Does the Loss of Teeth Have an Impact on Geriatric Patients’ Cognitive Status?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cognitive Dysfunction Assessment
2.3. Assessment of Covariates
2.4. Statistical Analysis
3. Results
4. Discussions
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontana, L.; Kennedy, B.K.; Longo, V.D.; Seals, D.; Melov, S. Medical research: Treat ageing. Nature 2014, 511, 405–407. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Verma, I.; Taegen, J. Ageing and Inclusion in Rural Areas. Stud. Health Technol. Inform. 2021, 282, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yin, P.; Wang, L.; Qu, M.; Kan, G.L.; Zhang, H.; Zhang, Q.; Xiao, Y.; Deng, Y.; Dong, Z.; et al. Prevalence of Parkinson’s Disease: A Community-Based Study in China. Mov. Disord. 2021, 36, 2940–2944. [Google Scholar] [CrossRef]
- Etgen, T.; Sander, D.; Bickel, H.; Förstl, H. Mild cognitive impairment and dementia: The importance of modifiable risk factors. Dtsch. Arztebl. Int. 2011, 108, 743–750. [Google Scholar] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.; Akinkugbe, A.; Sanders, A. Projections of U.S. Edentulism Prevalence Following 5 Decades of Decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Wåhlin, A. The many facets of brain ageing. eLife 2020, 9, e56640. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Voss, M.W.; Weng, T.B.; Burzynska, A.Z.; Wong, C.N.; Cooke, G.E.; Clark, R.; Fanning, J.; Awick, E.; Gothe, N.P.; Olson, E.A.; et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage 2016, 131, 113–125. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Gerstorf, D.; Herlitz, A.; Smith, J. Stability of Sex Differences in Cognition in Advanced Old Age: The Role of Education and Attrition. J. Gerontol. B Psychol. Sci. Soc. Sci. 2006, 61, P245–P249. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, R.F.; Akoudad, S.; Cremers, L.G.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Determinants, MRI Correlates, and Prognosis of Mild Cognitive Impairment: The Rotterdam Study. J. Alzheimer’s Dis. 2014, 42, S239–S249. [Google Scholar] [CrossRef] [PubMed]
- Fotuhi, M.; Hachinski, V.; Whitehouse, P.J. Changing perspectives regarding late-life dementia. Nat. Rev. Neurol. 2009, 5, 649–658. [Google Scholar] [CrossRef]
- Reyes-Ortiz, C.A.; Luque, J.S.; Eriksson, C.K.; Soto, L. Self-reported tooth loss and cognitive function: Data from the Hispanic Es-tablished Populations for Epidemiologic Studies of the Elderly (Hispanic EPESE). Colomb. Med. 2013, 44, 139–145. [Google Scholar] [CrossRef]
