Safety and Efficacy of Different Anticoagulant Doses for Patients with COVID-19 in the ICU: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
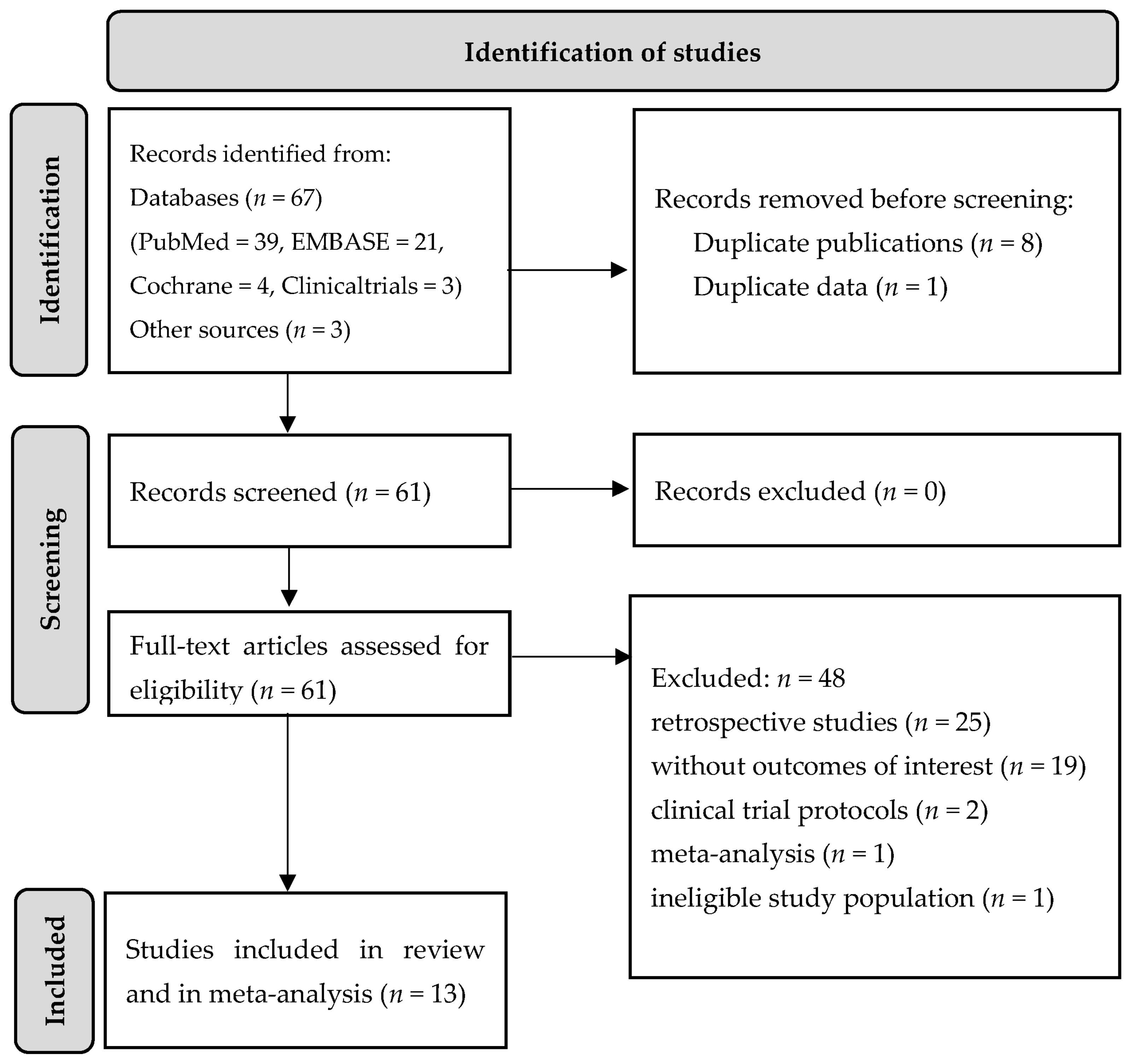
2.2. Eligibility Criteria
2.3. Data Extraction and Data Synthesis
2.4. Statistical Analyses
3. Results
3.1. Short-Term Mortality
3.2. Deep Vein Thrombosis
3.3. Pulmonary Embolism
3.4. Arterial Thrombosis
3.5. Major Bleeding
3.6. Minor Bleeding
3.7. Publication Bias and Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 15 November 2022).
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.C.; Goligher, E.C.; Lawler, P.R.; Slutsky, A.S.; Zarychanski, R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. Can. Med. Assoc. J. 2020, 192, E1156–E1161. [Google Scholar] [CrossRef] [PubMed]
- Moores, L.K.; Tritschler, T.; Brosnahan, S.; Carrier, M.; Collen, J.F.; Doerschug, K.; Holley, A.B.; Jimenez, D.; Le Gal, G.; Rali, P.; et al. Prevention, Diagnosis, and Treatment of VTE in Patients with Coronavirus Disease 2019, CHEST Guideline and Expert Panel Report. Chest 2020, 158, 1143–1163. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [Green Version]
- Valeriani, E.; Porfidia, A.; Ageno, W.; Spoto, S.; Pola, R.; Di Nisio, M. High-dose versus low-dose venous thromboprophylaxis in hospitalized patients with COVID-19, a systematic review and meta-analysis. Intern. Emerg. Med. 2022, 17, 1817–1825. [Google Scholar] [CrossRef]
- Kollias, A.; Kyriakoulis, K.G.; Lagou, S.; Kontopantelis, E.; Stergiou, G.S.; Syrigos, K. Venous thromboembolism in COVID-19, A systematic review and meta-analysis. Vasc. Med. 2021, 26, 415–425. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.; Wang, C.; Jin, J.; Qu, L. A systematic review and meta-analysis of incidence, prognosis, and laboratory indicators of venous thromboembolism in hospitalized patients with coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 1099–1111.e6. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19, A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Malato, A.; Dentali, F.; Siragusa, S.; Fabbiano, F.; Kagoma, Y.; Boddi, M.; Gensini, G.F.; Peris, A.; Crowther, M.; Napolitano, M. The impact of deep vein thrombosis in critically ill patients: A meta-analysis of major clinical outcomes. Blood Transfus. 2015, 13, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Nadkarni, G.N.; Lala, A.; Bagiella, E.; Chang, H.L.; Moreno, P.R.; Pujadas, E.; Arvind, V.; Bose, S.; Charney, A.W.; Chen, M.D.; et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1815–1826. [Google Scholar] [CrossRef]
- Thachil, J. The versatile heparin in COVID-19. J. Thromb. Haemost. 2020, 18, 1020–1022. [Google Scholar] [CrossRef] [Green Version]
- Clinical Management of COVID-19, Living Guideline, 15 September 2022. Geneva: World Health Organization. 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-2022.2 (accessed on 10 November 2022).
