Operative Digital Enhancement of Macular Pigment during Macular Surgery
Abstract
:1. Introduction
2. Materials and Methods
Technique
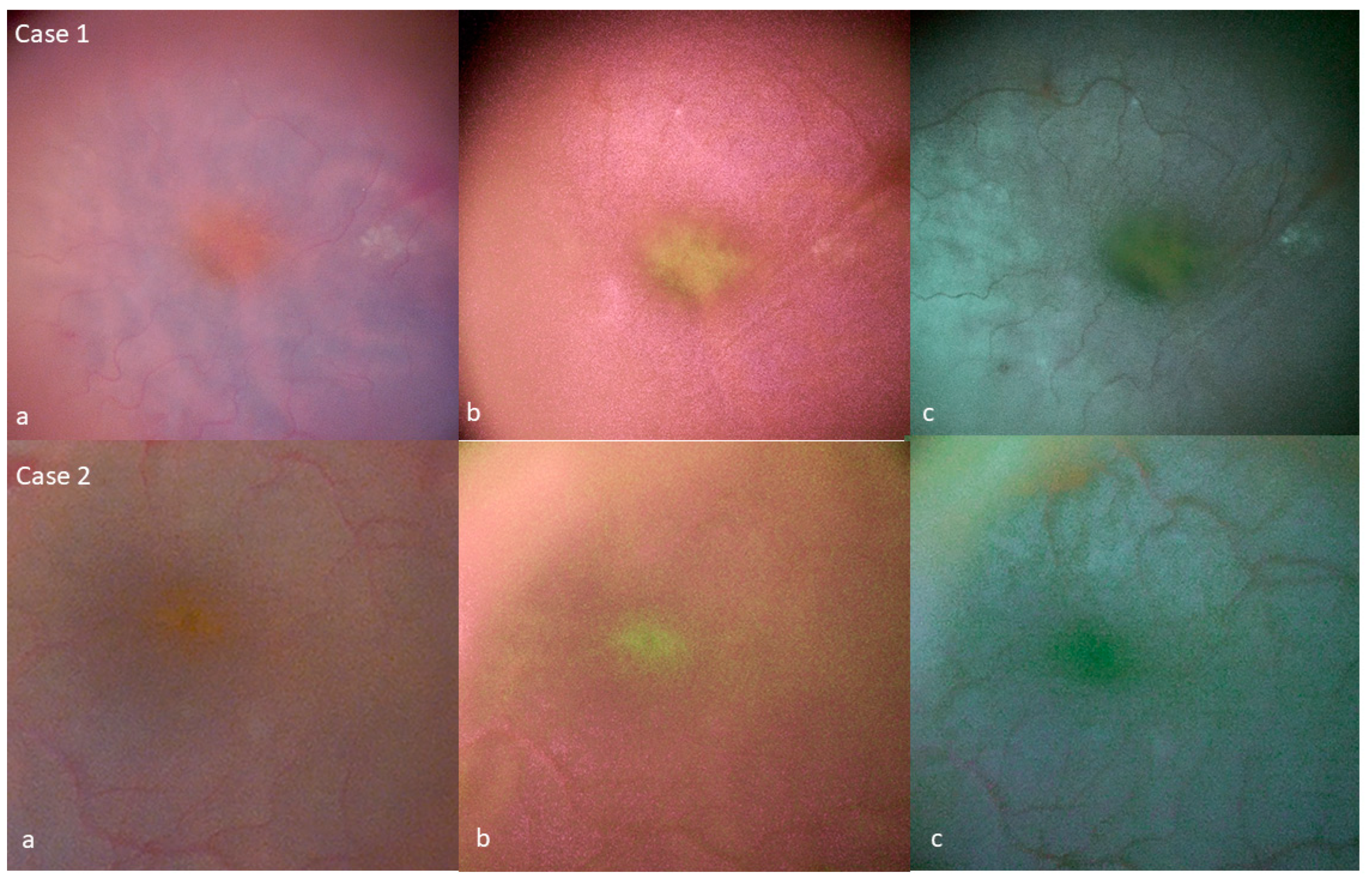
3. Results
Tissue Color Modifications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, E.; Nolan, J.; Howard, A.; Beatty, S. Macular Pigment and Its Contribution to Vision. Nutrients 2013, 5, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.C.; Rosen, R.B.; Farah, M. Macular pigment in retinal health and disease. Int. J. Retin. Vitr. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, L.; Vitale, A.S.; Modersitzki, N.K.; Bernstein, P.S. Fluorescence lifetime imaging ophthalmoscopy: Autofluorescence imaging and beyond. Eye 2021, 35, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Nakazawa, R.; Noma, S.; Sasano, H.; Gohto, Y. Macular Pigment in Eyes with Macular Hole Formation and Its Change after Surgery. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Obana, A.; Sasano, H.; Okazaki, S.; Otsuki, Y.; Seto, T.; Gohto, Y. Evidence of Carotenoid in Surgically Removed Lamellar Hole-Associated Epiretinal Proliferation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5157–5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.S.; Lee, J.S.; Son, G.; Sohn, J. Epiretinal Proliferation Associated with Lamellar Hole or Macular Hole: Origin and Surgical Prognosis. Korean J. Ophthalmol. 2019, 33, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Shiraga, F.; Takasu, I.; Fukuda, K.; Fujita, T.; Yamashita, A.; Hirooka, K.; Shirakata, Y.; Morizane, Y.; Fujiwara, A. Modified vitreous surgery for symptomatic lamellar macular hole with epiretinal membrane containing macular pigment. Retina 2013, 33, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Sandali, O.; El Sanharawi, M.; Tahiri, J.H.R.; Roux, H.; Bouheraoua, N.; Borderie, V. Early corneal pachymetry maps after cataract surgery and influence of 3D digital visualization system in minimizing corneal oedema. Acta Ophthalmol. 2022, 100, e1088–e1094. [Google Scholar] [CrossRef] [PubMed]