- Henke, K. A model for memory systems based on processing modes rather than consciousness. Nat. Rev. Neurosci. 2010, 11, 523–532. [Google Scholar] [CrossRef]
- Tulving, E.; Markowitsch, H.J. Episodic and declarative memory: Role of the hippocampus. Hippocampus 1998, 8, 198–204. [Google Scholar] [CrossRef]
- Batty, G.-D.; Li, Q.; Huxley, R.; Zoungas, S.; Taylor, B.-A.; Neal, B.; de Galan, B.; Woodward, M.; Harrap, S.-B.; Colagiuri, S.; et al. Oral Disease in Relation to Future Risk of Dementia and Cognitive Decline: Prospective Cohort Study Based on the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (Advance) Trial. Eur. Psychiatry 2013, 28, 49–52. [Google Scholar] [CrossRef]
- Onozuka, M.; Watanabe, K.; Mirbod, S.M.; Ozono, S.; Nishiyama, K.; Karasawa, N.; Nagatsu, I. Reduced mastication stimulates impairment of spatial memory and degeneration of hippocampal neurons in aged SAMP8 mice. Brain Res. 1999, 826, 148–153. [Google Scholar] [CrossRef]
- Makiura, T.; Ikeda, Y.; Hirai, T.; Terasawa, H.; Hamaue, N.; Minami, M. Influence of diet and occlusal support on learning memory in rats behavioral and biochemical studies. Res. Commun. Mol. Pathol. Pharmacol. 2000, 107, 269–277. [Google Scholar]
- Jiang, Q.-S.; Liang, Z.-L.; Wu, M.-J.; Feng, L.; Liu, L.-L.; Zhang, J.-J. Reduced brain-derived neurotrophic factor expression in cortex and hippocampus involved in the learning and memory deficit in molarless SAMP8 mice. Chin. Med. J. 2011, 124, 1540–1544. [Google Scholar] [PubMed]
- Terasawa, H.; Hirai, T.; Ninomiya, T.; Ikeda, Y.; Ishijima, T.; Yajima, T.; Hamaue, N.; Nagase, Y.; Kang, Y.; Minami, M. Influence of tooth-loss and concomitant masticatory alterations on cholinergic neurons in rats: Immunohistochemical and biochemical studies. Neurosci. Res. 2002, 43, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ozono, S.; Nishiyama, K.; Saito, S.; Tonosaki, K.; Fujita, M.; Onozuka, M. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav. Brain Res. 2002, 128, 19–25. [Google Scholar] [CrossRef]
- De Marchi, R.J.; Hugo, F.N.; Hilgert, J.B.; Padilha, D.M.P. Association between number of teeth, edentulism and use of dentures with percentage body fat in south Brazilian community-dwelling older people. Gerodontology 2012, 29, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Bastos, J.L.; Watt, R.G.; Xavier, A.J.; Barbato, P.R.; D’Orsi, E. Tooth loss is associated with severe cognitive impairment among older people: Findings from a population-based study in Brazil. Aging Ment. Health 2015, 19, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Koshino, H. Expectations of Prosthetic Dentistry as a Health Science. Nihon Hotetsu Shika Gakkai Zasshi 2006, 50, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Power, M.C.; Gottesman, R.F. