Are There Ethnic Differences in Hand Eczema? A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection of Articles: Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
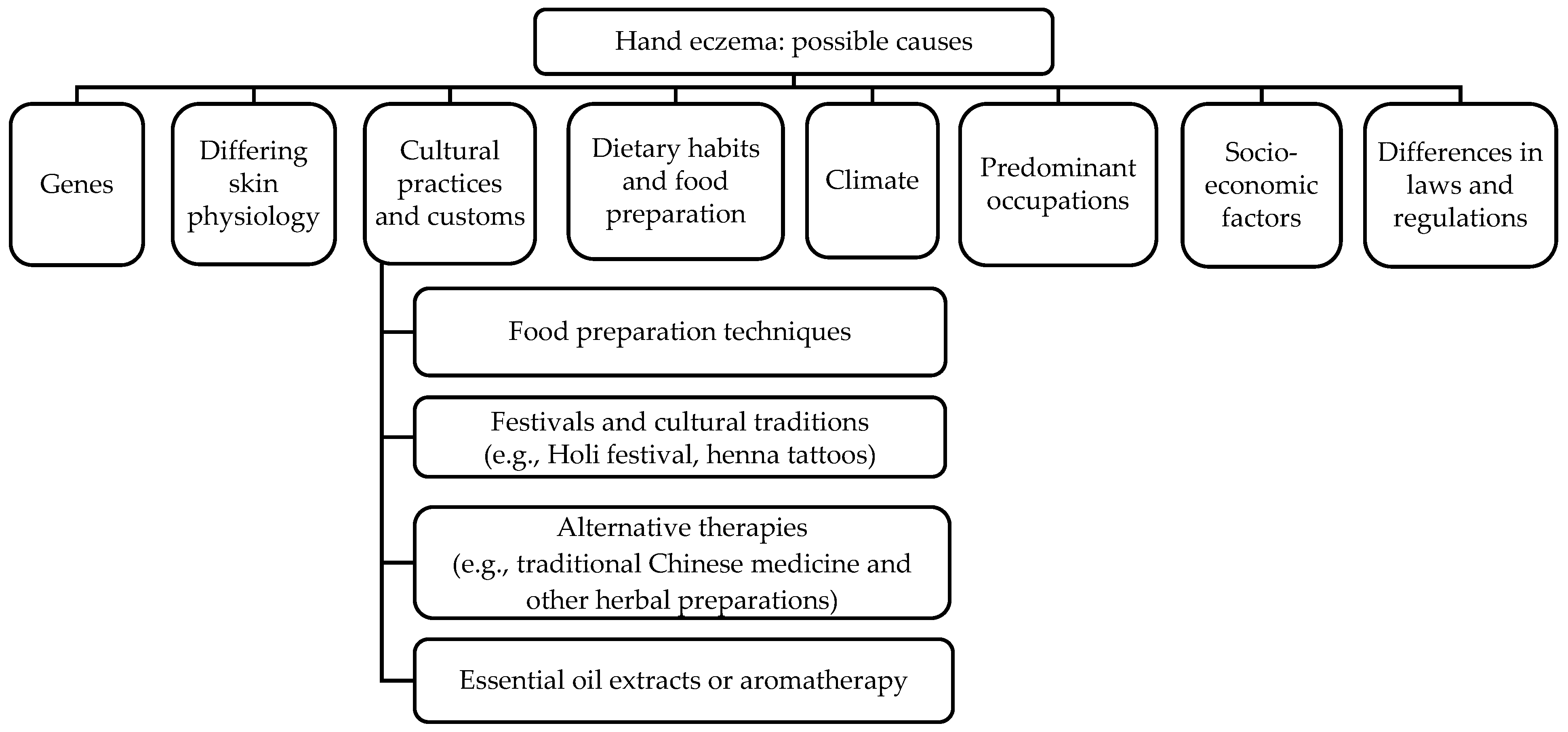
3. Results
3.1. Genetic Factors
3.2. Differing Skin Physiology
3.3. Cultural Practices and Customs Leading to Exposure to Chemicals
3.4. Dietary Habits and Food Preparation
3.5. Climate
3.6. Predominant Occupations in Various Ethnic Communities Affecting Exposure Risk
3.7. Socioeconomic Factors
3.8. Different Laws and Regulations
4. Discussion
4.1. Summary
4.2. Conclusions
4.3. Further Research Areas
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thyssen, J.P.; Schuttelaar, M.L.A.; Alfonso, J.H.; Andersen, K.E.; Angelova-Fischer, I.; Arents, B.W.M.; Bauer, A.; Brans, R.; Cannavo, A.; Christoffers, W.A.; et al. Guidelines for the Diagnosis, Treatment, and Prevention of Hand Eczema. Contact Dermat. 2022, 86, 357–378. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Incorporation of a barrier protection cream in the management of chronic hand dermatitis: Focus on data supporting an established hand protectant formulation and modifications designed to assist in barrier repair. J. Clin. Aesthetic Dermatol. 2014, 7, 40–48. [Google Scholar]
- Quaade, A.S.; Simonsen, A.B.; Halling, A.-S.; Thyssen, J.P.; Johansen, J.D. Prevalence, incidence, and severity of hand eczema in the general population–a systematic review and meta-analysis. Contact Dermat. 2021, 84, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.; Hahn-Pedersen, J.; Bartlett, C.; Glanville, J.; Thyssen, J.P. Economic Burden of Chronic Hand Eczema: A Review. Am. J. Clin. Dermatol. 2022, 23, 287–300. [Google Scholar] [CrossRef]
- Maden, S.; Ozbagcivan, O.; Aysevener, B.E.O.; Aktan, S. Quality of life, anxiety, depression, social anxiety and avoidance in patients with chronic hand eczema. Ital. J. Dermatol. Venerol. 2021, 156, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kouris, A.; Armyra, K.; Christodoulou, C.; Katoulis, A.; Potouridou, I.; Tsatovidou, R.; Rigopoulos, D.; Kontochristopoulos, G. Quality of life, anxiety, depression and obsessive-compulsive tendencies in patients with chronic hand eczema. Contact Dermat. 2015, 72, 367–370. [Google Scholar] [CrossRef]
- Coenraads, P.-J. Hand eczema. N. Engl. J. Med. 2012, 367, 1829–1837. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, S.H.; Kim, K.J.; Lee, G.-Y.; Yang, J.-M.; Kim, D.W.; Lee, S.J.; Lee, C.H.; Park, E.J.; Kim, K.H.; et al. Clinical Features and Awareness of Hand Eczema in Korea. Ann. Dermatol. 2016, 28, 335–343. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Elsner, P.; Schliemann, S.; Fartasch, M.; Köllner, A.; Skudlik, C.; John, S.M.; Worm, M. Deutsche Dermatologische Gesellschaft. Guideline on the management of hand eczema ICD-10 Code: L20. L23. L24. L25. L30. J. Dtsch. Dermatol. Ges. 2009, 7, S1–S16. [Google Scholar]
- James, G.H. Dinulos. Habif’s Clinical Dermatology: A Color Guide to Diagnosis and Therapy, 7th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 90–124. [Google Scholar]
- Mahler, V. Hand dermatitis—Differential diagnoses, diagnostics, and treatment options. J. Dtsch. Dermatol. Ges. 2016, 14, 7–26. [Google Scholar] [CrossRef]
