Insights on the Association between Thyroid Diseases and Colorectal Cancer
Abstract
1. Introduction
1.1. Colorectal Cancer: An Overview
1.2. Thyroid Diseases: An Overview
2. Epidemiological Evidence on Thyroid Dysfunctions and Colorectal Cancer
2.1. Hypothyroidism and Colorectal Cancer
2.2. Hyperthyroidism, Graves’ Disease, and Colorectal Cancer
2.3. The FT3/FT4 Ratio as Prognostic Factor in Metastatic Colorectal Cancer
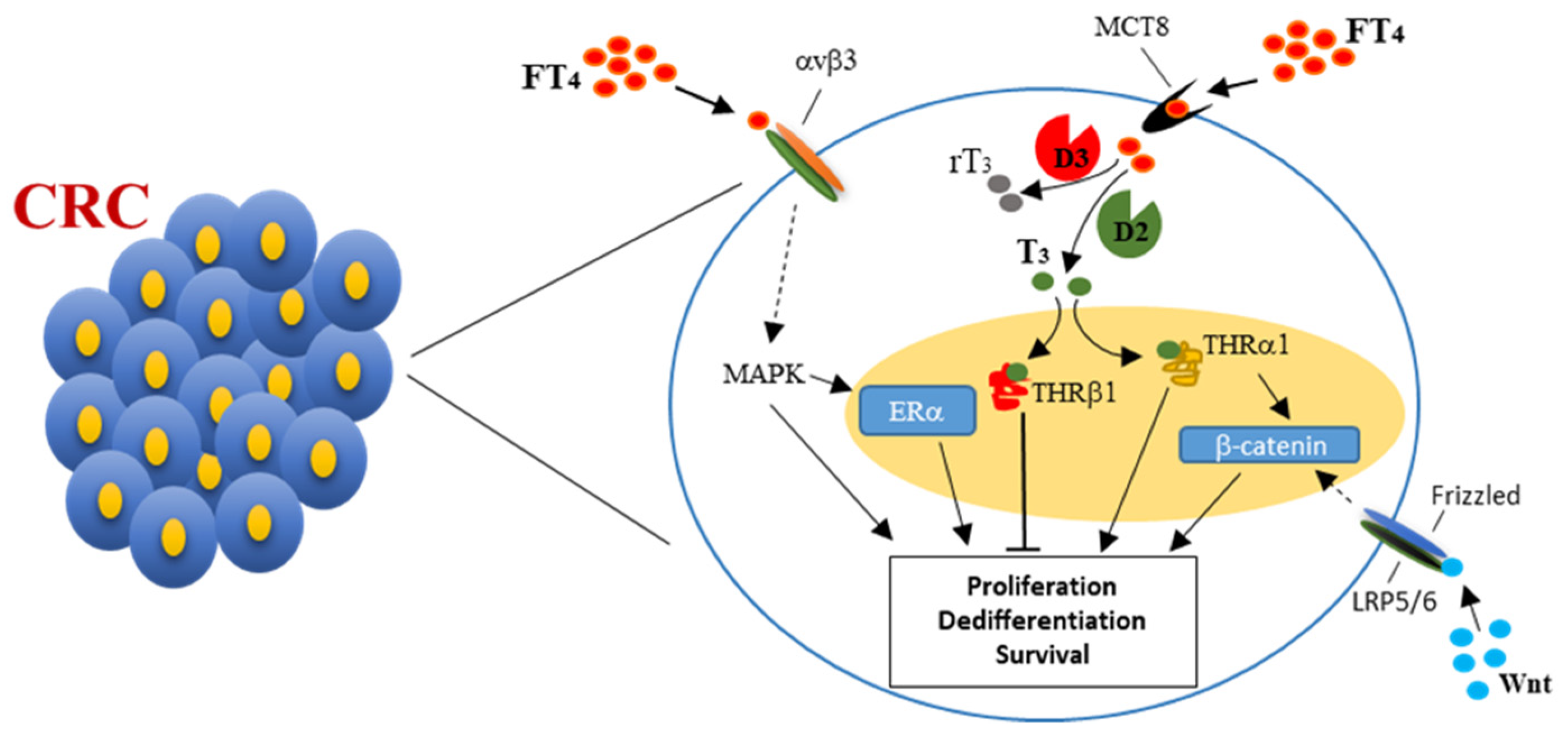
3. Thyroid Hormones, Thyroid Hormone Receptors, and Colorectal Cancer
4. Thyroid Hormones and Estrogens Cross-Talk in Colorectal Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prinzi, N.; Sorrenti, S.; Baldini, E.; De Vito, C.; Tuccilli, C.; Catania, A.; Coccaro, C.; Bianchini, M.; Nesca, A.; Grani, G.; et al. Association of thyroid diseases with primary extra-thyroidal malignancies in women: Results of a cross-sectional study of 6386 patients. PLoS ONE 2015, 10, e0122958. [Google Scholar] [CrossRef] [PubMed]
- Prinzi, N.; Baldini, E.; Sorrenti, S.; De Vito, C.; Tuccilli, C.; Catania, A.; Carbotta, S.; Mocini, R.; Coccaro, C.; Nesca, A.; et al. Prevalence of breast cancer in thyroid diseases: Results of a cross-sectional study of 3921 patients. Breast Cancer Res. Treat. 2014, 144, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, P.J.; Eslick, G.D.; Edirimanne, S. Benign thyroid disease is associated with breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2012, 133, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Lin, C.-L.; Chang, Y.-J.; Cheng, F.T.-F.; Peng, C.-L.; Sung, F.-C.; Cheng, Y.-H.; Kao, C.-H. Cancer Risk in Patients with Graves’ Disease: A Nationwide Cohort Study. Thyroid 2013, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-K.; Lin, C.-L.; Cheng, F.T.-F.; Sung, F.-C.; Kao, C.-H. Cancer risk in patients with Hashimoto’s thyroiditis: A nationwide cohort study. Br. J. Cancer 2013, 109, 2496–2501. [Google Scholar] [CrossRef]
- Bhatti, P.; Veiga, L.H.S.; Ronckers, C.M.; Sigurdson, A.J.; Stovall, M.; Smith, S.A.; Weathers, R.; Leisenring, W.; Mertens, A.C.; Hammond, S.; et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: An update from the childhood cancer survivor study. Radiat. Res. 2010, 174, 741–752. [Google Scholar] [CrossRef]
- Trinh, L.; Crawford, A.; Hussein, M.; Zerfaoui, M.; Toraih, E.; Randolph, G.; Kandil, E. Deciphering the Risk of Developing Second Primary Thyroid Cancer Following a Primary Malignancy—Who Is at the Greatest Risk? Cancers 2021, 13, 1402. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Li, H.; Cong, H.; Lin, Y. Risk of second primary breast cancer after radioactive iodine treatment in thyroid cancer: A systematic review and meta-analysis. Nucl. Med. Commun. 2016, 37, 110–115. [Google Scholar] [CrossRef]