- Sandali, O.; Tahiri, J.H.R.; Armia, A.B.; El Sanharawi, M.; Borderie, V. Facilitating Role of the 3D Viewing System in Tilted microscope Positions for Cataract Surgery in Patients Unable to Lie Flat. J. Clin. Med. 2022, 11, 1865. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, R.J.; Diakonis, V.F.; Schwartz, A.J.; Weinstock, A.J. Heads-up cataract surgery: Complication rates, surgical duration, and comparison with traditional microscopes. J. Refract. Surg. 2019, 35, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Sandali, O.; Tahiri, J.H.R.; Dulière, C.; El Sanharawi, M.; Borderie, V. Use of a 3D viewing system and microscope tilting to extend the peripheral retinal view. Retina 2022. [Google Scholar] [CrossRef]
- Sandali, O.; Tahiri, J.H.R.; Armia Balamoun, A.; Duliere, C.; El Sanharawi, M.; Borderie, V. Use of Black-and-White Digital Filters to Optimize Visualization in Cataract Surgery. J Clin Med. 2022, 13, 4056. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.G.R.; Conti, T.F.; Hom, G.L.; Greenlee, T.E.; Cella, W.P.; Talcott, K.E.; Rachitskaya, A.; Yuan, A.; Sood, A.; Milam, R.; et al. Optimizing Visualization of Membranes in Macular Surgery with Heads-Up Display. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Hardin, C.L. Red and yellow, green and blue, warm and cool: Explaining color appearance. J. Conscious. Stud. 2000, 7, 113–122. [Google Scholar]
- Pridmore, R.W. Complementary colors: A literature review. Color Res. Appl. 2021, 46, 482–488. [Google Scholar] [CrossRef]
- Jaggi, D.; Solberg, Y.; Dysli, C.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. Fluorescence lifetime imaging ophthalmoscopy: Findings after surgical reattachment of macula-off rhegmatogenous retinal detachment. Retina 2020, 40, 1929–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillbro, T.; Cogdell, R.J. Carotenoid fluorescence. Chem. Phys. Lett. 1989, 158, 312–316. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandali, O.; Tahiri Joutei Hassani, R.; Armia Balamoun, A.; Franklin, A.; Sallam, A.B.; Borderie, V. Operative Digital Enhancement of Macular Pigment during Macular Surgery. J. Clin. Med. 2023, 12, 2300. https://doi.org/10.3390/jcm12062300
Sandali O, Tahiri Joutei Hassani R, Armia Balamoun A, Franklin A, Sallam AB, Borderie V. Operative Digital Enhancement of Macular Pigment during Macular Surgery. Journal of Clinical Medicine. 2023; 12(6):2300. https://doi.org/10.3390/jcm12062300
Chicago/Turabian StyleSandali, Otman, Rachid Tahiri Joutei Hassani, Ashraf Armia Balamoun, Alan Franklin, Ahmed B. Sallam, and Vincent Borderie. 2023. "Operative Digital Enhancement of Macular Pigment during Macular Surgery" Journal of Clinical Medicine 12, no. 6: 2300. https://doi.org/10.3390/jcm12062300