- Ahmed, Z.H.; Agarwal, K. Rashmi SarkarHand Dermatitis: A Comprehensive Review with Special Emphasis on COVID-19 Pandemic. Indian J. Dermatol. 2021, 66, 508–519. [Google Scholar] [PubMed]
- Agarwal, U.S.; Besarwal, R.K.; Gupta, R.; Agarwal, P.; Napalia, S. Hand Eczema. Indian J. Dermatol. 2014, 59, 213–224. [Google Scholar] [CrossRef]
- Lerbaek, A.; Bisgaard, H.; Agner, T.; Ohm Kyvik, K.; Palmer, C.N.A.; Menné, T. Filaggrin null alleles are not associated with hand eczema or contact allergy. Br. J. Dermatol. 2007, 157, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, B.C.; Johansen, J.D.; Menné, T.; Meldgaard, M.; Szecsi, P.B.; Stender, S.; Thyssen, J.P. Filaggrin null mutations and association with contact allergy and allergic contact dermatitis: Results from a tertiary dermatology clinic. Contact Dermat. 2010, 63, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.; Carlsen, B.; Menné, T.; Linneberg, A.; Nielsen, N.; Meldgaard, M.; Szecsi, P.; Stender, S.; Johansen, J. Filaggrin null mutations increase the risk and persistence of hand eczema in subjects with atopic dermatitis: Results from a general population study. Br. J. Dermatol. 2010, 163, 115–120. [Google Scholar] [CrossRef]
- Bandier, J.; Ross-Hansen, K.; Carlsen, B.C.; Menné, T.; Linneberg, A.; Stender, S.; Szecsi, P.B.; Meldgaard, M.; Thyssen, J.P.; Johansen, J.D. Carriers of filaggrin gene (FLG) mutations avoid professional exposure to irritants in adulthood. Contact Dermat. 2013, 69, 355–362. [Google Scholar] [CrossRef]
- Molin, S.; Vollmer, S.; Weiss, E.; Ruzicka, T.; Prinz, J. Filaggrin mutations may confer susceptibility to chronic hand eczema characterized by combined allergic and irritant contact dermatitis. Br. J. Dermatol. 2009, 161, 801–807. [Google Scholar] [CrossRef]
- Brunner, P.M.; Guttman-Yassky, E. Racial differences in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 449–455. [Google Scholar] [CrossRef]
- Kaufman, B.P.; Guttman-Yassky, E.; Alexis, A.F. Atopic dermatitis in diverse racial and ethnic groups—Variations in epidemiology, genetics, clinical presentation and treatment. Exp. Dermatol. 2018, 27, 340–357. [Google Scholar] [CrossRef]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef]
- Rawlings, A.V. Ethnic skin types: Are there differences in skin structure and function? Int. J. Cosmet. Sci. 2006, 28, 79–93. [Google Scholar] [CrossRef]
- Wu, Y.; Wangari-Olivero, J.; Zhen, Y. Compromised Skin Barrier and Sensitive Skin in Diverse Populations. J. Drugs Dermatol. 2021, 20, 17–22. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Hellemans, L.; Van Overloop, L.; Corstjens, H.; Declercq, L.; Maes, D. Structural and functional differences in barrier properties of African American, Caucasian and East Asian skin. J. Dermatol. Sci. 2010, 59, 123–128. [Google Scholar] [CrossRef]
- Noor, H.A.; Suaini, C.P.T.; Tan, E.X.L.; Loo, E.H.T. Global differences in atopic dermatitis. Pediatr. Allergy Immunol. 2021, 32, 23–33. [Google Scholar]