- Mahmood, S.; Vu, K.; Tai, P.; Joseph, K.; Koul, R.; Dubey, A.; Yu, E. Radiation-induced second malignancies. Anticancer Res. 2015, 35, 2431–2434. [Google Scholar]
- Shu, X.; Ji, J.; Li, X.; Sundquist, J.; Hemminki, K.; Sundquist, K. Cancer risk in patients hospitalised for Graves’ disease: A population-based cohort study in Sweden. Br. J. Cancer 2010, 102, 1397–1399. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Hai, R.; You, Q.; Xie, L.; Shu, L.; Zhou, X. Thyroid disease is associated with an increased risk of breast cancer: A systematic review and meta-analysis. Gland Surg. 2021, 10, 336–346. [Google Scholar] [CrossRef]
- Pan, X.-F.; Ma, Y.-J.; Tang, Y.; Yu, M.-M.; Wang, H.; Fan, Y.-R. Breast cancer populations may have an increased prevalence of thyroglobulin antibody and thyroid peroxidase antibody: A systematic review and meta-analysis. Breast Cancer 2020, 27, 828–836. [Google Scholar] [CrossRef]
- Ellerhorst, J.; Cooksley, C.; Broemeling, L.; Johnson, M.; Grimm, E. High prevalence of hypothyroidism among patients with cutaneous melanoma. Oncol. Rep. 2003, 10, 1317–1320. [Google Scholar] [CrossRef]
- Shah, M.; Orengo, I.F.; Rosen, T. High prevalence of hypothyroidism in male patients with cutaneous melanoma. Dermatol. Online J. 2006, 12, 1. [Google Scholar] [CrossRef]
- Lazzara, D.R.; Zarkhin, S.G.; Rubenstein, S.N.; Glick, B.P. Melanoma and Thyroid Carcinoma: Our Current Understanding. J. Clin. Aesthetic Dermatol. 2019, 12, 39–41. [Google Scholar]
- Bellini, M.I.; Lori, E.; Forte, F.; Lauro, A.; Tripodi, D.; Amabile, M.I.; Cantisani, V.; Varanese, M.; Ferent, I.C.; Baldini, E.; et al. Thyroid and renal cancers: A bidirectional association. Front. Oncol. 2022, 12, 951976. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Pironi, D.; Gagliardi, F.; Tripodi, D.; Lauro, A.; Carbotta, S.; Tarroni, D.; D’Armiento, M.; Morrone, A.; et al. Is melanoma progression affected by thyroid diseases? Int. J. Mol. Sci. 2022, 23, 10036. [Google Scholar] [CrossRef]
- Baldini, E.; Lauro, A.; Tripodi, D.; Pironi, D.; Amabile, M.I.; Ferent, I.C.; Lori, E.; Gagliardi, F.; Bellini, M.I.; Forte, F.; et al. Thyroid diseases and breast cancer. J. Pers. Med. 2022, 12, 156. [Google Scholar] [CrossRef]
- American Cancer Society. Colorectal Cancer Statistics: How Common Is Colorectal Cancer? Available online: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html (accessed on 28 December 2022).