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: A Review. Curr. Hypertens. Rep. 2017, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Morikawa, M.; Tomioka, K.; Yanagi, M.; Amano, N.; Kurumatani, N. Association between Tooth Loss and the Development of Mild Memory Impairment in the Elderly: The Fujiwara-kyo Study. J. Alzheimer’s Dis. 2015, 44, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Folstein, F.M.; Folstein, S.E.; White, T.; Messer, M.A. MMSE-2: Mini-Mental State Examination, Ed. A 2-a-Manual de Utilizare a Testului; O.S.: Bucharest, Romania, 2013; Available online: https://testcentral.ro/upload/tests/LVrIQbIxZN8DZqHq9uw61tRQrANwgmxEKpHzPJCZ.pdf (accessed on 11 October 2022).
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, 13, CD011145. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, M. World Alzheimer Report 2015: The Global Impact of Dementia; Alzheimer’s Disease International (ADI): London, UK, 2015; pp. 1–87. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 21 December 2022).
- Sachdev, P.S.; Lipnicki, D.M.; Kochan, N.A.; Crawford, J.D.; Thalamuthu, A.; Andrews, G.; Brayne, C.; Matthews, F.E.; Stephan, B.C.M.; Lipton, R.B.; et al. The Prevalence of Mild Cognitive Impairment in Diverse Geographical and Ethnocultural Regions: The COSMIC Collaboration. PLoS ONE 2015, 10, e0142388. [Google Scholar] [CrossRef]
- Fukushima-Nakayama, Y.; Ono, T.; Hayashi, M.; Inoue, M.; Wake, H.; Ono, T.; Nakashima, T. Reduced Mastication Impairs Memory Function. J. Dent. Res. 2017, 96, 1058–1066. [Google Scholar] [CrossRef]
- Kumar, P.S. From focal sepsis to periodontal medicine: A century of exploring the role of the oral microbiome in systemic disease. J. Physiol. 2017, 595, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Peyron, M.A.; Woda, A.; Bourdiol, P.; Hennequin, M. Age-related changes in mastication. J. Oral Rehabil. 2017, 44, 299–312. [Google Scholar] [CrossRef]
