Clinical Significance of Corneal Striae in Thyroid Associated Orbitopathy
Abstract
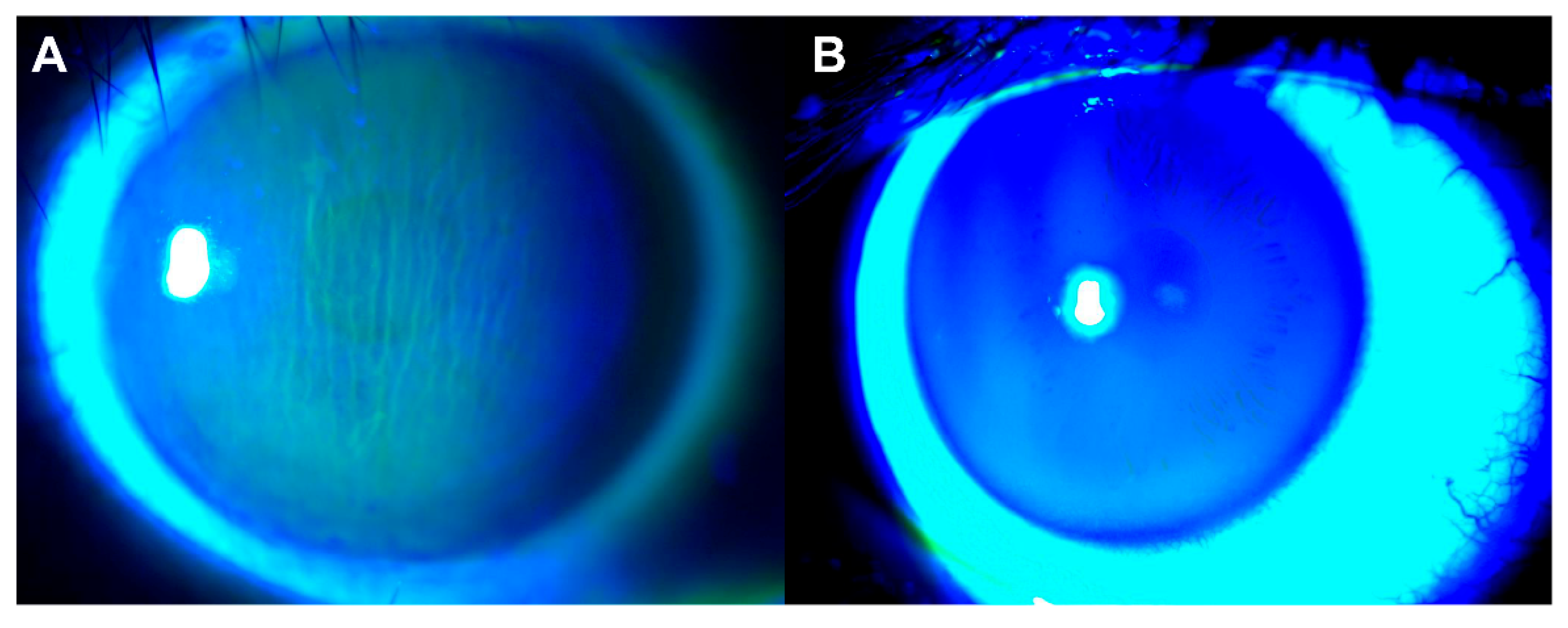
:1. Introduction
2. Methods
2.1. Orbital Examination
2.2. Magnetic Resonance Imaging
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahn, R.S. Graves’ Ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockerham, K.P.; Chan, S.S. Thyroid Eye Disease. Neurol. Clin. 2010, 28, 729–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.H.; Kahaly, G.J. Pathophysiology of thyroid-associated orbitopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 101620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Smith, T.J. Current Concepts in the Molecular Pathogenesis of Thyroid-Associated Ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1735–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Huang, Y.; Wang, S.; Zhang, Y.; Luo, X.; Liu, L.; Zhong, S.; Liu, X.; Li, D.; Liang, R.; et al. IL-17A Exacerbates Fibrosis by Promoting the Proinflammatory and Profibrotic Function of Orbital Fibroblasts in TAO. J. Clin. Endocrinol. Metab. 2016, 101, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A.H.; Nerad, J.A. Thyroid-Associated Orbitopathy. Semin. Plast. Surg. 2007, 21, 065–073. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Xiao, W.; Ye, H.; Chen, R.; Wu, J.; Mao, Y.; Yang, H. Ultrasonographic measurement of the optic nerve sheath diameter in dysthyroid optic neuropathy. Eye 2021, 35, 568–574. [Google Scholar] [CrossRef]
- Iñiguez-Ariza, N.M.; Sharma, A.; Garrity, J.A.; Stan, M.N. The “Quiet TED”—A Special Subgroup of Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 551–555. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; Ayvaz, G.; et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Kashani, S.; Papadopoulos, R.; Olver, J. Corneal striae in thyroid eye disease. Eye 2007, 21, 869–870. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.C.; Chong, K.K. Spontaneous intercalated corneal epithelial folds in thyroid eye disease: A case report. Hong Kong J. Ophthalmol. 2020, 24, 60–62. [Google Scholar] [CrossRef]
- Bartley, G.B.; Gorman, C.A. Diagnostic Criteria for Graves’ Ophthalmopathy. Am. J. Ophthalmol. 1995, 119, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Young, S.M.; Kim, Y.-D.; Woo, K.I. Course of upper eyelid retraction in thyroid eye disease. Br. J. Ophthalmol. 2020, 104, 254–259. [Google Scholar] [CrossRef]