- Kim, Y.; Blomberg, M.; Rifas-Shiman, S.L.; Camargo, C.A.; Gold, D.R.; Thyssen, J.P.; Litonjua, A.A.; Oken, E.; Asgari, M.M. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J. Investig. Dermatol. 2019, 139, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Hattangdi-Haridas, S.R.; A Lanham-New, S.; Wong, W.H.S.; Ho, M.H.K.; Darling, A.L. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019, 11, 1854. [Google Scholar] [CrossRef]
- Thuesen, B.H.; Heede, N.G.; Tang, L.; Skaaby, T.; Thyssen, J.P.; Friedrich, N.; Linneberg, A. No association between vitamin D and atopy, asthma, lung function or atopic dermatitis: A prospective study in adults. Allergy 2015, 70, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Han, T.Y.; Kong, T.S.; Kim, M.H.; Chae, J.D.; Lee, J.H.K.; Son, S.-J. Vitamin D Status and Its Association with the SCORAD Score and Serum LL-37 Level in Korean Adults and Children with Atopic Dermatitis. Ann. Dermatol. 2015, 27, 10–14. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Ali, F.A.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.-N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef]
- Mansour, N.O.; Mohamed, A.A.; Hussein, M.; Eldemiry, E.; Daifalla, A.; Hassanin, S.; Nassar, N.; Ghaith, D.; Salah, E.M. The impact of vitamin D supplementation as an adjuvant therapy on clinical outcomes in patients with severe atopic dermatitis: A randomized controlled trial. Pharmacol. Res. Perspect. 2020, 8, e00679. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.; Yew, Y.W. Effect of Vitamin D Serum Levels and Supplementation on Atopic Dermatitis: A Systematic Review and Meta-analysis. Am. J. Clin. Dermatol. 2022, 23, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Bae, J.-H. Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition 2016, 32, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Thappa, D.M.; Gupta, D. Dermatoses Due to Indian Cultural Practices. Indian J. Dermatol. 2015, 60, 3–12. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bandyopadhyay, D.; Chatterjee, G.; Saha, D. The ‘Holi’ dermatoses: Annual spate of skin diseases following the spring festival in India. Indian J. Dermatol. 2009, 54, 240–242. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bandyopadhyay, D.; Verma, S.B. Cultural practice and dermatology: The “Holi” dermatoses. Int. J. Dermatol. 2012, 51, 1385–1387. [Google Scholar] [CrossRef]
- Lilly, E.; Kundu, R.V. Dermatoses secondary to Asian cultural practices. Int. J. Dermatol. 2012, 51, 372–379. [Google Scholar] [CrossRef]
- Budair, F.M. Dermatoses due to Arabic cultural and traditional practices. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 448–454. [Google Scholar] [CrossRef]
- Vashi, N.A.; Patzelt, N.; Wirya, S.; Maymone, M.B.C.; Kundu, R.V. Dermatoses caused by cultural practices: Cosmetic cultural practices. J. Am. Acad. Dermatol. 2018, 79, 19–30. [Google Scholar] [CrossRef]
- Niggemann, B.; Gruber, C. Side-effects of complementary and alternative medicine. Allergy 2003, 58, 707–716. [Google Scholar] [CrossRef]
- Haw, S.; Cho, H.-R.; Lee, M.-H. Allergic contact dermatitis associated with mugwort (Artemisia vulgaris). Contact Dermat. 2010, 62, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Bingham, L.J.; Tam, M.M.; Palmer, A.M.; Cahill, J.L.; Nixon, R.L. Contact allergy and allergic contact dermatitis caused by lavender: A retrospective study from an Australian clinic. Contact Dermat. 2019, 81, 37–42. [Google Scholar] [CrossRef]