- Bergdahl, M.; Habib, R.; Bergdahl, J.; Nyberg, L.; Nilsson, L.-G. Natural teeth and cognitive function in humans. Scand. J. Psychol. 2007, 48, 557–565. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Lopez-Chaichio, L.; Padial-Molina, M.; Avila-Ortiz, G.; O’Valle, F.; Ravida, A.; Catena, A. The impact of tooth loss on cognitive function. Clin. Oral Investig. 2021, 26, 3493–3500. [Google Scholar] [CrossRef]
- Okamoto, N.; Morikawa, M.; Okamoto, K.; Habu, N.; Hazaki, K.; Harano, A.; Iwamoto, J.; Tomioka, K.; Saeki, K.; Kurumatani, N. Tooth loss is associated with mild memory impairment in the elderly: The Fujiwara-kyo study. Brain Res. 2010, 1349, 68–75. [Google Scholar] [CrossRef]
- Onozuka, M.; Fujita, M.; Watanabe, K.; Hirano, Y.; Niwa, M.; Nishiyama, K.; Saito, S. Mapping Brain Region Activity during Chewing: A Functional Magnetic Resonance Imaging Study. J. Dent. Res. 2002, 81, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Obata, T.; Kashikura, K.; Nonaka, H.; Tachibana, A.; Ikehira, H.; Onozuka, M. Effects of chewing in working memory processing. Neurosci. Lett. 2008, 436, 189–192. [Google Scholar] [CrossRef]
- Fontijn-Tekamp, F.A.; Slagter, A.P.; Van Der Bilt, A.; Van ’T Hof, M.A.; Witter, D.J.; Kalk, W.; Jansen, J.A. Biting and chewing in overdentures, full dentures, and natural dentitions. J. Dent. Res. 2000, 79, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, K.; Gondo, Y.; Kamide, K.; Masui, Y.; Ishizaki, T.; Arai, Y.; Inagaki, H.; Nakagawa, T.; Kabayama, M.; Ryuno, H.; et al. Occlusal force is correlated with cognitive function directly as well as indirectly via food intake in community-dwelling older Japanese: From the SONIC study. PLoS ONE 2018, 13, e0190741. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Chaichio, L.; Padial-Molina, M.; O’Valle, F.; Gil-Montoya, J.A.; Catena, A.; Galindo-Moreno, P. Oral health and healthy chewing for healthy cognitive ageing: A comprehensive narrative review. Gerodontology 2021, 38, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Cerutti-Kopplin, D.; Feine, J.; Padilha, D.M.; de Souza, R.F.; Ahmadi, M.; Rompré, P.; Booij, L.; Emami, E. Tooth loss increases the risk of diminished cognitive function: A systematic review and meta-analysis. JDR Clin. Transl. Res. 2016, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Morikawa, M.; Amano, N.; Yanagi, M.; Takasawa, S.; Kurumatani, N. Effects of Tooth Loss and the Apolipoprotein E ɛ4 Allele on Mild Memory Impairment in the Fujiwara-kyo Study of Japan: A Nested Case-Control Study. J. Alzheimer’s Dis. 2017, 55, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Soh, I.; Saito, T.; Yamashita, Y.; Koga, T.; Miyazaki, H.; Takehara, T. Influence of dentition status on physical disability, mental impairment and mortality in in-stitutionalized elderly people. J. Dent. Res. 2001, 80, 340–345. [Google Scholar] [CrossRef]