- Kozaki, A.; Inoue, R.; Komoto, N.; Maeda, T.; Inoue, Y.; Inoue, T.; Ayaki, M. Proptosis in Dysthyroid Ophthalmopathy: A Case Series of 10,931 Japanese Cases. Optom. Vis. Sci. 2010, 87, 200–204. [Google Scholar] [CrossRef]
- Feizi, S.; Faramarzi, A.; Kheiri, B. Goldmann applanation tonometer versus ocular response analyzer for measuring intraocular pressure after congenital cataract surgery. Eur. J. Ophthalmol. 2018, 28, 582–589. [Google Scholar] [CrossRef]
- Ma, R.; Geng, Y.; Gan, L.; Peng, Z.; Cheng, J.; Guo, J.; Qian, J. Quantitative T1 mapping MRI for the assessment of extraocular muscle fibrosis in thyroid-associated ophthalmopathy. Endocrine 2022, 75, 456–464. [Google Scholar] [CrossRef]
- Tamçelik, N.; Oto, B.B.; Mergen, B.; Kiliçarslan, O.; Gönen, B.; Arici, C. Corneal Endothelial Changes in Patients With Primary Congenital Glaucoma. J. Glaucoma 2022, 31, 123–128. [Google Scholar] [CrossRef]
- Rakhshandadi, T.; Sedaghat, M.-R.; Askarizadeh, F.; Momeni-Moghaddam, H.; Khabazkhoob, M.; Yekta, A.; Narooie-Noori, F. Refractive characteristics of keratoconus eyes with corneal Vogt’s striae: A contralateral eye study. J. Optom. 2021, 14, 183–188. [Google Scholar] [CrossRef]
- Thuret, G.; Ain, A.; Koizumi, N.; Okumura, N.; Gain, P.; He, Z. Radial Endothelial Striae over 360 Degrees in Fuchs Corneal Endothelial Dystrophy: New Pathophysiological Findings. Cornea 2021, 40, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Donnenfeld, E.D.; Perry, H.D.; Doshi, S.J.; Biser, S.A.; Solomon, R. Hyperthermic treatment of post-LASIK corneal striae. J. Cataract. Refract. Surg. 2004, 30, 620–625. [Google Scholar] [CrossRef]
- Mandal, A.K.; Raghavachary, C.; Peguda, H.K. Haab’s Striae. Ophthalmology 2017, 124, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.P.; Vemuri, S.; Patel, A.D.; Compton, C.; Nunery, W.R. Lateral Tarsoconjunctival Onlay Flap Lower Eyelid Suspension in Facial Nerve Paresis. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.V.C.; Glória, A.L.F. Lagophthalmos. Semin. Ophthalmol. 2010, 25, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zheng, L.; Shuo, Z.; Xingtong, L.; Mengda, J.; Lin, Z.; Ziyang, S.; Huifang, Z. Evaluation of extraocular muscles in patients with thyroid associated ophthalmopathy using apparent diffusion coefficient measured by magnetic resonance imaging before and after radiation therapy. Acta Radiol. 