Urinary Oxidative Stress Biomarker Levels Might Be Useful in Identifying Functional Bladder Disorders in Women with Frequency and Urgency Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Urinary Biomarker Investigations
2.2. Statistical Analysis
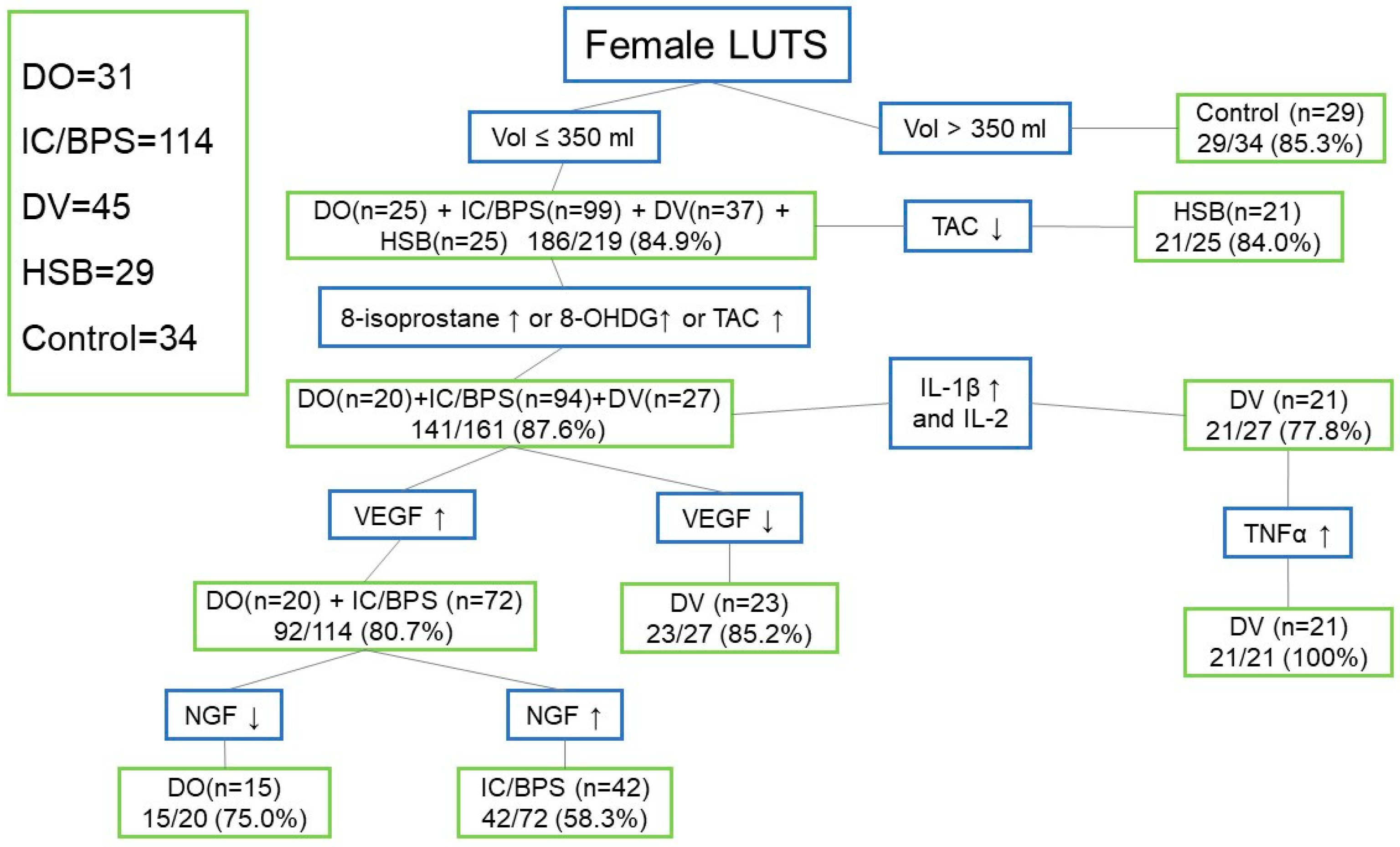
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chuang, F.C.; Huang, K.H.; Kuo, H.C. Lower urinary tract symptoms and video-urodynamic characteristics of women with clinically unsuspected bladder outlet obstruction. Low Urin. Tract Symptoms 2013, 5, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Clinical symptoms are not reliable in the diagnosis of lower urinary tract dysfunction in women. J. Formos Med. Assoc. 2012, 111, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Chen, S.F.; Jiang, Y.H.; Kuo, H.C. Effect of videourodynamic subtypes on treatment outcomes of female dysfunctional voiding. Int. Urogynecol. J. 2022, 33, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Lopes, T.; Cruz, F. Urinary biomarkers in overactive bladder: Revisiting the evidence in 2019. Eur. Urol. Focus 2019, 5, 329–336. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lu, J.H.; Chuang, S.M.; Chueh, K.S.; Juan, T.J.; Liu, Y.C.; Juan, Y.S. Urinary biomarkers in interstitial cystitis/bladder pain syndrome and its impact on therapeutic outcome. Diagnostics 2021, 12, 75. [Google Scholar] [CrossRef]
- Lai, H.H.; Pickersgill, N.A.; Vetter, J.M. Hunner lesion phenotype in interstitial cystitis/bladder pain syndrome: A systematic review and meta-analysis. J. Urol. 2020, 204, 518–523. [Google Scholar] [CrossRef]
- Bilé Silva, A.; Dinis, P.J.; Abranches Monteiro, L. Systematic review of urinary biomarkers of female bladder outlet obstruction (fBOO). Arch. Ital. Urol. Androl. 2022, 94, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.M.; Kuo, H.C. Performance of urinary biomarkers in differentiating dysfunctional voiding in women with overactive bladder syndrome: A prospective pilot study. Int. Urol. Nephrol. 2022, 54, 2497–2502. [Google Scholar] [CrossRef]
