exogenous Delivery of Nanoformulated Butyrylcholinesterase to the Central Nervous System. Chem. Biol. Interact. 2010, 187, 295–298. [Google Scholar] [CrossRef]
- Byers, M.; Russell, M.; Lockey, D.J. Clinical Care in the “Hot Zone”. Emerg. Med. J. 2008, 25, 108–112. [Google Scholar] [CrossRef]
- Levitin, H.W.; Siegelson, H.J. Hazardous Materials. Disaster Medical Planning and Response. Emerg. Med. Clin. N. Am. 1996, 14, 327–348. [Google Scholar] [CrossRef]
- Kumar, V.; Goel, R.; Chawla, R.; Silambarasan, M.; Sharma, R. Chemical, Biological, Radiological, and Nuclear Decontamination: Recent Trends and Future Perspective. J. Pharm. Bioall. Sci. 2010, 2, 220. [Google Scholar] [CrossRef]
- Trzos, A.; Jurowski, K. Emergency Medical Services in CBRNE/HAZMAT Incidents. SFT 2019, 54, 142–159. [Google Scholar] [CrossRef]
- Melnikova, N.; Wu, J.; Yang, A.; Orr, M. Acute Chemical Incidents With Injured First Responders, 2002–2012. Disaster Med. Public Health Prep. 2018, 12, 211–221. [Google Scholar] [CrossRef]
- Arnold, J.L.; Dembry, L.-M.; Tsai, M.-C.; Dainiak, N.; Rodoplu, Ü.; Schonfeld, D.J.; Parwani, V.; Paturas, J.; Cannon, C.; Selig, S. Recommended Modifications and Applications of the Hospital Emergency Incident Command System for Hospital Emergency Management. Prehosp. Disaster Med. 2005, 20, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Peplow, M. Nerve Agent Attack Used ‘Novichok’ Poison. Chem. Eng. News 2018, 96, 3. [Google Scholar]
- Okumura, T.; Takasu, N.; Ishimatsu, S.; Miyanoki, S.; Mitsuhashi, A.; Kumada, K.; Tanaka, K.; Hinohara, S. Report on 640 Victims of the Tokyo Subway Sarin Attack. Ann. Emerg. Med. 1996, 28, 129–135. [Google Scholar] [CrossRef]
- Nakajima, T.; Sato, S.; Morita, H.; Yanagisawa, N. Sarin Poisoning of a Rescue Team in the Matsumoto Sarin Incident in Japan. Occup. Environ. Med. 1997, 54, 697–701. [Google Scholar] [CrossRef]
- Chai, P.R.; Berlyand, Y.; Goralnick, E.; Goldfine, C.E.; VanRooyen, M.J.; Hryhorczuk, D.; Erickson, T.B. Wartime Toxicology: The Spectre of Chemical and Radiological Warfare in Ukraine. Toxicol. Commun. 2022, 6, 52–58. [Google Scholar] [CrossRef]
- NHS England Clinical Guidelines for Major Incidents and Mass Casualty Events. Available online: https://www.england.nhs.uk/publication/clinical-guidelines-for-major-incidents-and-mass-casualty-events/ (accessed on 20 February 2023).
- NHS England Hazardous Materials (HAZMAT) and Chemical, Biological, Radiological and Nuclear (CBRN). Available online: https://www.england.nhs.uk/ourwork/eprr/hm/#ior (accessed on 20 February 2023).
- Balali-Mood, M.; Mathews, R.; Pita, R.; Rice, P.; Romano, J.; Thiermann, H.; Willems, J. Practical Guide for Medical Management of Chemical Warfare Casualties; International Cooperation and Assistance Division, Assistance and Protection Branch, OPCW: Hague, The Netherlands, 2016. [Google Scholar]
- Hulse, E.J.; Haslam, J.D.; Emmett, S.R.; Woolley, T. Organophosphorus Nerve Agent Poisoning: Managing the Poisoned Patient. Br. J. Anaesth. 2019, 123, 457–463. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noga, M.; Michalska, A.; Jurowski, K. Review of Possible Therapies in Treatment of Novichoks Poisoning and HAZMAT/CBRNE Approaches: State of the Art. J. Clin. Med. 2023, 12, 2221. https://doi.org/10.3390/jcm12062221
Noga M, Michalska A, Jurowski K. Review of Possible Therapies in Treatment of Novichoks Poisoning and HAZMAT/CBRNE Approaches: State of the Art. Journal of Clinical Medicine. 2023; 12(6):2221. https://doi.org/10.3390/jcm12062221
Chicago/Turabian StyleNoga, Maciej, Agata Michalska, and Kamil Jurowski. 2023. "Review of Possible Therapies in Treatment of Novichoks Poisoning and HAZMAT/CBRNE Approaches: State of the Art" Journal of Clinical Medicine 12, no. 6: 2221. https://doi.org/10.3390/jcm12062221
APA StyleNoga, M., Michalska, A., & Jurowski, K. (2023). Review of Possible Therapies in Treatment of Novichoks Poisoning and HAZMAT/CBRNE Approaches: State of the Art. Journal of Clinical Medicine, 12(6), 2221. https://doi.org/10.3390/jcm12062221