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.J. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin. Gastroenterol. Hepatol. 2021, 19, 955–966.e61. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.S.; Bradbury, K.E.; Cross, A.J.; Morris, J.S.; Gunter, M.J.; Murphy, N.A. Prospective investigation of body size, body fat composition and colorectal cancer risk in the UK biobank. Sci. Rep. 2017, 7, 17807. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorectal Cancer. Available online: https://www.wcrf.org/dietandcancer (accessed on 28 December 2022).
- Robsahm, T.E.; Aagnes, B.; Hjartåker, A.; Langseth, H.; Bray, F.I.; Larsen, I.K. Body mass index, physical activity, and colorectal cancer by anatomical subsites: A systematic review and meta-analysis of cohort studies. Eur. J. Cancer Prev. 2013, 22, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Secretan, B.; Straif, K.; Baan, R.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009, 10, 1033–1034. [Google Scholar] [CrossRef]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Migliore, L.; Migheli, F.; Spisni, R.; Coppedè, F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J. Biomed. Biotechnol. 2011, 2011, 792362. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Plateroti, M.; Kress, E.; Mori, J.I.; Samarut, J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol. Cell. Biol. 2006, 26, 3204–3214. [Google Scholar] [CrossRef]
- Kress, E.; Skah, S.; Sirakov, M.; Nadjar, J.; Gadot, N.; Scoazec, J.Y.; Samarut, J.; Plateroti, M. Cooperation between the thyroid hormone receptor TRalpha1 and the WNT pathway in the induction of intestinal tumorigenesis. Gastroenterology 2010, 138, 1863–1874. [Google Scholar] [CrossRef]
- Beck, B.; Blanpain, C. Unravelling cancer stem cell potential. Nat. Rev. Cancer 2013, 13, 727–738. [Google Scholar] [CrossRef]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef]
- Cunningham, L.A.; Gasior, A.; Kalady, M.F. Management of colorectal cancer in hereditary syndromes. Surg. Oncol. Clin. N. Am. 2022, 31, 307–319. [Google Scholar] [CrossRef]
- Cerretelli, G.; Ager, A.; Arends, M.J.; Frayling, I.M. Molecular pathology of Lynch syndrome. J. Pathol. 2020, 250, 518–531. [Google Scholar] [CrossRef]
- Waller, A.; Findeis, S.; Lee, M.J. Familial Adenomatous Polyposis. J. Pediatr. Genet. 2016, 5, 78–83. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 6, 2044–2058. [Google Scholar] [CrossRef]
- Ahadi, M.; Sokolova, A.; Brown, I.; Chou, A.; Gill, A.J. The 2019 World Health Organization classification of appendiceal, colorectal and anal canal tumours: An update and critical assessment. Pathology 2021, 53, 454–461. [Google Scholar] [CrossRef]
- Vanderpump, M.P. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Yang, B.; Wang, Q.; Kuang, H. The management and metabolic characterization: Hyperthyroidism and hypothyroidism. Neuropeptides 2023, 97, 102308. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P. Thyroid carcinoma: Epidemiology, histology, and diagnosis. Clin. Adv. Hematol. Oncol. 2015, 13, 3–6. [Google Scholar] [PubMed]
- Bogović Crnčić, T.; Ilić Tomaš, M.; Girotto, N.; Grbac Ivanković, S. Risk factors for thyroid cancer: What do we know so far? Acta Clin. Croat. 2020, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Schmidbauer, B.; Menhart, K.; Hellwig, D.; Grosse, J. Differentiated thyroid cancer-treatment: State of the art. Int. J. Mol. Sci. 2017, 18, 1292. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Yoo, S.-K.; Lee, S.; Kim, S.-J.; Jee, H.-G.; Kim, B.-A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.-K.; Shin, J.-Y.; et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef]
- Soares, P.; Lima, J.; Preto, A.; Castro, P.; Vinagre, J.; Celestino, R.; Couto, J.P.; Prazeres, H.; Eloy, C.; Maximo, V.; et al. Genetic alterations in poorly differentiated and undifferentiated thyroid carcinomas. Curr. Genom. 2011, 12, 609–617. [Google Scholar] [CrossRef]
