Discovering the Physio-Pathological Mechanisms of Interaction between Bone Mineral Density, Muscle Mass, and Visceral Adipose Tissue in Female Older Adults through Structural Equation Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Outcomes
2.4. Anthropometric Parameters and Body Composition
2.5. Anthropometric Parameters and Body Composition
2.6. Muscle Function
2.7. Cognitive Performance and Functional Status
2.8. Biochemical Analysis
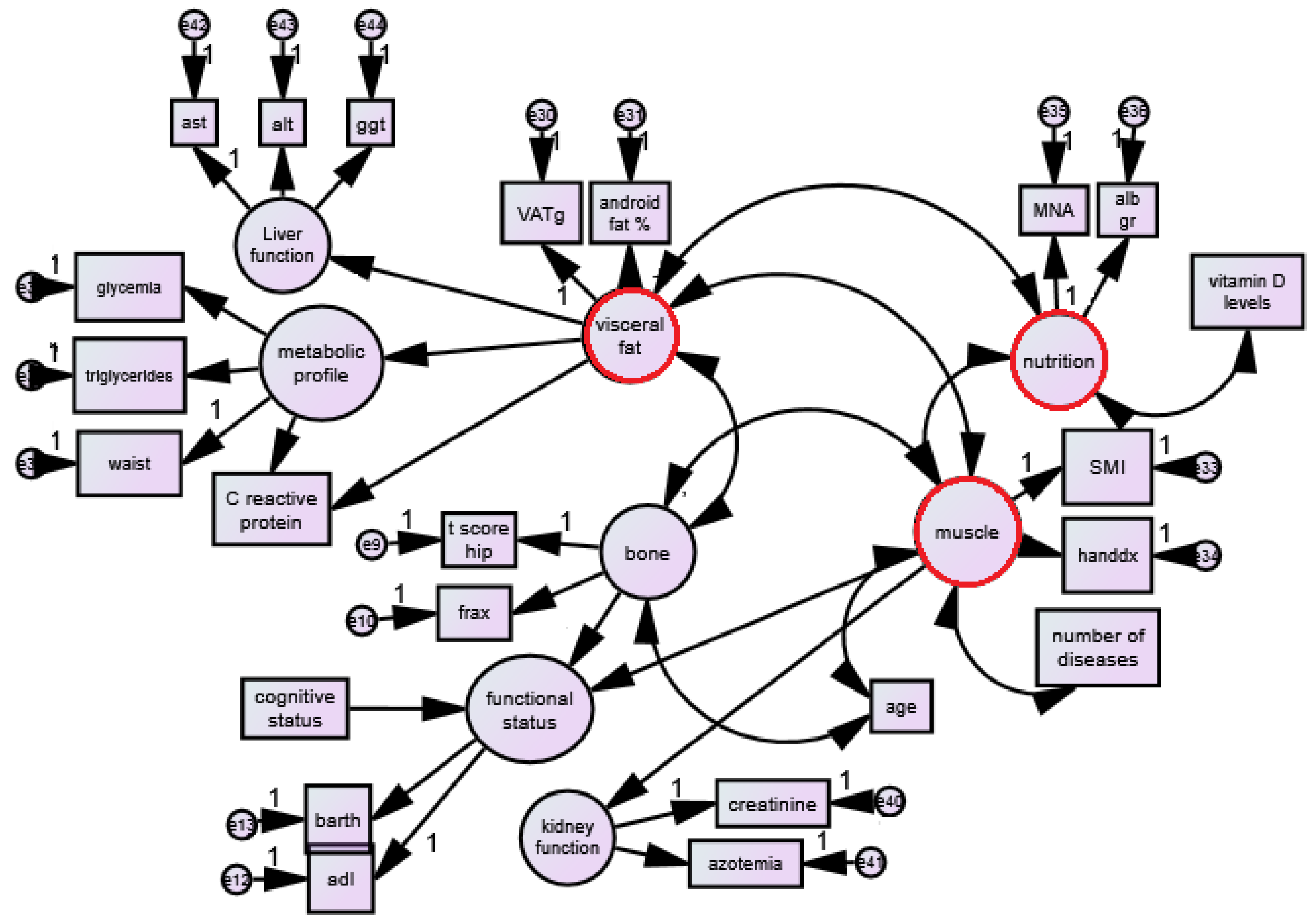
2.9. Prespecified Structural Equation Modeling (SEM) of the Visceral Obesity–Muscle Mass–Bone Mineral Density Interactions
2.10. Statistical Analysis
- (1)
- Muscle mass (latent variable) includes ALM/h2 and handgrip strength;
- (2)
- Visceral fat (latent variable) includes VAT (grams) and android fat (%);
- (3)
- Bone mineral density (latent variable) includes T-score for hip and femur and hip FRAX (%).