- Vashi, N.A.; Patzelt, N.; Wirya, S.; Maymone, M.B.C.; Zancanaro, P.; Kundu, R.V. Dermatoses caused by cultural practices: Therapeutic cultural practices. J. Am. Acad. Dermatol. 2018, 79, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Schmidt, E.; Geier, J.; Lessmann, H.; Schnuch, A.; Frosch, P. Contact allergy to essential oils: Current patch test results (2000–2008) from the Information Network of Departments of Dermatology (IVDK). Contact Dermat. 2010, 63, 277–283. [Google Scholar] [CrossRef]
- Brans, R.; Schröder-Kraft, C.; Bauer, A.; Weisshaar, E.; Skudlik, C.; Geier, J. Contact sensitizations in massage therapists with occupational contact dermatitis: Patch test data of the Information Network of Departments of Dermatology, 2008–2020. Contact Dermat. 2023, 88, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tsimpidakis, A.; Rigopoulos, D.; Gregoriou, S. Aromatherapy: Cure or curse? A case report of allergic contact dermatitis caused by essential oils. Contact Dermat. 2020, 83, 141–143. [Google Scholar] [CrossRef]
- Sergoynne, L.; Mertens, M.; Dendooven, E.; Leysen, J.; Aerts, O. Allergic contact dermatitis, mimicking atopic dermatitis, associated with the use of essential oils in “home-made” cosmetics and aromatherapy diffusers. Contact Dermat. 2020, 83, 311–313. [Google Scholar] [CrossRef]
- Schaller, M.; Korting, H.C. Allergic airborne contact dermatitis from essential oils used in aromatherapy. Clin. Exp. Dermatol. 1995, 20, 143–145. [Google Scholar] [CrossRef]
- Weller, R.B.; Hunter, H.J.A.; Mann, M.W. Clinical Dermatology, 5th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; p. 206. [Google Scholar]
- Brancaccio, R.R.; Alvarez, M.S. Contact allergy to food. Dermatol. Ther. 2004, 17, 302–313. [Google Scholar] [CrossRef]
- Lembo, S.; Lembo, C.; Patruno, C.; Balato, A.; Balato, N.; Ayala, F. Pizza Makers’ Contact Dermatitis. Dermatitis 2014, 25, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Loman, L.; Schuttelaar, M.L.A. Hand eczema and lifestyle factors in the Dutch general population: Evidence for smoking, chronic stress, and obesity. Contact Dermat. 2022, 86, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Loman, L.; Brands, M.J.; Patsea, A.A.L.M.; Politiek, K.; Arents, B.W.M.; Schuttelaar, M.L.A. Lifestyle factors and hand eczema: A systematic review and meta-analysis of observational studies. Contact Dermat. 2022, 87, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Disulfiram and low nickel diet in the management of hand eczema: A clinical study. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 113–118. [Google Scholar] [CrossRef]
- Sharma, A. Relationship between nickel allergy and diet. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 307–312. [Google Scholar] [CrossRef]
- Sargen, M.R.; Hoffstad, O.; Margolis, D.J. Warm, Humid, and High Sun Exposure Climates are Associated with Poorly Controlled Eczema: PEER (Pediatric Eczema Elective Registry) Cohort, 2004–2012. J. Investig. Dermatol. 2014, 134, 51–57. [Google Scholar] [CrossRef]