- Siddiqui, N.Y.; Helfand, B.T.; Andreev, V.P.; Kowalski, J.T.; Bradley, M.S.; Lai, H.H.; Berger, M.B.; Mueller, M.G.; Bickhaus, J.A.; Packiam, V.T.; et al. Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN). Biomarkers implicated in lower urinary tract symptoms: Systematic review and pathway analyses. J. Urol. 2019, 202, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A. Standardisation Sub-committee of the International Continence Society. The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-Committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine biomarkers in ESSIC type 2 interstitial cystitis/bladder pain syndrome and overactive bladder with developing a novel diagnostic algorithm. Sci. Rep. 2021, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine cytokines as biomarkers for diagnosing interstitial cystitis/bladder pain syndrome and mapping its clinical characteristics. Am. J. Physiol. Ren. Physiol. 2020, 318, F1391–F1399. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Ho, H.C.; Chiou, D.Y.; Kuo, H.C. Urine oxidative stress biomarkers as novel biomarkers in interstitial cystitis/bladder pain syndrome. Biomedicines 2022, 10, 1701. [Google Scholar] [CrossRef]
- Wang, H.J.; Kuo, H.C. Effects of different urodynamic characteristics on therapeutic outcomes of overactive bladder medication in a real-life clinical practice. Tzu Chi Med. J. 2022, 34, 441–447. [Google Scholar]
- Clemens, J.Q.; Erickson, D.R.; Varela, N.P.; Lai, H.H. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J. Urol. 2022, 208, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo Gomez, M.F.; Gomez Castro, S. Physiopathologic relationship between interstitial cystitis and rheumatic, autoimmune, and chronic inflammatory diseases. Arch. Esp. Urol. 2004, 57, 25–34. [Google Scholar]
- Peng, C.H.; Chen, S.F.; Kuo, H.C. Videourodynamic analysis of the urethral sphincter overactivity and the poor relaxing pelvic floor muscles in women with voiding dysfunction. Neurourol. Urodyn. 2017, 36, 2169–2175. [Google Scholar] [CrossRef]
- Chess-Williams, R.; McDermott, C.; Sellers, D.J.; West, E.G.; Mills, K.A. Chronic psychological stress and lower urinary tract symptoms. Low Urin. Tract Symptoms 2021, 13, 414–424. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, V.; Qu, X.; Chuang, Y.C.; Kuo, H.C.; Chancellor, M. Elevated CXC chemokines in urine noninvasively discriminate OAB from UTI. Am. J. Physiol. Ren. Physiol. 2016, 311, F548–F554. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.H.; Jhang, J.F.; Ho, H.C.; Hsu, Y.H.; Kuo, H.C. Diagnostic and prognostic value of urine biomarkers among women with dysfunctional voiding. Sci. Rep. 2022, 12, 6608. [Google Scholar] [CrossRef]