- (1)
- Nutritional status (latent variable) includes Mini Nutritional Assessment and albumin and vitamin D (single variable);
- (2)
- Liver status (latent variable) includes ALT, GGT, ALP;
- (3)
- Functional status (latent variable) includes Barthel test and ADL;
- (4)
- Cognitive performance includes MMSE;
- (5)
- Age, gender;
- (6)
- Kidney function (latent variable) includes creatinine and azotemia (blood urea nitrogen) (BUN));
- (7)
- Metabolic profile (latent variable) includes triglycerides, glycemia, and waist circumference.
3. Results
4. Discussion
4.1. The Effect of VAT on Muscular Status and Strenght
4.2. The Nutritional Status’ Mediation between Visceral Fat and Muscle Mass
4.3. Muscle Deterioriation with Age and Comorbidities
4.4. Interaction between Muscle and Bone
4.5. The Effects of Cognitive Status on Muscle Deterioriation
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Rondanelli, M.; Guido, D.; Opizzi, A.; Faliva, M.A.; Perna, S.; Grassi, M. A path model of sarcopenia on bone mass loss in elderly subjects. J. Nutr. Health Aging 2014, 18, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H. Abdominal Obesity Is Associated with an Increased Risk of All-Cause Mortality in Patients With HFpEF. J. Am. Coll. Cardiol. 2017, 70, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Zupo, R.; Castellana, F.; Guerra, V.; Donghia, R.; Bortone, I.; Griseta, C.; Lampignano, L.; Dibello, V.; Lozupone, M.; Coelho-Júnior, H.J.; et al. Associations between nutritional frailty and 8-year all-cause mortality in older adults: The Salus in Apulia Study. J. Intern. Med. 2021, 290, 1071–1082. [Google Scholar] [CrossRef]
- Perna, S.; Spadaccini, D.; Rondanelli, M. Sarcopenic obesity: Time to target the phenotypes. J. Cachexia Sarcopenia Muscle 2019, 10, 710–711. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Zhu, Y.; Chang, H.; Wang, X.; Liu, W.; Zhang, Y.; Huang, G. Higher visceral fat area increases the risk of vitamin D insufficiency and deficiency in Chinese adults. Nutr. Metab. 2015, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, C.; Gaggini, M.; Carli, F.; Gastaldelli, A. The Subtle Balance between Lipolysis and Lipogenesis: A Critical Point in Metabolic Homeostasis. Nutrients 2015, 7, 9453–9474. [Google Scholar] [CrossRef]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Gunawardene, P.; Demontiero, O.; Duque, G. Comprehensive nutritional status in sarco-osteoporotic older fallers. J. Nutr. Health Aging 2015, 19, 474–480. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M.; Zanetti, M.; Riso, S.; Riso, D.S. Linea guida pratica ESPEN: Nutrizione Clinica nelle malattie di fegato. Clin. Nutr. 2019, 38, 485–521. [Google Scholar]