- Sato, T.; Katayama, C.; Hayashida, Y.; Asanuma, Y.; Aoyama, Y. Role of basal sweating in maintaining skin hydration in the finger: A long-standing paradox in dry skin resolved. Exp. Dermatol. 2022, 31, 1891–1899. [Google Scholar] [CrossRef]
- Uter, W.; Gefeller, O.; Schwanitz, H.J. An epidemiological study of the influence of season (cold and dry air) on the occurrence of irritant skin changes of the hands. Br. J. Dermatol. 1998, 138, 266–272. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Johansen, J.D.; Linneberg, A.; Menné, T. The epidemiology of hand eczema in the general population—Prevalence and main findings. Contact Dermat. 2010, 62, 75–87. [Google Scholar] [CrossRef]
- Febriana, S.A.; Jungbauer, F.; Soebono, H.; Coenraads, P.-J. Occupational allergic contact dermatitis and patch test results of leather workers at two Indonesian tanneries. Contact Dermat. 2012, 67, 277–283. [Google Scholar] [CrossRef]
- Olumide, Y. Contact dermatitis in Nigeria—(I) Hand dermatitis in women. Contact Dermat. 1987, 17, 85–88. [Google Scholar] [CrossRef]
- Vester, L.; Thyssen, J.P.; Menné, T.; Johansen, J.D. Consequences of occupational food-related hand dermatoses with a focus on protein contact dermatitis. Contact Dermat. 2012, 67, 328–333. [Google Scholar] [CrossRef]
- Rocha, J.; Pereira, T.; Sousa-Basto, A.; Brito, C. Occupational Protein Contact Dermatitis: Two Case Reports. Case Rep. Med. 2010, 2010, 489627. [Google Scholar] [CrossRef] [PubMed]
- Meding, B. Skin symptoms among workers in a spice factory. Contact Dermat. 1993, 29, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Akker, T.W.V.D.; Roesyanto-Mahadi, I.D.; van Toorenenbergen, A.W.; van Joost, T. Contact allergy to spices. Contact Dermat. 1990, 22, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, L.; Estlander, T.; Jolanki, R. Occupational allergic contact dermatitis from spices. Contact Dermat. 1996, 35, 157–162. [Google Scholar] [CrossRef]
- Fischer, A.H.; Shin, D.B.; Margolis, D.J.; Takeshita, J. Racial and ethnic differences in healthcare utilization for childhood eczema: An analysis of the 2001–2013 Medical Expenditure Panel Surveys. J. Am. Acad. Dermatol. 2017, 77, 1060–1067. [Google Scholar] [CrossRef]
- Wan, J.; Oganisian, A.; Spieker, A.J.; Hoffstad, O.J.; Mitra, N.; Margolis, D.J.; Takeshita, J. Ethnic Variation in Use of Ambulatory and Emergency Care for Atopic Dermatitis among US Children. J. Investig. Dermatol. 2019, 139, 1906–1913.e1. [Google Scholar] [CrossRef]
- Elbert, N.J.; Duijts, L.; Den Dekker, H.T.; Jaddoe, V.W.V.; Sonnenschein-van der Voort, A.M.M.; De Jongste, J.C.; Pasmans, S.G.M.A. Role of environmental exposures and filaggrin mutations on associations of ethnic origin with risk of childhood eczema. The Generation R Study. Pediatr. Allergy Immunol. 2016, 27, 627–635. [Google Scholar] [CrossRef]
- Croce, E.A.; Levy, M.L.; Adamson, A.S.; Matsui, E.C. Reframing racial and ethnic disparities in atopic dermatitis in Black and Latinx populations. J. Allergy Clin. Immunol. 2021, 148, 1104–1111. [Google Scholar] [CrossRef]