- Kim, J.; De Hoedt, A.; Wiggins, E.; Haywood, K.; Jin, P.; Greenwood, B.; Narain, N.R.; Tolstikov, V.; Bussberg, V.; Barbour, K.E.; et al. Diagnostic utility of serum and urinary metabolite analysis in patients with interstitial cystitis/painful bladder syndrome. Urology 2021, 157, 85–92. [Google Scholar] [CrossRef]
- Speich, J.E.; Tarcan, T.; Hashitani, H.; Vahabi, B.; McCloskey, K.D.; Andersson, K.E.; Wein, A.J.; Birder, L.A. Are oxidative stress and ischemia significant causes of bladder damage leading to lower urinary tract dysfunction? Report from the ICI-RS 2019. Neurourol. Urodyn. 2020, 39 (Suppl. 3), S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Nomiya, M.; Sagawa, K.; Yazaki, J.; Takahashi, N.; Kushida, N.; Haga, N.; Aikawa, K.; Matsui, T.; Oka, M.; Fukui, T.; et al. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol. Urodyn. 2012, 31, 185–189. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chueh, K.S.; Chuang, S.M.; Long, C.Y.; Lu, J.H.; Juan, Y.S. Bladder hyperactivity induced by oxidative stress and bladder ischemia: A review of treatment strategies with antioxidants. Int. J. Mol. Sci. 2021, 22, 6014. [Google Scholar] [CrossRef]
- Dokumacioglu, E.; Demiray, O.; Dokumacioglu, A.; Sahin, A.; Sen, T.M.; Cankaya, S. Measuring urinary 8-hydroxy-2′-deoxyguanosine and malondialdehyde levels in women with overactive bladder. Investig. Clin. Urol. 2018, 59, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Irwin, P.; Galloway, N.T. Impaired bladder perfusion in interstitial cystitis: A study of blood supply using laser Doppler flowmetry. J. Urol. 1993, 149, 890–892. [Google Scholar] [CrossRef]
- Pontari, M.A.; Hanno, P.M.; Ruggieri, M.R. Comparison of bladder blood flow in patients with and without interstitial cystitis. J. Urol. 1999, 162, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Lee, M.H. Increased expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor associated with glomerulation formation in patients with interstitial cystitis. Urology 2011, 78, 971.e11–971.e15. [Google Scholar] [CrossRef]
- Kiuchi, H.; Tsujimura, A.; Takao, T.; Yamamoto, K.; Nakayama, J.; Miyagawa, Y.; Nonomura, N.; Takeyama, M.; Okuyama, A. Increased vascular endothelial growth factor expression in patients with bladder pain syndrome/interstitial cystitis: Its association with pain severity and glomerulations. BJU Int. 2009, 104, 826–831; discussion 831. [Google Scholar] [CrossRef]