- Vahanian, A.; Baumgartner, H.; Bax, J.; Butchart, E.; Dion, R.; Filippatos, G.; Flachskampf, F.; Hall, R.; Iung, B.; Kasprzak, J.; et al. Guidelines on the management of valvular heart diseaseThe Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J. 2007, 28, 230–268. [Google Scholar]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8. [Google Scholar] [CrossRef]
- Frisancho, A.R. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; De Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal Muscle Cutpoints Associated with Elevated Physical Disability Risk in Older Men and Women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef] [PubMed]
- Spijkerman, D.C.; Snijders, C.J.; Stijnen, T.; Lankhorst, G.J. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand. J. Rehabil. Med. 1991, 23, 203–206. [Google Scholar] [PubMed]
- Monroe, T.; Carter, M. Using the Folstein Mini Mental State Exam (MMSE) to explore methodological issues in cognitive aging research. Eur. J. Ageing 2012, 9, 265–274. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md. State Med. J. 1965, 14, 56–61. [Google Scholar]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA J. Am. Med. Assoc. 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Kirk, B.; Bani Hassan, E.; Brennan-Olsen, S.; Vogrin, S.; Bird, S.; Zanker, J.; Phu, S.; Meerkin, J.D.; Heymsfield, S.B.; Duque, G. Body composition reference ranges in community-dwelling adults using dual-energy X-ray absorptiometry: The Australian Body Composition (ABC) Study. J. Cachexia Sarcopenia Muscle 2021, 12, 880–890. [Google Scholar] [CrossRef]
- Murai, J.; Nishizawa, H.; Otsuka, A.; Fukuda, S.; Tanaka, Y.; Nagao, H.; Sakai, Y.; Suzuki, M.; Yokota, S.; Tada, H.; et al. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc. Diabetol. 2018, 17, 112. [Google Scholar] [CrossRef]
- Alalwan, T.A. Phenotypes of Sarcopenic Obesity: Exploring the Effects on Peri-Muscular Fat, the Obesity Paradox, Hormone-Related Responses and the Clinical Implications. Geriatrics 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Chang, Y.F.; Liu, P.Y.; Wu, S.J.; Chiu, C.J.; Chen, C.Y.; Wu, C.H. Interaction of central obesity and sarcopenia on nutritional status in the community-dwelling older people. Arch. Gerontol. Geriatr. 2020, 87, 104003. [Google Scholar] [CrossRef] [PubMed]