- Avivi-Arber, L.; Sessle, B.J. Jaw sensorimotor control in healthy adults and effects of ageing. J. Oral Rehabil. 2018, 45, 50–80. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Ansai, T.; Soh, I.; Akifusa, S.; Sonoki, K.; Fujisawa, K.; Yoshida, A.; Kagiyama, S.; Hamasaki, T.; Nakamichi, I.; et al. Relationship between chewing ability and high-level functional capacity in an 80-year-old population in Japan. Gerodontology 2008, 25, 147–154. [Google Scholar] [CrossRef]
- Utsugi, C.; Miyazono, S.; Osada, K.; Matsuda, M.; Kashiwayanagi, M. Impaired mastication reduced newly generated neurons at the accessory olfactory bulb and pheromonal responses in mice. Arch. Oral Biol. 2014, 59, 1272–1278. [Google Scholar] [CrossRef]
- Kumar, A.; Kothari, M.; Grigoriadis, A.; Trulsson, M.; Svensson, P. Bite or brain: Implication of sensorimotor regulation and neu-roplasticity in oral rehabilitation procedures. J. Oral Rehabil. 2018, 45, 323–333. [Google Scholar] [CrossRef] [PubMed]
| No. | % | ||
|---|---|---|---|
| Age | 67.79 year (SD 14.44), (min. 28 years, max. 87 years) | ||
| Sex | Female | 62 | 57.4 |
| Male | 46 | 42.6 | |
| Education level | Elementary school | 38 | 35.2 |
| Secondary school | 58 | 53.7 | |
| University studies | 12 | 11.1 | |
| Place of residence | Urban | 70 | 64.8 |
| Rural | 38 | 35.2 | |
| Type of edentation | Partially extended edentulism | 14 | 13.0 |
| Total edentulism | 38 | 35.2 | |
| Combined edentulism | 56 | 51.9 | |
| Treatment of dental edentation | Yes | 54 | 50.0 |
| No | 54 | 50.0 | |
| Type of treatment of dental edentation | Untreated edentulism | 54 | 50.0 |
| Removable prosthesis | 28 | 25.9 | |
| Composite prosthesis | 26 | 24.1 | |
| Subjective masticatory efficiency | Adequate mastication | 50 | 46.3 |
| Inadequate mastication | 58 | 53.7 | |
| Comorbidities | Yes | 92 | 85.2 |
| No | 16 | 14.8 | |
| Questions | Mean ± SD Maxim Value | MMSE Scores | Edentulism Treatment | p | |
|---|---|---|---|---|---|
| No | Yes | ||||
| 1. Orientation: Which (year), (season), (day of the week), (date), (month) is it? | 4.15 ± 0.873 | 2 3 4 5 | 18.5% 7.4% 63.0% 11.1% | 0.0% 0.0% 37.0% 63.0% | 0.000 |
| Max. 5 | |||||
| Where are we—(country), (town), (district), (hospital), (floor)? | 4.35 ± 0.701 | 3 4 5 | 25.9% 51.9% 22.2% | 0.0% 25.9% 74.1% | 0.000 |