- Tsiapakidou, S.; Apostolidis, A.; Pantazis, K.; Grimbizis, G.F.; Mikos, T. The use of urinary biomarkers in the diagnosis of overactive bladder in female patients. A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 3143–3155. [Google Scholar] [CrossRef]
- Ochodnicky, P.; Cruz, C.D.; Yoshimura, N.; Cruz, F. Neurotrophins as regulators of urinary bladder function. Nat. Rev. Urol. 2012, 9, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, W.T.; Kim, W.J. Advances in urinary biomarker discovery in urological research. Investig. Clin. Urol. 2020, 61 (Suppl. 1), S8–S22. [Google Scholar] [CrossRef]
- Furuta, A.; Yamamoto, T.; Suzuki, Y.; Gotoh, M.; Egawa, S.; Yoshimura, N. Comparison of inflammatory urine markers in patients with interstitial cystitis and overactive bladder. Int. Urogynecol. J. 2018, 29, 961–966. [Google Scholar] [CrossRef]
- Shen, Y.C.; Tyagi, P.; Lee, W.C.; Chancellor, M.; Chuang, Y.C. Improves symptoms and urinary biomarkers in refractory interstitial cystitis/bladder pain syndrome patients randomized to extracorporeal shock wave therapy versus placebo. Sci. Rep. 2021, 11, 7558. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, N.; Katsura, R.; Hamada, K.; Suzutani, T. Blueberry prevents the bladder dysfunction in bladder outlet obstruction rats by attenuating oxidative stress and suppressing bladder remodeling. Nutrients 2020, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Siroky, M.; Yang, J.H.; Zhao, Z.; Azadzoi, K. Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology 2014, 84, 1249.e1–1249.e7. [Google Scholar] [CrossRef]
- Rada, M.P.; Ciortea, R.; Măluţan, A.M.; Doumouchtsis, S.K.; Bucuri, C.E.; Clim, A.; Roman, A.; Mihu, D. The profile of urinary biomarkers in overactive bladder. Neurourol. Urodyn. 2020, 39, 2305–2313. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.I.; Griendling, K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015, 116, 531–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Urine Cytokines | (A) DO N = 31 | (B) IC/BPS N = 114 | (C) DV N = 45 | (D) HSB N = 29 | (E) Normal N = 34 | p Value # | Post Hoc $ |
|---|---|---|---|---|---|---|---|
| Pdet (cmH2O) | 18.0 ± 11.0 | 22.3 ± 17.6 | 47.8 ± 42.7 | 11.5 ± 9.49 | 15.1 ± 7.12 | <0.001 | ABDE v C; B v DE |