- Hudzik, B.; Nowak, J.; Szkodziński, J.; Zubelewicz-Szkodzińska, B. Visceral Adiposity in Relation to Body Adiposity and Nutritional Status in Elderly Patients with Stable Coronary Artery Disease. Nutrients 2021, 13, 2351. [Google Scholar] [CrossRef]
- Morrison, S.A.; Goss, A.M.; Azziz, R.; Raju, D.A.; Gower, B.A. Peri-muscular adipose tissue may play a unique role in determining insulin sensitivity/resistance in women with polycystic ovary syndrome. Hum. Reprod. 2017, 32, 185–192. [Google Scholar] [CrossRef]
- Larsson, L.; Grimby, G.; Karlsson, J. Muscle strength and speed of movement in relation to age and muscle morphology. J. Appl. Physiol. 1979, 46, 451–456. [Google Scholar] [CrossRef]
- Kaji, H. Interaction between Muscle and Bone. J. Bone Metab. 2014, 21, 29. [Google Scholar] [CrossRef]
- Ilich, J.Z.; Brownbill, R.A. Habitual and low-impact activities are associated with better bone outcomes and lower body fat in older women. Calcif. Tissue Int. 2008, 83, 260–271. [Google Scholar] [CrossRef]
- Foldvari, M.; Clark, M.; Laviolette, L.C.; Bernstein, M.A.; Kaliton, D.; Castaneda, C.; Pu, C.T.; Hausdorff, J.M.; Fielding, R.A.; Fiatarone Singh, M.A. Association of muscle power with functional status in community-dwelling elderly women. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55. [Google Scholar] [CrossRef]
- Lin, R.C.; Chiang, S.L.; Heitkemper, M.M.L.; Weng, S.M.; Lin, C.F.; Yang, F.C.; Lin, C.H. Effectiveness of Early Rehabilitation Combined with Virtual Reality Training on Muscle Strength, Mood State, and Functional Status in Patients with Acute Stroke: A Randomized Controlled Trial. Worldviews Evid.-Based Nurs. 2020, 17, 158–167. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Invest. 2019, 129, 3214. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean | Std. Deviation |
|---|---|---|
| Age (years) | 82.13 | 6.32 |
| BMI (kg/m2) | 24.99 | 5.00 |
| Mini Mental State Examination (score) | 18.13 | 7.02 |
| Barthel test (score) | 61.03 | 25.93 |
| Adl (score) | 3.13 | 1.81 |
| Iron (mg/dL) | 65.60 | 31.27 |
| Triglycerides (mg/dL) | 123.07 | 59.33 |
| Cholesterol (mg/dL) | 190.10 | 44.52 |
| Albumin (g) | 3.96 | 4.19 |
| Creatinine (mg/dL) | 0.93 | 2.09 |
| Blood urea nitrogen (BUN) (mg/dL) | 43.96 | 20.21 |