2021, 63, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Abualhasan, H.; Mimouni, M.; Safuri, S.; Blumenthal, E.Z. Anterior Corneal Folds Correlate With Low Intraocular Pressure and May Serve as a Marker for Ocular Hypotony. J. Glaucoma 2019, 28, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, F.A.; Mirzania, D.; Swaminathan, S.S.; Davis, A.R.; Perez, V.L.; Herndon, L.W. Risk Factors for Corneal Striae in Eyes After Glaucoma Surgery. J. Glaucoma 2022, 31, 116–122. [Google Scholar] [CrossRef]
- Thia, B.; McGuinness, M.B.; Ebeling, P.R.; Khong, J.J. Diagnostic accuracy of Immulite® TSI immunoassay for thyroid-associated orbitopathy in patients with recently diagnosed Graves’ hyperthyroidism. Int. Ophthalmol. 2022, 42, 863–870. [Google Scholar] [CrossRef]
- Das, T.; Roos, J.C.P.; Patterson, A.J.; Graves, M.J.; Murthy, R. T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: An initial feasibility study. Eye 2019, 33, 235–243. [Google Scholar] [CrossRef] [Green Version]



| Parameters | Patients with TAO |
|---|---|
| Number of patients | 53 |
| Number of eyes | 106 |
| Number of CS eyes (N%) | 53 (50%) |
| Age (years) | 48.83 ± 14.46 |
| Female:Male | 36:17 |
| Onset age of TAO (years) | 46.47 ± 14.73 |
| Clinical activity score | 1.77 ± 1.25 |
| Graves’ disease (N%) | 49 (92.5%) |
| Smoker (N%) | 17 (32.1%) |
| TAO with CS | TAO without CS | p-Value | |
|---|---|---|---|
| Number of eyes | 53 | 53 | |
| BCVA (Log MAR) | 0.13 ± 0.20 | 0.08 ± 0.18 | 0.143 |
| IOP Primary (mmHg) | 17.15 ± 3.77 | 16.94 ± 3.34 | 0.765 |
| IOP Upgaze (mmHg) | 25.53 ± 6.37 | 24.91 ± 6.56 | 0.704 |
| Orbital parameters | |||
| MRD1 (mm) | 5.60 ± 1.98 | 5.15 ± 2.06 | 0.277 |
| MRD2 (mm) | 5.15 ± 1.22 | 5.11 ± 1.12 | 0.974 |
| Lagophthalmos (mm) | 0.83 ± 1.10 | 0.42 ± 0.69 | 0.022 |
| Exophthalmos (mm) | 18.84 ± 2.43 | 18.52 ± 2.77 | 0.484 |
| Extraocular muscles | |||
| LPS/SR (cm2) | 0.52 ± 0.21 | 0.42 ± 0.21 | 0.003 |
| Medial rectus (cm2) | 0.36 ± 0.20 | 0.33 ± 0.13 | 0.401 |
| Lateral rectus (cm2) | 0.39 ± 0.15 | 0.41 ± 0.15 | 0.270 |
| Inferior rectus (cm2) | 0.56 ± 0.25 | 0.54 ± 0.23 | 0.845 |
| Univariate Model | Multivariate Model * | |
|---|---|---|
| OR (95% CI) p-Value | OR (95% CI) p-Value | |
| IOP Primary (mmHg) | 1.02 (0.95, 1.09) 0.645 | 1.02 (0.94, 1.10) 0.646 |
| IOP Upgaze (mmHg) | 1.02 (0.98, 1.05) 0.358 | 1.02 (0.98, 1.05) 0.360 |
| Orbital parameters | ||
| MRD1 (mm) | 1.12 (0.98, 1.28) 0.103 | 1.13 (0.98, 1.32) 0.101 |