| Max. 5 | |||||
| 2. Memory: Say the names of three unrelated objects loudly and clearly, with one-second pauses between them. Ask the patient to repeat all three (1 point for each correct answer). If it does not work the first time, repeat the test until the patient repeats all three words (try up to 5 times). If the patient cannot learn them all, immediate memory cannot be properly assessed | 2.28 ± 0.653 | 1 2 3 | 7.4% 59.3% 33.3% | 14.8% 40.7% 44.4% | 0.133 |
| Max. 3 | |||||
| 3. Attention and calculation Subtract 7 from 100, then repeat from the result. Continue five times: 100, 93, 86, 79, 72, 65 | 2.65 ± 1.328 | 0 1 2 3 4 5 | 22.2% 3.7% 33.3% 29.6% 7.4% 3.7% | 0.0% 0.0% 25.9% 40.7% 18.5% 14.8% | 0.001 |
| Max. 5 | |||||
| 4. Recall Ask for the names of the three objects learned earlier (1 point for each correct answer). | 1.98 ± 0.875 | 1 2 3 | 51.9% 18.5% 29.6% | 25.9% 29.6% 44.4% | 0.022 |
| Max. 3 | |||||
| 5. Language: “Make up and write a sentence about anything.” (This sentence must contain a noun and a verb.) | 1 ± 0.820 | 0 1 2 | 51.9% 29.6% 18.5% | 14.8% 37.0% 48.1% | 0.000 |
| Max. 2 | |||||
| Repeat “No ifs, and or buts.” | 1 ± 0.00 | 1 | 100% | 100% | - |
| Max. 1 | |||||
| Follow a 3-stage command: “Take the paper in your right hand, fold it in half, and put it on the floor.” (The examiner gives the patient a piece of blank paper.) Score 1 for each stage. | 2.41 ± 0.737 | 1 2 3 | 18.5% 33.3% 48.1% | 11.1% 25.9% 63.0% | 0.277 |
| Max. 3 | |||||
| Read and obey the following: “Please read this and do what it says.” (Written instruction is “Close your eyes.”) | 0.85 ± 0.35 | 0 1 | 29.6% 70.4% | 0.0% 100% | 0.000 |
| Max. 1 | |||||
| Name a pencil and watch. | 0.94 ± 0.23 | 0 1 | 11.1% 88.9% | 0.0% 100% | 0.012 |
| Max. 1 | |||||
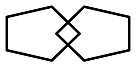 6. Copying: | 0.52 ± 0.50 | 0 1 | 55.6% 44.4% | 40.7% 59.3% | 0.177 |
| Max. 1 | |||||
| SCOR MMSE | 21.81 ± 3.872 | 16 17 18 19 21 22 23 24 25 27 29 30 | 22.2% 3.7% 14.8% 33.3% 3.7% 18.5% 0.0% 0.0% 0.0% 0.0% 3.7% 0.0% | 0.0% 0.0% 0.0% 0.0% 11.1% 0.0% 40.7% 11.1% 14.8% 7.4% 0.0% 14.8% | 0.000 |
| Max. 30 | |||||
| Cognitive Impairment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | p * | No MMSE % | MMSE Mild Form % | MMSE Moderate Form % | p ** | ||
| Sex | Female Male | 21.10 22.78 | 3.696 3.932 | 0.025 | 6.5 8.7 | 58.1 65.2 | 35.5 26.1 | 0.565 |