| AST (IU/L) | 19.72 | 12.89 |
| ALT (IU/L) | 17.31 | 14.62 |
| GGT (U/L) | 31.46 | 38.52 |
| Glycemia (mg/dL) | 106.72 | 39.96 |
| CRP (mg/dL) | 1.11 | 2.38 |
| 25 OH vitamin D (ng/mL) | 13.98 | 11.87 |
| Waist circumference (cm) | 90.02 | 12.12 |
| MNA (score) | 17.47 | 3.58 |
| Handgrip dx (kg) | 14.80 | 5.43 |
| SMI (Kg/m2) | 6.37 | 1.09 |
| Femur T score (pt) | −2.39 | 1.22 |
| Hip FRAX (%) | 8.61 | 7.80 |
| Android fat (%) | 36.90 | 13.55 |
| VAT (g) | 911.62 | 607.33 |
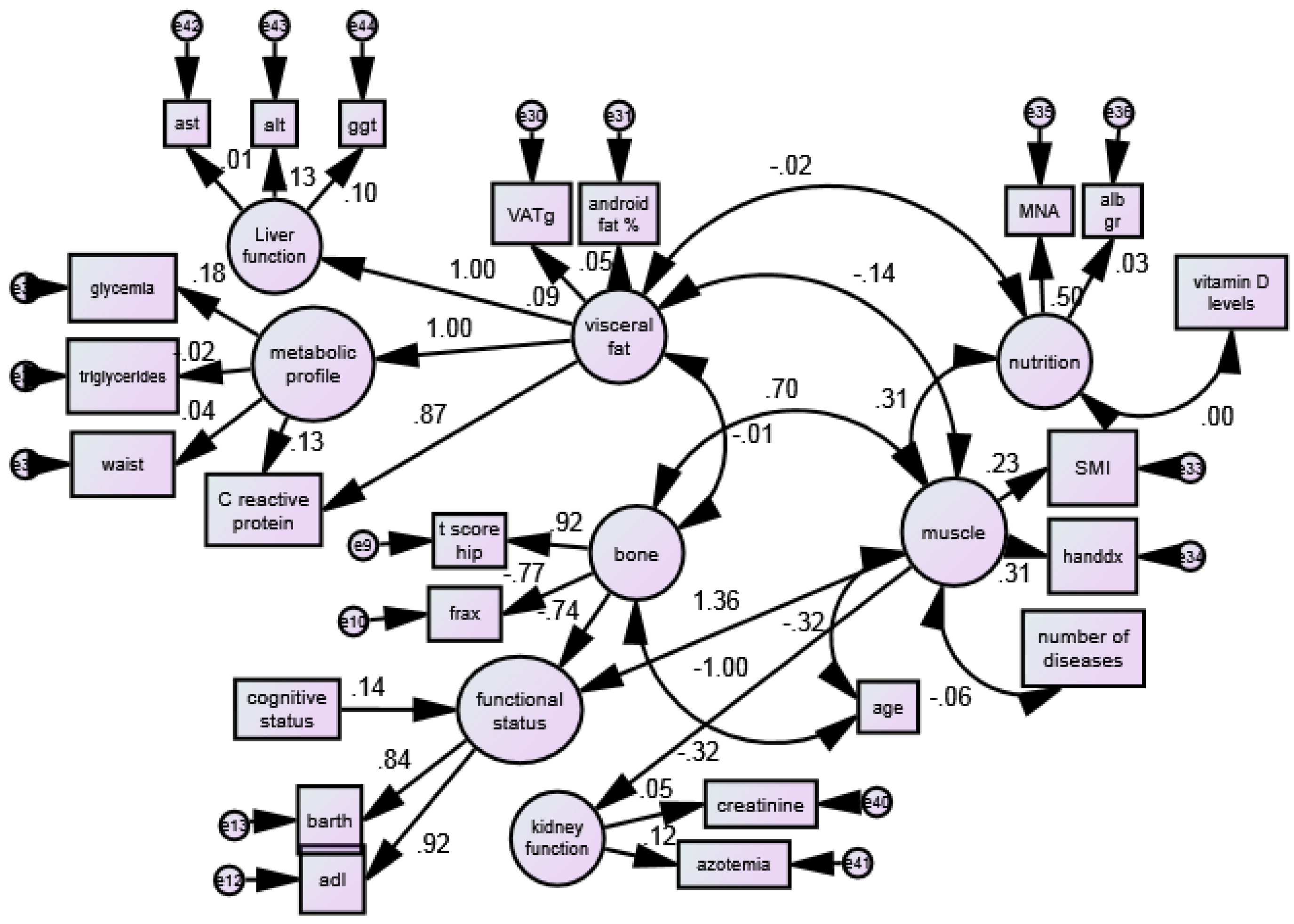
| Variable | Estimate | S.E. | C.R. | p | ||
|---|---|---|---|---|---|---|
| BONE | ||||||
| tscorefemore | <--- | Bone | 1.000 | |||
| fraxanca | <--- | Bone | −5.287 | 0.561 | −9.415 | <0.001 |
| FUNCTIONAL STATUS | ||||||
| ADL (score) | <--- | Functional status | 1.000 | |||
| Barthel test (score) | <--- | Functional status | 13.143 | 0.971 | 13.538 | <0.001 |
| VISCERAL FAT | ||||||
| VAT (grams) | <--- | Visceral fat | 1.000 | |||
| Android fat (%) | <--- | Visceral fat | 0.013 | 0.012 | 1.098 | <0.001 |
| MUSCLE | ||||||
| SMI (kg/m2) | <--- | Muscle | 1.000 | |||
| Handgrip (kg) | <--- | Muscle | 6.716 | 1.576 | 4.261 | <0.001 |
| NUTRITIONAL STATUS | ||||||
| MNA (score) | <--- | Nutrition | 1.000 | |||
| Albumin gr | <--- | Nutrition | 0.062 | 0.232 | 0.267 | 0.790 |
| METABOLIC LE PROFILE | ||||||
| Waist circ.(cm) | <--- | Metabolic profile | 1.000 | |||
| Triglyceride (mg/dL) | <--- | Metabolic profile | −1.971 | 5.506 | −0.358 | 0.720 |
| Glycemia (mg/dL) | <--- | Metabolic profile | 14.446 | 17.154 | 0.842 | 0.400 |
| KIDNEY FUNCTION | ||||||
| Creatinine (mg/dL) | <--- | Kidney function | 1.000 | |||
| Blood urea nitrogen (BUN) (mg/dL) | <--- | Kidney function | 23.651 | 21.744 | 1.088 | 0.277 |
| LIVER FUNCTION | ||||||
| AST (mg/dL) | <--- | Liver function | 1.000 | |||
| ALT (mg/dL) | <--- | Liver function | 11.201 | 33.260 | 0.337 | 0.736 |
| GGT (mg/dL) | <--- | Liver function | 21.632 | 64.608 | 0.335 | 0.738 |
| Variable | Estimate | S.E. | C.R. | p | ||
|---|---|---|---|---|---|---|
| Bone | <--> | Visceral fat | −0.541 | 2.487 | −0.218 | 0.828 |
| Bone | <--> | Muscle | 0.195 | 0.044 | 4.466 | <0.001 |
| Visceral fat | <--> | Muscle | −1.889 | 1.090 | −1.733 | 0.083 * |
| Nutrition | <--> | Muscle | 0.139 | 0.042 | 3.280 | <0.001 |
| Nutrition | <--> | Visceral fat | −1.684 | 8.167 | −0.206 | 0.837 |
| N’ of diseases | <--> | Muscle | −0.046 | 0.024 | −1.897 | 0.058 |