- Kyriakoulis, K.G.; Dimakakos, E.; Kyriakoulis, I.G.; Catalano, M.; Spyropoulos, A.C.; Schulman, S.; Douketis, J.; Falanga, A.; Maraveyas, A.; Olinic, D.M.; et al. Practical Recommendations for Optimal Thromboprophylaxis in Patients with COVID-19, A Consensus Statement Based on Available Clinical Trials. J. Clin. Med. 2022, 11, 5997. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Connors, J.M.; Douketis, J.D.; Goldin, M.; Hunt, B.J.; Kotila, T.R.; Lopes, R.D.; Schulman, S.; International Society on Thrombosis and Haemostasis. Good practice statements for antithrombotic therapy in the management of COVID-19, Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2022, 20, 2226–2236, Does High-Dose Thromboprophylaxis Improve Outcomes in COVID-19 Patients. [Google Scholar] [CrossRef]
- Hasan, S.S.; Radford, S.; Kow, C.S.; Zaidi, S.T.R. Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: A systematic review and meta-analysis. J. Thromb. Thrombolysis 2020, 50, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Santoliquido, A.; Porfidia, A.; Nesci, A.; De Matteis, G.; Marrone, G.; Porceddu, E.; Cammà, G.; Giarretta, I.; Fantoni, M.; Landi, F.; et al. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J. Thromb. Haemost. 2020, 18, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19, An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Patell, R.; Chiasakul, T.; Bauer, E.; Zwicker, J.I. Pharmacologic Thromboprophylaxis and Thrombosis in Hospitalized Patients with COVID-19, A Pooled Analysis. Thromb. Haemost. 2021, 121, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, F.; Jaiyesimi, I.; Petrescu, I.; Lawler, P.R.; Castillo, E.; Munoz-Maldonado, Y.; Imam, Z.; Narasimhan, M.; Abbas, A.E.; Konde, A.; et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: A retrospective propensity score-weighted analysis. Eur. J. Haematol. 2021, 106, 165–174. [Google Scholar] [CrossRef]
- Flumignan, R.L.; Civile, V.T.; Tinôco, J.D.S.; Pascoal, P.I.; Areias, L.L.; Matar, C.F.; Tendal, B.; Trevisani, V.F.; Atallah, Á.N.; Nakano, L.C. Anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst. Rev. 2022, 3, CD013739. [Google Scholar] [CrossRef]
- Yasuda, H.; Mayumi, T.; Okano, H. Efficacy of different anticoagulant doses for patients with COVID-19, a systematic review and network meta-analysis. Infection 2022, 50, 1453–1463. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Risk of Bias Tools Website. Available online: https://www.riskofbias.info/ (accessed on 10 May 2022).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021; 544p. [Google Scholar]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators; Goligher, E.C.; Bradbury, C.A.; McVerry, B.J.; Lawler, P.R.; Berger, J.S.; Gong, M.N.; Carrier, M.; et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar] [CrossRef]
- Lemos, A.C.B.; do Espírito Santo, D.A.; Salvetti, M.C.; Gilio, R.N.; Agra, L.B.; Pazin-Filho, A.; Miranda, C.H. Therapeutic versus prophylactic anticoagulation for severe COVID-19, A randomized phase II clinical trial (HESACOVID). Thromb. Res. 2020, 196, 359–366. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19, A multi-center, open-label, randomized controlled trial. J. Thromb. Haemost. 2021, 19, 2225–2234. [Google Scholar] [CrossRef]