- Tawfik, M.; Rodriguez-Homs, L.G.M.; Alexander, T.; Patterson, S.; Okoye, G.; Atwater, A.R. Allergen Content of Best-Selling Ethnic Versus Nonethnic Shampoos, Conditioners, and Styling Products. Dermatitis 2021, 32, 101–110. [Google Scholar] [CrossRef]
- Petherick, E.S.; Pearce, N.; Sunyer, J.; Wright, J. Ethnic and socio-economic differences in the prevalence of wheeze, severe wheeze, asthma, eczema and medication usage at 4 years of age: Findings from the Born in Bradford birth cohort. Respir. Med. 2016, 119, 122–129. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Celedón, J.C.; Hausmann, J.; Nikolov, M.; Sredl, D.; Ryan, L.; Platts-Mills, T.A.; Weiss, S.T.; Gold, D.R. Variation in total and specific IgE: Effects of ethnicity and socioeconomic status. J. Allergy Clin. Immunol. 2005, 115, 751–757. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Johansen, J.D.; Menné, T.; Nielsen, N.H.; Linneberg, A. Nickel Allergy in Danish Women before and after Nickel Regulation. N. Engl. J. Med. 2009, 360, 2259–2260. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Thyssen, J.P.; Uter, W.; Schnuch, A.; Johansen, J.D.; Menné, T.; Fortina, A.B.; Statham, B.; Gawkrodger, D.J. Nickel allergy following European Union regulation in Denmark, Germany, Italy and the U.K. Br. J. Dermatol. 2013, 169, 854–858. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, T.T.; Tan, G.H. Nickel Sensitivity in Singapore. Int. J. Dermatol. 1986, 25, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Sevagamoorthy, A.; Sockler, P.; Akoh, C.; Takeshita, J. Racial and ethnic diversity of US participants in clinical trials for acne, atopic dermatitis, and psoriasis: A comprehensive review. J. Dermatol. Treat. 2022, 33, 3086–3097. [Google Scholar] [CrossRef]
- Hirano, S.A.; Murray, S.B.; Harvey, V.M. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr. Dermatol. 2012, 29, 749–755. [Google Scholar] [CrossRef]
- Chen, V.; Akhtar, S.; Zheng, C.; Kumaresan, V.; Nouri, K. Assessment of Changes in Diversity in Dermatology Clinical Trials Between 2010-2015 and 2015-2020: A Systematic Review. JAMA Dermatol. 2022, 158, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Silverberg, J.I. Efficacy of Systemic Treatments for Atopic Dermatitis in Racial and Ethnic Minorities in the United States. JAMA Dermatol. 2014, 150, 1232–1234. [Google Scholar] [CrossRef]
- Daya, M.; Barnes, K.C. African American ancestry contribution to asthma and atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 456–462. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, E.S.X.; Tey, H.L.; Lim, Z.V. Are There Ethnic Differences in Hand Eczema? A Review. J. Clin. Med. 2023, 12, 2232. https://doi.org/10.3390/jcm12062232
Chai ESX, Tey HL, Lim ZV. Are There Ethnic Differences in Hand Eczema? A Review. Journal of Clinical Medicine. 2023; 12(6):2232. https://doi.org/10.3390/jcm12062232
Chicago/Turabian StyleChai, Eleanor Shu Xian, Hong Liang Tey, and Ziying Vanessa Lim. 2023. "Are There Ethnic Differences in Hand Eczema? A Review" Journal of Clinical Medicine 12, no. 6: 2232. https://doi.org/10.3390/jcm12062232
APA StyleChai, E. S. X., Tey, H. L., & Lim, Z. V. (2023). Are There Ethnic Differences in Hand Eczema? A Review. Journal of Clinical Medicine, 12(6), 2232. https://doi.org/10.3390/jcm12062232