- Baldini, E.; Tuccilli, C.; Pironi, D.; Catania, A.; Tartaglia, F.; Di Matteo, F.M.; Palumbo, P.; Arcieri, S.; Mascagni, D.; Palazzini, G.; et al. Expression and clinical utility of transcription factors involved in epithelial–mesenchymal transition during thyroid cancer progression. J. Clin. Med. 2021, 10, 4076. [Google Scholar] [CrossRef]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA consensus statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Jannin, A.; Escande, A.; Al Ghuzlan, A.; Blanchard, P.; Hartl, D.; Chevalier, B.; Deschamps, F.; Lamartina, L.; Lacroix, L.; Dupuy, C.; et al. Anaplastic thyroid carcinoma: An update. Cancers 2022, 14, 1061. [Google Scholar] [CrossRef]
- Ulisse, S.; Baldini, E.; Lauro, A.; Pironi, D.; Tripodi, D.; Lori, E.; Ferent, I.C.; Amabile, M.I.; Catania, A.; Di Matteo, F.M.; et al. Papillary thyroid cancer prognosis: An evolving field. Cancers 2021, 13, 5567. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Santini, F.; Corrado, A.; Materazzi, G.; Ulisse, S.; Miccoli, P.; Antonelli, A. Sorafenib and thyroid cancer. BioDrugs 2013, 27, 615–628. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Bocci, G.; Di Desidero, T.; Elia, G.; Ruffilli, I.; Ragusa, F.; Orlandi, P.; Paparo, S.R.; Patrizio, A.; Piaggi, S.; et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol. Rep. 2018, 39, 2225–2234. [Google Scholar] [CrossRef]
- Ulisse, S.; Tuccilli, C.; Sorrenti, S.; Antonelli, A.; Fallahi, P.; D’Armiento, E.; Catania, A.; Tartaglia, F.; Amabile, M.I.; Giacomelli, L.; et al. PD-1 ligand expression in epithelial thyroid cancers: Potential clinical implications. Int. J. Mol. Sci. 2019, 20, 1405. [Google Scholar] [CrossRef]
- Ragusa, F.; Ferrari, S.M.; Elia, G.; Paparo, S.R.; Balestri, E.; Botrini, C.; Patrizio, A.; Mazzi, V.; Guglielmi, G.; Foddis, R.; et al. Combination strategies involving immune checkpoint inhibitors and tyrosine kinase or braf inhibitors in aggressive thyroid cancer. Int. J. Mol. Sci. 2022, 23, 5731. [Google Scholar] [CrossRef]
- Rennert, G.; Rennert, H.S.; Pinchev, M.; Gruber, S.B. A case-control study of levothyroxine and the risk of colorectal cancer. J. Natl. Cancer Inst. 2010, 102, 568–572. [Google Scholar] [CrossRef]
- Friedman, G.D.; Schwalbe, J.S.; Habel, L.A. Re: A case-control study of levothyroxine and the risk of colorectal cancer. J. Natl. Cancer Inst. 2011, 103, 1637–1639. [Google Scholar] [CrossRef]
- Mu, G.; Mu, X.; Xing, H.; Xu, R.; Sun, G.; Dong, C.; Pan, Q.; Xu, C. Subclinical hypothyroidism as an independent risk factor for colorectal neoplasm. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 261–266. [Google Scholar] [CrossRef]
- Boursi, B.; Haynes, K.; Mamtani, R.; Yang, Y.X. Thyroid dysfunction, thyroid hormone replacement and colorectal cancer risk. J. Natl. Cancer Inst. 2015, 107, djv084. [Google Scholar] [CrossRef]
- Kuiper, J.G.; Fenneman, A.C.; van der Spek, A.H.; Rampanelli, E.; Nieuwdorp, M.; van Herk-Sukel, M.P.P.; Lemmens, V.E.P.P.; Kuipers, E.J.; Herings, R.M.C.; Fliers, E. Levothyroxine use and the risk of colorectal cancer: A large population-based case-control study. Endocr. Connect. 2022, 11, e210463. [Google Scholar] [CrossRef]
- L’Heureux, A.; Wieland, D.R.; Weng, C.H.; Chen, Y.H.; Lin, C.H.; Lin, T.H.; Weng, C.H. Association between thyroid disorders and colorectal cancer risk in adult patients in taiwan. JAMA Netw. Open 2019, 2, e193755. [Google Scholar] [CrossRef] [PubMed]
- Petranović Ovčariček, P.; Verburg, F.A.; Hoffmann, M.; Iakovou, I.; Mihailovic, J.; Vrachimis, A.; Luster, M.; Giovanella, L. Higher thyroid hormone levels and cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Hellevik, A.I.; Asvold, B.O.; Bjøro, T.; Romundstad, P.R.; Nilsen, T.I.; Vatten, L.J. Thyroid function and cancer risk: A prospective population study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2010, 205, 1–13. [Google Scholar] [CrossRef]