- INSPIRATION Investigators; Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients with COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19, The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620. [Google Scholar] [CrossRef]
- Helms, J.; Severac, F.; Merdji, H.; Schenck, M.; Clere-Jehl, R.; Baldacini, M.; Ohana, M.; Grunebaum, L.; Castelain, V.; Anglés-Cano, E.; et al. Higher anticoagulation targets and risk of thrombotic events in severe COVID-19 patients: Bi-center cohort study. Ann. Intensive Care 2021, 11, 14. [Google Scholar] [CrossRef]
- Stessel, B.; Vanvuchelen, C.; Bruckers, L.; Geebelen, L.; Callebaut, I.; Vandenbrande, J.; Pellens, B.; Van Tornout, M.; Ory, J.P.; van Halem, K.; et al. Impact of implementation of an individualised thromboprophylaxis protocol in critically ill ICU patients with COVID-19, A longitudinal controlled before-after study. Thromb. Res. 2020, 194, 209–215. [Google Scholar] [CrossRef]
- Voicu, S.; Chousterman, B.G.; Bonnin, P.; Deye, N.; Malissin, I.; Gall, A.L.; Barthélémy, R.; Sutterlin, L.; Naim, G.; Mrad, A.; et al. Increased anticoagulation reduces proximal deep vein thrombosis in mechanically ventilated COVID-19 patients: Venous thrombosis prevention & COVID-19. J. Infect. 2021, 82, 186–230. [Google Scholar] [CrossRef]
- Ferrandis, R.; Escontrela, B.; Ferrando, C.; Hernandez, M.; Herrera, J.; Hidalgo, F.; Librero, J.; Llau, J.V.; Martinez, A.; Pajares, A.; et al. Eficacia de la tromboprofilaxis con heparina de bajo peso molecular en pacientes críticos con COVID-19, estudio observacional, prospectivo y multicéntrico [Effectiveness of thromboprophylaxis with low molecular weight heparin in critically ill patients with COVID-19. An observational, prospective, multicenter study.]. Rev. Esp. Anestesiol. Reanim. 2022, in press. [Google Scholar] [CrossRef]
- Ren, B.; Yan, F.; Deng, Z.; Zhang, S.; Xiao, L.; Wu, M.; Cai, L. Extremely High Incidence of Lower Extremity Deep Venous Thrombosis in 48 Patients with Severe COVID-19 in Wuhan. Circulation 2020, 142, 181–183. [Google Scholar] [CrossRef]
- Stattin, K.; Lipcsey, M.; Andersson, H.; Pontén, E.; Bülow Anderberg, S.; Gradin, A.; Larsson, A.; Lubenow, N.; von Seth, M.; Rubertsson, S.; et al. Inadequate prophylactic effect of low-molecular weight heparin in critically ill COVID-19 patients. J. Crit. Care 2020, 60, 249–252. [Google Scholar] [CrossRef]
- Voicu, S.; Bonnin, P.; Stépanian, A.; Chousterman, B.G.; Le Gall, A.; Malissin, I.; Deye, N.; Siguret, V.; Mebazaa, A.; Mégarbane, B. High Prevalence of Deep Vein Thrombosis in Mechanically Ventilated COVID-19 Patients. J. Am. Coll. Cardiol. 2020, 76, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- The SAPS 3 Research Group. Available online: https://www.saps3.org/ (accessed on 16 January 2023).