| MRD2 (mm) | 1.03 (0.85, 1.24) 0.770 | 1.03 (0.85, 1.25) 0.770 |
| Lagophthalmos (mm) | 1.69 (1.17, 2.45) 0.005 | 1.75 (1.18, 2.61) 0.006 |
| Exophthalmos (mm) | 1.05 (0.98, 1.12) 0.172 | 1.06 (0.98, 1.14) 0.171 |
| Extraocular muscles | ||
| LPS/SR (cm2) | 12.70 (1.15, 140.47) 0.038 | 19.27 (1.43, 259.32) 0.026 |
| Medial rectus (cm2) | 3.61 (1.00, 12.95) 0.049 | 5.18 (0.93, 28.94) 0.061 |
| Lateral rectus (cm2) | 0.38 (0.08, 1.87) 0.235 | 0.31 (0.04, 2.16) 0.238 |
| Inferior rectus (cm2) | 1.43 (0.53, 3.85) 0.474 | 1.65 (0.42, 6.47) 0.476 |
| AUC | 95% CI | p-Value | |
|---|---|---|---|
| IOP Primary (mmHg) | 0.517 | 0.406–0.628 | 0.678 |
| IOP Upgaze (mmHg) | 0.479 | 0.367–0.590 | 0.390 |
| Orbital parameters | |||
| MRD1 (mm) | 0.560 | 0.452–0.669 | 0.089 |
| MRD2 (mm) | 0.498 | 0.392–0.604 | 0.752 |
| Lagophthalmos (mm) | 0.598 | 0.503–0.692 | 0.004 |
| Exophthalmos (mm) | 0.539 | 0.429–0.650 | 0.074 |
| Extraocular muscles | |||
| LPS/SR (cm2) | 0.666 | 0.562–0.769 | 0.001 |
| Medial rectus (cm2) | 0.547 | 0.436–0.658 | 0.179 |
| Lateral rectus (cm2) | 0.562 | 0.451–0.673 | 0.305 |
| Inferior rectus (cm2) | 0.511 | 0.400–0.622 | 0.227 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Aljufairi, F.M.A.A.; Lai, K.K.H.; Chan, K.K.W.; Jia, R.; Chen, W.; Hu, Z.; Wei, Y.; Chu, W.C.W.; Tham, C.C.Y.; et al. Clinical Significance of Corneal Striae in Thyroid Associated Orbitopathy. J. Clin. Med. 2023, 12, 2284. https://doi.org/10.3390/jcm12062284
Liao X, Aljufairi FMAA, Lai KKH, Chan KKW, Jia R, Chen W, Hu Z, Wei Y, Chu WCW, Tham CCY, et al. Clinical Significance of Corneal Striae in Thyroid Associated Orbitopathy. Journal of Clinical Medicine. 2023; 12(6):2284. https://doi.org/10.3390/jcm12062284
Chicago/Turabian StyleLiao, Xulin, Fatema Mohamed Ali Abdulla Aljufairi, Kenneth Ka Hei Lai, Karen Kar Wun Chan, Ruofan Jia, Wanxue Chen, Zhichao Hu, Yingying Wei, Winnie Chiu Wing Chu, Clement Chee Yung Tham, and et al. 2023. "Clinical Significance of Corneal Striae in Thyroid Associated Orbitopathy" Journal of Clinical Medicine 12, no. 6: 2284. https://doi.org/10.3390/jcm12062284
APA StyleLiao, X., Aljufairi, F. M. A. A., Lai, K. K. H., Chan, K. K. W., Jia, R., Chen, W., Hu, Z., Wei, Y., Chu, W. C. W., Tham, C. C. Y., Pang, C. P., & Chong, K. K. L. (2023). Clinical Significance of Corneal Striae in Thyroid Associated Orbitopathy. Journal of Clinical Medicine, 12(6), 2284. https://doi.org/10.3390/jcm12062284