- De Alfieri, W.; Nisticò, F.; Borgogni, T.; Riello, F.; Cellai, F.; Mori, C.; Nante, N.; Di Bari, M. Thyroid hormones as predictors of short- and long-term mortality in very old hospitalized patients. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 68, 1122–1128. [Google Scholar] [CrossRef]
- Shigihara, S.; Shirakabe, A.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Sawatani, T.; Okajima, F.; Asai, K.; et al. Clinical significance of low-triiodothyronine syndrome in patients requiring non-surgical intensive care—Triiodothyronine is a comprehensive prognostic marker for critical patients with cardiovascular disease. Circ. Rep. 2021, 3, 578–588. [Google Scholar] [CrossRef]
- Adawiyah, J.; Norasyikin, A.W.; Mat, N.H.; Shamsul, A.S.; Azmi, K.N. The non-thyroidal illness syndrome in acute coronary syndrome is associated with increased cardiac morbidity and mortality. Heart Asia 2010, 2, 11–14. [Google Scholar] [CrossRef]
- Boelen, A.; Kwakkel, J.; Fliers, E. Beyond low plasma T3: Local thyroid hormone metabolism during inflammation and infection. Endocr. Rev. 2011, 32, 670–693. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Zhang, K.; Tang, Y.D. Association between low T3 syndrome and poor prognosis in adult patients with acute myocarditis. Front. Endocrinol. 2021, 12, 571765. [Google Scholar] [CrossRef]
- Lamba, N.; Liu, C.; Zaidi, H.; Broekman, M.L.D.; Simjian, T.; Shi, C.; Doucette, J.; Ren, S.; Smith, T.R.; Mekary, R.A.; et al. A prognostic role for Low tri-iodothyronine syndrome in acute stroke patients: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 169, 55–63. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Schirripa, M.; Dochy, E.; Fassan, M.; Ziranu, P.; Puzzoni, M.; Scartozzi, M.; Alberti, G.; Lonardi, S.; Zagonel, V.; et al. Thyroid hormones ratio is a major prognostic marker in advanced metastatic colorectal cancer: Results from the phase III randomised CORRECT trial. Eur. J. Cancer 2020, 133, 66–73. [Google Scholar] [CrossRef]
- Schirripa, M.; Pasqualetti, G.; Giampieri, R.; Scartozzi, M.; Lonardi, S.; Rumanò, L.; Bergamo, F.; Stragliotto, S.; Murgioni, S.; Alberti, G.; et al. Prognostic Value of Thyroid Hormone Ratios in Patients with Advanced Metastatic Colorectal Cancer Treated With Regorafenib: The TOREADOR Study. Clin. Colorectal Cancer 2018, 17, e601–e615. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Maruzzo, M.; Verzoni, E.; Vitale, M.G.; Dionese, M.; Buti, S.; Galli, L.; Zivi, A.; Watutantrige-Fernando, S.; Zielli, T.; Zanardi, E.; et al. Prognostic Value of Thyroid Hormone Ratio in Patients with Advanced Metastatic Renal Cell Carcinoma: Results From the Threefour Study (Meet-URO 14). Front. Oncol. 2021, 11, 787835. [Google Scholar] [CrossRef]
- Gao, R.; Chen, R.Z.; Xia, Y.; Liang, J.H.; Wang, L.; Zhu, H.Y.; Wu, J.-Z.; Fan, L.; Li, J.Y.; Yang, T.; et al. Low T3 syndrome as a predictor of poor prognosis in chronic lymphocytic leukemia. Int. J. Cancer 2018, 143, 466–477. [Google Scholar] [CrossRef]
- Pan, Q.; Jian, Y.; Zhang, Y.; Zhang, W.; Chen, Z.; Yang, Y.; Liu, A.; Wang, G. The Association Between Low T3 Syndrome and Survival in Patients with Newly Diagnosed Multiple Myeloma: A Retrospective Study. Technol. Cancer Res. Treat. 2022, 21, 1–10. [Google Scholar] [CrossRef]
- Xue, L.G.; Shen, H.R.; Gao, R.; Du, K.X.; Xing, T.Y.; Wang, W.T.; Wang, L.; Li, J.Y.; Liang, J.H.; Xu, W. Low T3 syndrome as a predictor of poor outcomes in patients with follicular lymphoma. Ann. Hematol. 2023, 102, 851–862. [Google Scholar] [CrossRef]