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients with Coronavirus Disease 2019, A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar] [CrossRef]
- Loffredo, L.; Di Castelnuovo, A.; Chiariello, G.A.; Pignatelli, P.; Violi, F. Full versus prophylactic-intermediate doses of anticoagulants in COVID-19, a meta-analysis. Haematologica 2022, 107, 1933–1939. [Google Scholar] [CrossRef]
- Elsebaie, M.A.T.; Baral, B.; Elsebaie, M.; Shrivastava, T.; Weir, C.; Kumi, D.; Birch, N.W. Does High-Dose Thromboprophylaxis Improve Outcomes in COVID-19 Patients? A Meta-analysis of Comparative Studies. TH Open 2022, 6, e323–e334. [Google Scholar] [CrossRef]
- Parisi, R.; Costanzo, S.; Di Castelnuovo, A.; de Gaetano, G.; Donati, M.B.; Iacoviello, L. Different Anticoagulant Regimens, Mortality, and Bleeding in Hospitalized Patients with COVID-19, A Systematic Review and an Updated Meta-Analysis. Semin. Thromb. Hemost. 2021, 47, 372–391. [Google Scholar] [CrossRef]
- Sofia, R.; Carbone, M.; Landoni, G.; Zangrillo, A.; Dagna, L. Anticoagulation as secondary prevention of massive lung thromboses in hospitalized patients with COVID-19. Eur. J. Intern. Med. 2022, 100, 21–24. [Google Scholar] [CrossRef]
- Vedovati, M.C.; Graziani, M.; Agnelli, G.; Becattini, C. Efficacy and safety of two heparin regimens for prevention of venous thromboembolism in hospitalized patients with COVID-19, a meta-analysis. Intern. Emerg. Med. 2022, 29, 1–15. [Google Scholar] [CrossRef]






| Study (Author, Year) | Country | Study Design | Patients in ICU, % | Comparisons | Type of Anticoagulation | Sample, n | Mean/Median Age, y | Male, % | Average APACHE II Score [46] | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short-Term Mortality | DVT | PE | AT | Major Bleeding | Minor Bleeding | ||||||||||
| Ferrandis, 2022 [42] | Spain | Observational | 100 | Treatment dose | UFH | 462 | 63.5 | 32 | 12 | + | + | + | + | ||
| Prophylactic dose | UFH | 258 | 63 | 33 | 12 | ||||||||||
| Goligher, 2021 [34] | Canada, multinational | Open-label RCT | 100 | Treatment dose | UFH LMWH | 534 | 60.4 | 72.2 | 14 | + | + | + | |||
| Prophylactic dose | UFH LMWH | 564 | 61.7 | 67.9 | 13 | ||||||||||
| Helms, 2021 [39] | France | Before/after study | 100 | Treatment dose | LMWH, UFH | 71 | 64 | 66.2 | 47 * | + | + | + | + | ||
| Prophylactic dose | LMWH, UFH | 108 | 61 | 76.9 | 48 * | ||||||||||
| Klok, 2020 [10] | Norway | Observational | 100 | Prophylactic dose | LMWH (nadroparin) | 184 | 64 | 76 | na | + | + | + | + | ||
| Lemos, 2020 [35] | Brazil | Open-label RCT | 100 | Treatment dose | LMWH (enoxaparin) | 10 | 55 | 90 | 56 ** | + | + | + | + | + | + |
| Prophylactic dose | LMWH (enoxaparin) | 10 | 58 | 70 | 56 ** | ||||||||||
| Perepu, 2021 [36] | USA | Multi-center, open-label RCT | 62 | Intermediate dose | LMWH (enoxaparin) | 86 | 65 | 54 | na | + | + | + | + | ||