| Qmax (mL/s) | 16.1 ± 7.35 | 10.3 ± 6.41 | 10.6 ± 6.78 | 11 ± 6.69 | 18.9 ± 8.44 | <0.001 | AE v BCD, |
| Volume (mL) | 272 ± 134 | 212 ± 117 | 229 ± 116 | 232 ± 126 | 416 ± 152 | <0.001 | ABCD v E; A v B |
| PVR (mL) | 14.7 ± 40.8 | 50.8 ± 103 | 56.4 ± 66 | 75 ± 105 | 17.1 ± 71.9 | 0.012 | A v BC |
| FSF (mL) | 109 ± 48.6 | 131 ± 59.9 | 125 ± 55.2 | 185 ± 207 | 170 ± 65.8 | 0.002 | ABC v DE, |
| FS (mL) | 172 ± 74.2 | 201 ± 86.1 | 200 ± 82.5 | 243 ± 58.6 | 293 ± 95.8 | <0.001 | ABC v DE, D v E |
| CBC (mL) | 286 ± 134.7 | 251.7 ± 138 | 279 ± 134 | 302 ± 96 | 407 ± 166 | <0.001 | ABCD v E |
| Compliance | 61.8 ± 42.9 | 70.3 ± 52.9 | 75.9 ± 83.8 | 98.4 ± 84.3 | 160 ± 101 | <0.001 | ABC v E |
| BCI | 98.1 ± 36.4 | 70.5 ± 36.3 | 97.6 ± 51.5 | 65.3 ± 37.2 | 98.7 ± 50.4 | <0.001 | BD v ACE |
| Urine Cytokines | (A) DO (N = 31) | (B) IC/BPS (N = 114) | (C) DV (N = 45) | (D) HSB (N = 29) | (E) Control (N = 34) | p-Value | Post Hoc $ |
|---|---|---|---|---|---|---|---|
| Age | 63.9 ± 8.96 | 54.6 ± 12.4 | 53.2 ± 4.2 | 63.0 ± 11.2 | 59.8 ± 11.1 | <0.001 | AD v BC |
| 8-isoprostane | 32.5 ± 29.8 | 39.1 ± 29.6 | 12.9 ± 14.7 | 22.8 ± 17.3 | 17.5 ± 15.5 | <0.001 | A v C, B v CDE |
| TAC | 1559 ± 1359 | 1658 ± 1190 | 604 ± 420 | 388 ± 279 | 1107 ± 1017 | <0.001 | AB v CD, D v E |
| 8-OHDG | 26.0 ± 17.7 | 33.2 ± 17.9 | 32.4 ± 19.4 | 18.4 ± 16.6 | 17.7 ± 13.6 | <0.001 | A v B, BC v DE |
| IL-1β | 0.61 ± 0.54 | 0.64 ± 0.49 | 1.16 ± 1.4 | 0.71 ± 0.63 | 0.56 ± 0.26 | 0.001 | ABDE v C |
| IL-2 | 0.74 ± 0.19 | 0.76 ± 0.18 | 0.28 ± 0.22 | 0.64 ± 0.14 | 0.79 ± 0.19 | <0.001 | ABDE v C, D v BE |
| IL-6 | 2.05 ± 2.62 | 1.72 ± 1.53 | 2.14 ± 5.16 | 1.53 ± 1.71 | 1.22 ± 1.29 | 0.582 | |
| IL-8 | 20.7 ± 34.4 | 14.2 ± 15.8 | 31.0 ± 63.9 | 48.3 ± 97.7 | 13.6 ± 22.8 | 0.060 | |
| TNF-α | 0.87 ± 0.4 | 0.78 ± 0.42 | 1.21 ± 0.33 | 0.92 ± 0.56 | 0.79 ± 0.31 | <0.001 | ABDE v C |
| VEGF | 14.6 ± 5.96 | 14.4 ± 6.81 | 5.56 ± 4.91 | 8.44 ± 7.84 | 11.2 ± 5.3 | <0.001 | AB v CD, C v E |
| NGF | 0.26 ± 0.07 | 0.37 ± 0.17 | 0.21 ± 0.05 | 0.22 ± 0.07 | 0.27 ± 0.07 | <0.001 | A v BC, B v CDE |
| BDNF | 0.6 ± 0.22 | 0.5 ± 0.17 | 0.63 ± 0.15 | 0.61 ± 0.29 | 0.57 ± 0.14 | 0.004 | B v C |
| PGE2 | 262 ± 175 | 239 ± 168 | 218 ± 187 | 283 ± 259 | 171 ± 107 | 0.077 |
| Urine Biomarkers | DO + IC + DV (N = 190) | HSB + Normal (N = 63) | p Value | Cut-Off Value | AUC |
|---|---|---|---|---|---|
| 8-isoprostane | 31.8 ± 28.8 | 20.0 ± 16.4 | <0.001 | ≥20.8 | 0.610 |