- Yasar, Z.A.; Kirakli, C.; Yilmaz, U.; Ucar, Z.Z.; Talay, F. Can non-thyroid illness syndrome predict mortality in lung cancer patients? A prospective cohort study. Horm. Cancer 2014, 5, 240–246. [Google Scholar] [CrossRef]
- Bunevicius, A.; Deltuva, V.P.; Tamasauskas, S.; Smith, T.; Laws, E.R.; Bunevicius, R.; Iervasi, G.; Tamasauskas, A. Preoperative low tri-iodothyronine concentration is associated with worse health status and shorter five year survival of primary brain tumor patients. Oncotarget 2017, 8, 8648–8656. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Visser, T.J. Thyroid hormone transporters and resistance. Endocr. Dev. 2013, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, F.S. The Thyroid Gland. In Basic & Clinical Endocrinology, 7th ed.; Greenspan, F.S., Gardner, D.G., Eds.; Lange Medical Books/McGraw-Hill: New York, NY, USA, 2004; pp. 215–294. [Google Scholar]
- Chin, W.W. Nuclear Thyroid Hormone Receptors. In Nuclear Hormone Receptor; Parker, M.G., Ed.; Academic Press: London, UK, 1991; pp. 79–102. [Google Scholar]
- Ortiga-Carvalho, T.M.; Sidhaye, A.R.; Wondisford, F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014, 10, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Chin, W.W. Nuclear thyroid hormone receptors. J. Clin. Investig. 1990, 86, 1777–1782. [Google Scholar] [CrossRef]
- Sap, J.; Munoz, A.; Damm, K.; Goldberg, Y.; Ghysdael, J.; Leutz, A.; Beug, H.; Vennström, B. The c-erbA protein is a high-affinity receptor for thyroid hormone. Nature 1986, 324, 635–640. [Google Scholar] [CrossRef]
- Weinberger, C.; Thompson, C.C.; Ong, E.S.; Lebo, R.; Gruol, D.J.; Evans, R.M. The c-erbA gene encodes a thyroid hormone receptor. Nature 1986, 324, 641–646. [Google Scholar] [CrossRef]
- Chassande, O.; Fraichard, A.; Gauthier, K.; Flamant, F.; Legrand, C.; Savatier, P.; Laudet, V.; Samarut, J. Identification of transcripts initiated from an internal promoter in the c-erbA alpha locus that encode inhibitors of retinoic acid receptor-alpha and triiodothyronine receptor activities. Mol. Endocrinol. 1997, 11, 1278–1290. [Google Scholar] [CrossRef]
- Gauthier, K.; Chassande, O.; Plateroti, M.; Roux, J.P.; Legrand, C.; Pain, B.; Rousset, B.; Weiss, R.; Trouillas, J.; Samarut, J. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 1999, 18, 623–631. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.S.H.; Lai, H.Y.; Whang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid hormone, cancer, and apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [CrossRef]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol.-Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. (Lausanne) 2019, 10, 59. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Okuda, S.; Taniguchi, H. Enhancement by thyroxine of experimental carcinogenesis induced in rat colon by azoxymethane. Int. J. Cancer 1992, 50, 974–976. [Google Scholar] [CrossRef]
- Markowitz, S.; Haut, M.; Stellato, T.; Gerbic, C.; Molkentin, K. Expression of the ErbA-beta class of thyroid hormone receptors is selectively lost in human colon carcinoma. J. Clin. Investig. 1989, 84, 1683–1687. [Google Scholar] [CrossRef]
- Zhu, L.; Tian, G.; Yang, Q.; De, G.; Zhang, Z.; Wang, Y.; Nie, H.; Zhang, Y.; Yang, X.; Li, J. Thyroid hormone receptor β1 suppresses proliferation and migration by inhibiting PI3K/Akt signaling in human colorectal cancer cells. Oncol. Rep. 2016, 36, 1419–1426. [Google Scholar] [CrossRef]
- Kim, W.G.; Cheng, S.-Y. Thyroid hormone receptors and cancer. Biochim. Biophys. Acta 2013, 1830, 3928–3936. [Google Scholar] [CrossRef]
- Sabatino, L.; Vassalle, C.; Del Seppia, C.; Iervasi, G. Deiodinases and the three types of thyroid hormone deiodination reactions. Endocrinol. Metab. 2021, 36, 952–964. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Ambrosio, R.; Dentice, M. Thyroid hormone promotes differentiation of colon cancer stem cells. Mol. Cell. Endocrinol. 2017, 459, 84–89. [Google Scholar] [CrossRef]