| Prophylactic dose | LMWH (enoxaparin) | 87 | 63.5 | 58 | na | ||||||||||
| Ren, 2020 [43] | China | Cross-sectional | 100 | Prophylactic dose | LMWH | 48 | 70 | 54.2 | 16 | + | + | ||||
| Sadeghipour, 2021 [37] | Iran | Multicenter RCT with a 2 × 2 factorial design | 100 | Intermediate dose | LMWH (enoxaparin) | 276 | 62 | 57 | 8 | + | + | + | + | + | |
| Prophylactic dose | LMWH (enoxaparin) | 286 | 61 | 58.7 | 8 | ||||||||||
| Spyropoulos, 2021 [38] | USA | RCT | 100 | Treatment dose | LMWH (enoxaparin) | 45 | 65.8 | 52.7 | na | + | |||||
| Stattin, 2020 [44] | Sweden | Observational | 100 | Prophylactic dose | LMWH (dalteparin) | 31 | 65 | 81 | 53 ** | + | + | + | + | + | |
| Stessel, 2020 [40] | Belgium | Longitudinal controlled before/after study | 100 | Intermediate dose | LMWH (nadroparin) | 26 | 62 | 57.7 | 11 | + | + | ||||
| Prophylactic dose | LMWH (nadroparin) | 46 | 69.5 | 73.9 | 13 | ||||||||||
| Voicu, 2020 [45] | France | Observational | 100 | Prophylactic dose | LMWH (enoxaparin), UFH | 56 | na | 75 | na | + | |||||
| Voicu, 2021 [41] | France | Before/after observational exploratory study | 100 | Prophylactic dose | LMWH (enoxaparin), UFH | 50 | 62 | 72 | na | + | + | ||||
| Outcome | Prophylactic Dose | Intermediate Dose | Therapeutic Dose | Total | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | No. of Patients | No. of Studies | No. of Patients | No. of Studies | No. of Patients | No. of Studies | No. of Patients | |
| Short-term mortality | 10 | 1640 | 3 | 389 | 4 | 1077 | 10 | 3106 |
| Deep vein thrombosis | 11 | 1672 | 3 | 389 | 4 | 1073 | 11 | 3134 |
| Pulmonary embolism | 4 | 333 | 0 | 0 | 2 | 81 | 4 | 414 |
| Arterial thrombosis | 5 | 619 | 1 | 276 | 2 | 81 | 5 | 976 |
| Major bleeding | 7 | 1283 | 2 | 363 | 4 | 1046 | 8 | 2692 |
| Minor bleeding | 4 | 585 | 2 | 363 | 3 | 558 | 5 | 1506 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachina, S.; Belkova, Y.; Shchendrygina, A.; Suvorov, A.; Bourgeois, D.; Karuk, M.; Sitnikova, V.; Dyatlov, N. Safety and Efficacy of Different Anticoagulant Doses for Patients with COVID-19 in the ICU: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2222. https://doi.org/10.3390/jcm12062222
Rachina S, Belkova Y, Shchendrygina A, Suvorov A, Bourgeois D, Karuk M, Sitnikova V, Dyatlov N. Safety and Efficacy of Different Anticoagulant Doses for Patients with COVID-19 in the ICU: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(6):2222. https://doi.org/10.3390/jcm12062222
Chicago/Turabian StyleRachina, Svetlana, Yuliya Belkova, Anastasia Shchendrygina, Aleksandr Suvorov, Denis Bourgeois, Marina Karuk, Violetta Sitnikova, and Nikita Dyatlov. 2023. "Safety and Efficacy of Different Anticoagulant Doses for Patients with COVID-19 in the ICU: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 6: 2222. https://doi.org/10.3390/jcm12062222