| 25 OH vitamin D | <--> | Nutrition | −0.032 | 2.363 | −0.014 | 0.989 |
| Age (years) | <--> | Muscle | −0.509 | 0.116 | −4.388 | <0.001 |
| Age (years) | <--> | Bone | −2.264 | 0.313 | −7.226 | <0.001 |
| Estimate | S.E. | C.R. | p | |||
|---|---|---|---|---|---|---|
| Functional status | <--- | Bone | −1.081 | 0.340 | −3.182 | <0.001 |
| Functional status | <--- | Muscle | 9.000 | 1.985 | 4.534 | <0.001 |
| Functional status | <--- | MMSE | 0.032 | 0.010 | 3.022 | <0.001 |
| Metabolic profile | <--- | Visceral fat | 0.010 | 0.012 | 0.797 | 0.425 |
| Kidney function | <--- | Muscle | −0.422 | 0.368 | −1.147 | 0.251 |
| Liver function | <--- | Visceral fat | 0.003 | 0.010 | 0.334 | 0.738 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perna, S.; Gasparri, C.; Allehdan, S.; Riva, A.; Petrangolini, G.; Ferraris, C.; Guido, D.; Alalwan, T.A.; Rondanelli, M. Discovering the Physio-Pathological Mechanisms of Interaction between Bone Mineral Density, Muscle Mass, and Visceral Adipose Tissue in Female Older Adults through Structural Equation Modeling. J. Clin. Med. 2023, 12, 2269. https://doi.org/10.3390/jcm12062269
Perna S, Gasparri C, Allehdan S, Riva A, Petrangolini G, Ferraris C, Guido D, Alalwan TA, Rondanelli M. Discovering the Physio-Pathological Mechanisms of Interaction between Bone Mineral Density, Muscle Mass, and Visceral Adipose Tissue in Female Older Adults through Structural Equation Modeling. Journal of Clinical Medicine. 2023; 12(6):2269. https://doi.org/10.3390/jcm12062269
Chicago/Turabian StylePerna, Simone, Clara Gasparri, Sabika Allehdan, Antonella Riva, Giovanna Petrangolini, Cinzia Ferraris, Davide Guido, Tariq A. Alalwan, and Mariangela Rondanelli. 2023. "Discovering the Physio-Pathological Mechanisms of Interaction between Bone Mineral Density, Muscle Mass, and Visceral Adipose Tissue in Female Older Adults through Structural Equation Modeling" Journal of Clinical Medicine 12, no. 6: 2269. https://doi.org/10.3390/jcm12062269
APA StylePerna, S., Gasparri, C., Allehdan, S., Riva, A., Petrangolini, G., Ferraris, C., Guido, D., Alalwan, T. A., & Rondanelli, M. (2023). Discovering the Physio-Pathological Mechanisms of Interaction between Bone Mineral Density, Muscle Mass, and Visceral Adipose Tissue in Female Older Adults through Structural Equation Modeling. Journal of Clinical Medicine, 12(6), 2269. https://doi.org/10.3390/jcm12062269