- Dentice, M. Hedgehog-mediated regulation of thyroid hormone action through iodothyronine deiodinases. Expert Opin. Ther. Targets 2011, 15, 493–504. [Google Scholar] [CrossRef]
- Ciavardelli, D.; Bellomo, M.; Crescimanno, C.; Vella, V. Type 3 deiodinase: Role in cancer growth, stemness, and metabolism. Front. Endocrinol. 2014, 5, 215. [Google Scholar] [CrossRef]
- Dentice, M.; Antonini, D.; Salvatore, D. Type 3 deiodinase and solid tumors: An intriguing pair. Expert Opin. Ther. Targets 2013, 17, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M.; Luongo, C.; Ambrosio, R.; Sibilio, A.; Casillo, A.; Iaccarino, A.; Troncone, G.; Fenzi, G.; Larsen, P.R.; Salvatore, D. β-Catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology 2012, 143, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Davis, T.E. Plasma thyronine levels in carcinoma of the breast and colon. Arch. Intern. Med. 1981, 141, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Dentice, M.; Ambrosio, R.; Luongo, C.; Carollo, R.; Benfante, A.; Todaro, M.; Stassi, G.; Salvatore, D. Activated thyroid hormone promotes differentiation and chemotherapeutic sensitization of colorectal cancer stem cells by regulating Wnt and BMP4 signaling. Cancer Res. 2016, 76, 1237–1244. [Google Scholar] [CrossRef]
- Yang, Y.S.H.; Ko, P.J.; Pan, Y.S.; Lin, H.Y.; Whang-Peng, J.; Davis, P.J.; Wang, K. Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers. J. Biomed. Sci. 2021, 28, 24. [Google Scholar] [CrossRef]
- Davis, P.J.; Lin, H.Y.; Sudha, T.; Yalcin, M.; Tang, H.Y.; Hercbergs, A.; Leith, J.T.; Luidens, M.K.; Ashur-Fabian, O.; Incerpi, S.; et al. Nanotetrac targets integrin αvβ3 on tumor cells to disorder cell defense pathways and block angiogenesis. OncoTargets Ther. 2014, 7, 1619–1624. [Google Scholar] [CrossRef]
- Nana, A.W.; Chin, Y.T.; Lin, C.Y.; Ho, Y.; Bennett, J.A.; Shih, Y.J.; Chen, Y.R.; Changou, C.A.; Pedersen, J.Z.; Incerpi, S.; et al. Tetrac downregulates β-catenin and HMGA2 to promote the effect of resveratrol in colon cancer. Endocr. Relat. Cancer 2018, 25, 279–293. [Google Scholar] [CrossRef]
- Lin, H.Y.; Cody, V.; Davis, F.B.; Hercbergs, A.A.; Luidens, M.K.; Mousa, S.A.; Davis, P.J. Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discov. Med. 2011, 11, 337–347. [Google Scholar]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef]
- Wele, P.; Wu, X.; Shi, H. Sex-Dependent Differences in Colorectal Cancer: With a Focus on Obesity. Cells 2022, 11, 3688. [Google Scholar] [CrossRef]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Estrogen receptors and their implications in colorectal carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Murphy, G.; Devesa, S.S.; Cross, A.J.; Inskip, P.D.; McGlynn, K.A.; Cook, M.B. Sex Disparities in Colorectal Cancer Incidence by Anatomic Subsite, Race and Age. Int. J. Cancer 2011, 128, 1668–1675. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Brenner, H.; Hoffmeister, M.; Arndt, V.; Haug, U. Gender differences in colorectal cancer: Implications for age at initiation of screening. Br. J. Cancer 2007, 96, 828–831. [Google Scholar] [CrossRef]
- Chen, J.; Iverson, D. Estrogen in obesity-associated colon cancer: Friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control 2012, 23, 1767–1773. [Google Scholar] [CrossRef]
- Kennelly, R.; Kavanagh, D.O.; Hogan, A.M.; Winter, D.C. Oestrogen and the colon: Potential mechanisms for cancer prevention. Lancet Oncol. 2008, 9, 385–391. [Google Scholar] [CrossRef]
- Das, P.K.; Saha, J.; Pillai, S.; Lam, A.K.Y.; Gopalan, V.; Islam, F. Implications of Estrogen and Its Receptors in Colorectal Carcinoma. Cancer Med. 2023, 12, 4367–4379. [Google Scholar] [CrossRef]
- Ulisse, S.; Tata, J.R. Thyroid hormone and glucocorticoid independently regulate the expression of estrogen receptor in male Xenopus liver cells. Mol. Cell. Endocrinol. 1994, 105, 45–53. [Google Scholar] [CrossRef]