| Education level | Elementary school Secondary school University studies | 21.16 21.90 23.50 | 3.309 4.233 3.398 | 0.184 | 0.0 10.3 16.7 | 65.8 55.2 75.0 | 34.2 34.5 8.3 | 0.091 |
| Place of residence | Urban Rural | 22.09 21.32 | 3.907 3.807 | 0.326 | 8.6 5.3 | 57.1 68.4 | 34.3 26.3 | 0.503 |
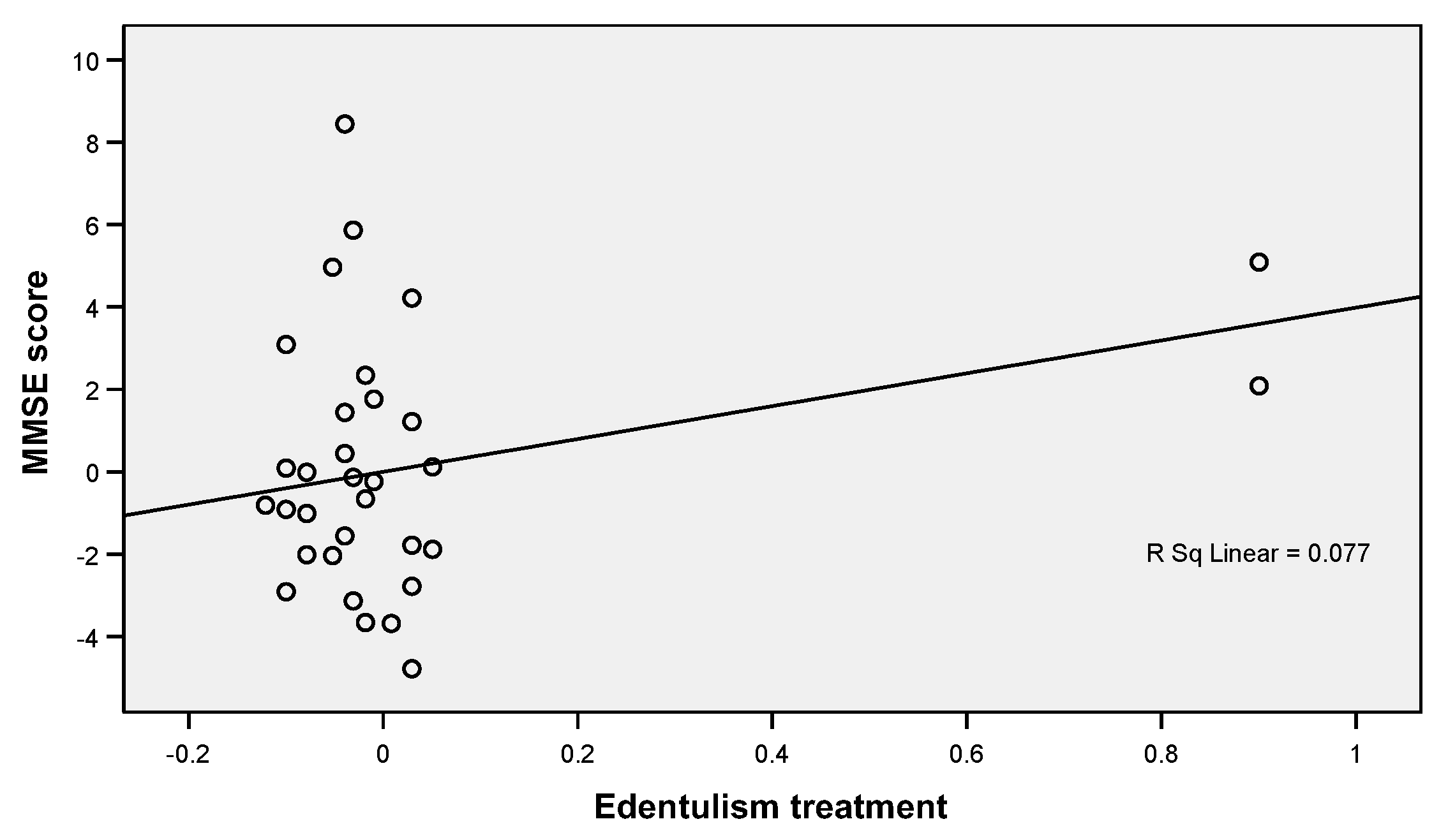
| Treatment of dental edentation | No Yes | 19.11 24.52 | 2.820 2.725 | 0.000 | 0.0 14.8 | 37.0 85.2 | 63.0 0.0 | 0.000 * |
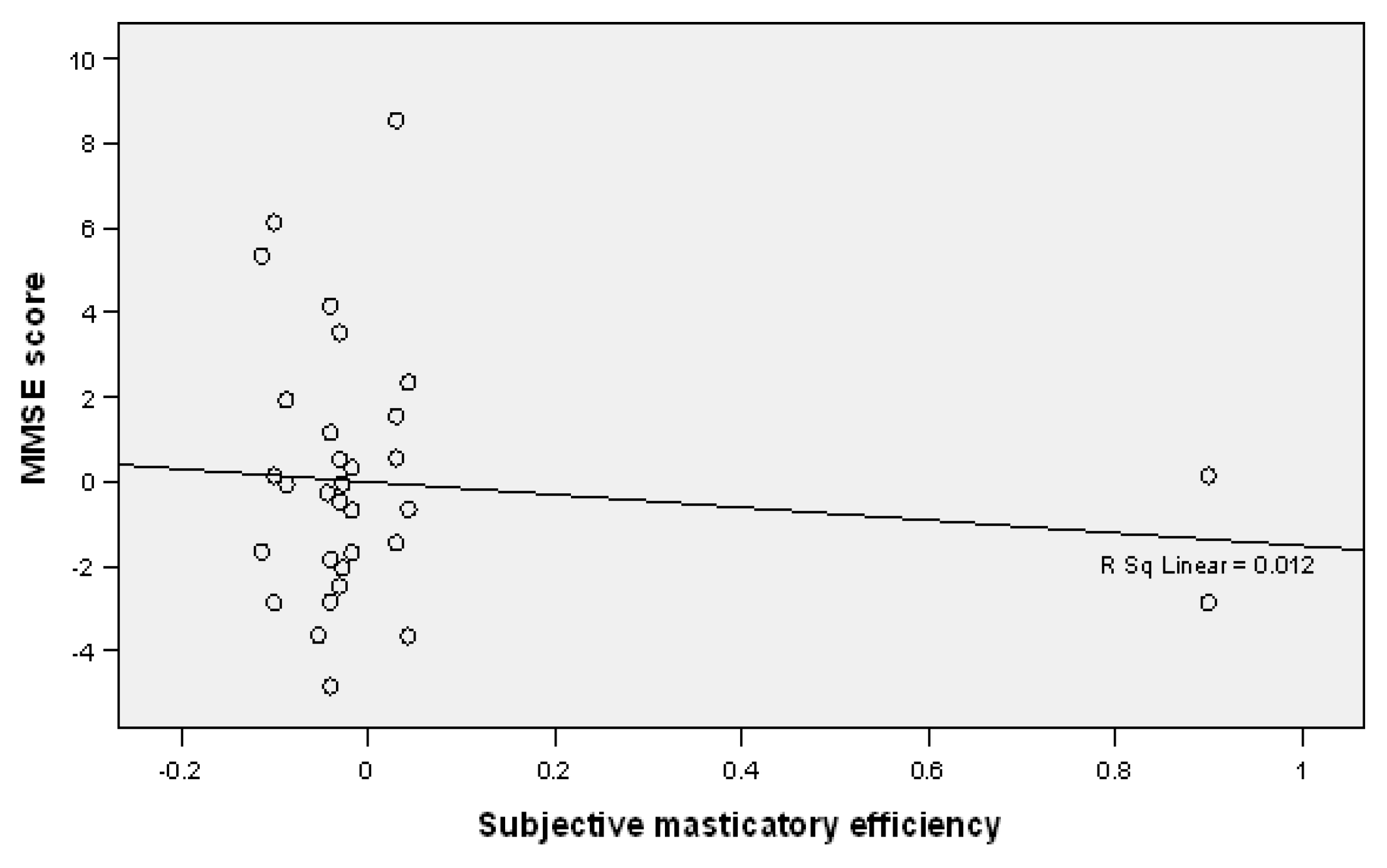
| Subjective masticatory efficiency | Adequate mastication Inadequate mastication | 24.68 19.34 | 2.736 2.881 | 0.000 | 16.0 0.0 | 84.0 41.4 | 0.0 58.6 | 0.000 * |
| Comorbidities | No Yes | 24.60 21.18 | 5.051 3.268 | 0.000 | 40.0 0.0 | 40.0 65.9 | 20.0 34.1 | 0.000 * |
| Dependent Variable | Independent Variables | 95% CI for B | p | ||||
|---|---|---|---|---|---|---|---|
| B | SE B | Beta | Lower Bound | Upper Bound | |||
| MMSE | (Constant) | 18.022 | 3.011 | 12.051 | 23.994 | 0.000 | |
| Sex | 1.887 | 0.511 | 0.242 | 0.874 | 2.899 | 0.000 | |
| Education | 0.815 | 0.393 | 0.135 | 0.036 | 1.595 | 0.041 | |
| Edentulism treatment | 3.986 | 1.359 | 0.517 | 1.291 | 6.681 | 0.004 | |
| Subjective masticatory efficiency | −1.513 | 1.360 | −0.196 | −4.210 | 1.184 | 0.268 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budală, D.G.; Balcoș, C.; Armencia, A.; Virvescu, D.I.; Lupu, C.I.; Baciu, E.R.; Vasluianu, R.I.; Tatarciuc, M.; Luchian, I. Does the Loss of Teeth Have an Impact on Geriatric Patients’ Cognitive Status? J. Clin. Med. 2023, 12, 2328. https://doi.org/10.3390/jcm12062328
Budală DG, Balcoș C, Armencia A, Virvescu DI, Lupu CI, Baciu ER, Vasluianu RI, Tatarciuc M, Luchian I. Does the Loss of Teeth Have an Impact on Geriatric Patients’ Cognitive Status? Journal of Clinical Medicine. 2023; 12(6):2328. https://doi.org/10.3390/jcm12062328
Chicago/Turabian StyleBudală, Dana Gabriela, Carina Balcoș, Adina Armencia, Dragoș Ioan Virvescu, Costin Iulian Lupu, Elena Raluca Baciu, Roxana Ionela Vasluianu, Monica Tatarciuc, and Ionuț Luchian. 2023. "Does the Loss of Teeth Have an Impact on Geriatric Patients’ Cognitive Status?" Journal of Clinical Medicine 12, no. 6: 2328. https://doi.org/10.3390/jcm12062328
APA StyleBudală, D. G., Balcoș, C., Armencia, A., Virvescu, D. I., Lupu, C. I., Baciu, E. R., Vasluianu, R. I., Tatarciuc, M., & Luchian, I. (2023). Does the Loss of Teeth Have an Impact on Geriatric Patients’ Cognitive Status? Journal of Clinical Medicine, 12(6), 2328. https://doi.org/10.3390/jcm12062328