- Alarid, E.T.; Preisler-Mashek, M.T.; Solodin, N.M. Thyroid hormone is an inhibitor of estrogen-induced degradation of estrogen receptor-alpha protein: Estrogen-dependent proteolysis is not essential for receptor transactivation function in the pituitary. Endocrinology 2003, 144, 3469–3476. [Google Scholar] [CrossRef]
- Tang, H.Y.; Lin, H.Y.; Zhang, S.; Davis, F.B.; Davis, P.J. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 2004, 145, 3265–3272. [Google Scholar] [CrossRef]
- Hammes, S.R.; Davis, P.J. Overlapping nongenomic and genomic actions of thyroid hormone and steroids. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
| First Author (Ref.) | Year | Country | Thyroid Disease | Cancer | Patients/ Control | Risk (95% CI) | p |
|---|---|---|---|---|---|---|---|
| Rennert et al. [58] | 2010 | Israel | Hypothyroidism on levothyroxine | CRC | 2566/2566 | OR 0.59 (0.43–0.82) | 0.001 |
| Friedman et al. [59] | 2011 | California | Hypothyroidism on levothyroxine | Rectum Colon | 4729/235,925 12,207/608,296 | OR Male 0.66 (0.45–0.97) OR Female 0.97 (0.78–1.19) | 0.03 0.74 0.18 0.06 |
| OR Male 0.87 (0.71–1.07) OR Female 0.90 (0.81–1.07) | |||||||
| Chen et al. [5] | 2013 | Taiwan | Hashimoto | CRC | 1521/6084 | HR 4.76 (1.36–16.6) | <0.05 |
| Mu et al. [60] | 2015 | China | Subclinical Hypothyroidism | CRC | 273/819 | OR 1.689 (1.207–2.362) | 0.002 |
| Boursi et al. [61] | 2015 | United Kingdom | Hypothyroidism | CRC | 20,990/82,504 | OR 1.16 (1.08–1.24) | <0.001 |
| Hypothyroidism on levothyroxine | OR 0.92 (0.86–0.98) | 0.009 | |||||
| L’Heureux et al. [63] | 2019 | Taiwan | Hypothyroidism | CRC | 69,713/69,713 | 0.78 (0.65–0.94 | <0.001 |
| Kuiper et al. [62] | 2022 | Netherlands | Hypothyroidism | CRC | 28,121/106,086 | OR 0.95 (0.88–1.01) | >0.05 |
| Hellevik et al. [64] | 2009 | Norway | Hyperthyroidism | Colon | 29,691 | HR 1.42 | >0.05 |
| Boursi et al. [61] | 2015 | United Kingdom | Hyperthyroidism | CRC | 20,990/82,504 | OR 1.21 (1.08–1.36) | 0.001 |
| L’Heureux et al. [63] | 2019 | Taiwan | Hyperthyroidism | CRC | 69,713/69,713 | OR 0.77 (0.69–0.86) | <0.001 |
| Shu et al. [10] | 2010 | Sweden | Graves’ disease | Colon | 18,156 | SIR 0.78 (0.61–0.97) | <0.05 |
| Chen et al. [4] | 2013 | Taiwan | Graves’ disease | Colon | 5025/20,100 | IRR 0.61 (0.53–0.69) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, F.; Baldini, E.; Lori, E.; Cardarelli, S.; Pironi, D.; Lauro, A.; Tripodi, D.; Palumbo, P.; D’Armiento, E.; Cavallaro, G.; et al. Insights on the Association between Thyroid Diseases and Colorectal Cancer. J. Clin. Med. 2023, 12, 2234. https://doi.org/10.3390/jcm12062234
Gagliardi F, Baldini E, Lori E, Cardarelli S, Pironi D, Lauro A, Tripodi D, Palumbo P, D’Armiento E, Cavallaro G, et al. Insights on the Association between Thyroid Diseases and Colorectal Cancer. Journal of Clinical Medicine. 2023; 12(6):2234. https://doi.org/10.3390/jcm12062234
Chicago/Turabian StyleGagliardi, Federica, Enke Baldini, Eleonora Lori, Silvia Cardarelli, Daniele Pironi, Augusto Lauro, Domenico Tripodi, Piergaspare Palumbo, Eleonora D’Armiento, Giuseppe Cavallaro, and et al. 2023. "Insights on the Association between Thyroid Diseases and Colorectal Cancer" Journal of Clinical Medicine 12, no. 6: 2234. https://doi.org/10.3390/jcm12062234
APA StyleGagliardi, F., Baldini, E., Lori, E., Cardarelli, S., Pironi, D., Lauro, A., Tripodi, D., Palumbo, P., D’Armiento, E., Cavallaro, G., Polistena, A., D’Orazi, V., Sibio, S., Fallahi, P., Antonelli, A., D’Andrea, V., Ulisse, S., & Sorrenti, S. (2023). Insights on the Association between Thyroid Diseases and Colorectal Cancer. Journal of Clinical Medicine, 12(6), 2234. https://doi.org/10.3390/jcm12062234