| TAC | 1396 ± 1175 | 776 ± 846 | <0.001 | ≥844.3 | 0.704 |
| 8-OHDG | 31.8 ± 18.3 | 18.0 ± 14.9 | <0.001 | ≥24.13 | 0.719 |
| IL-1β | 0.76 ± 0.84 | 0.62± 0.47 | 0.245 | ≥0.645 | 0.592 |
| IL-2 | 0.64 ± 0.28 | 0.72 ± 0.18 | 0.015 | ≤0.39 | 0.549 |
| IL-6 | 1.87 ± 2.94 | 1.36 ± 1.49 | 0.194 | ≥0.825 | 0.575 |
| IL-8 | 19.2 ± 36.5 | 29.5 ± 69.8 | 0.272 | ≥1.87 | 0.587 |
| TNF-α | 0.89 ± 0.43 | 0.85 ± 0.44 | 0.520 | ≥1.045 | 0.531 |
| VEGF | 12.4 ± 7.30 | 9.96 ± 6.66 | 0.022 | ≥11.24 | 0.599 |
| NGF | 0.31 ± 0.15 | 0.25 ± 0.08 | <0.001 | ≥0.315 | 0.642 |
| BDNF | 0.55 ± 0.18 | 0.59 ± 0.22 | 0.210 | ≤0.315 | 0.524 |
| PGE2 | 236 ± 182 | 171 ± 107 | 0.022 | ≥173.1 | 0.573 |
| Urine Biomarker | Cut-Off Value | DO (N = 31) | IC/BPS (N = 114) | DV (N = 45) | HSB (N = 29) | Normal (N = 34) |
|---|---|---|---|---|---|---|
| 8-isoprostane | ≥19.08 | 19 (61.3%) | 80 (70.2%) | 8 (17.8%) | 12 (41.4%) | 11 (32.4%) |
| TAC | ≥592.2 | 23 (74.2%) | 96 (84.2%) | 16 (35.6%) | 6 (20.7%) | 23 (67.6%) |
| 8-OHDG | ≥24.13 | 16 (51.6%) | 77 (67.5%) | 31 (68.9%) | 7 (24.1%) | 9 (26.5%) |
| IL-1β | ≥0.615 | 9 (29.0%) | 31 (27.2%) | 39 (86.7%) | 7 (24.1%) | 11 (32.4%) |
| IL-2 | ≤0.39 | 0 | 0 | 34 (75.6%) | 0 | 0 |
| TNF-α | ≥1.115 | 6 (19.4%) | 14 (12.3%) | 35 (77.8%) | 5 (17.2%) | 5 (14.7%) |
| VEGF | ≥9.08 | 27 (87.1%) | 87 (76.3%) | 6 (13.3%) | 11 (37.9%) | 20 (58.8%) |
| NGF | ≥0.315 | 9 (29.0%) | 63 (55.3%) | 4 (8.9%) | 3 (10.3%) | 7 (20.6%) |
| PGE2 | ≥173.1050 | 19 (61.3%) | 66 (57.9%) | 18 (40.0%) | 14 (48.3%) | 9 (26.5%) |
| Urine Biomarker | Bladder Disorders | Cut-Off Value | AUC | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 8-isoprostane | DO + IC | ≥19.08 | 0.751 | 67.8% | 72.6% |
| TAC | DV + HSB | ≤592.2 | 0.818 | 72.2% | 79.0% |
| 8-OHDG | DO + IC + DV | ≥24.13 | 0.719 | 65.3% | 74.6% |
| IL-1β | DV | ≥0.615 | 0.837 | 86.4% | 74.6% |
| IL-2 | DV | ≤0.39 | 0.925 | 75.6% | 100% |
| TNF-α | DV | ≥1.115 | 0.837 | 76.7% | 88.1% |
| VEGF | DO + IC | ≥9.08 | 0.768 | 78.5% | 67.0% |
| NGF | DO + IC + DV | ≥0.315 | 0.642 | 38.4% | 84.1% |
| PGE2 | DO + IC + DV | ≥173.1 | 0.573 | 53.2% | 64.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-H.; Jhang, J.-F.; Kuo, H.-C. Urinary Oxidative Stress Biomarker Levels Might Be Useful in Identifying Functional Bladder Disorders in Women with Frequency and Urgency Syndrome. J. Clin. Med. 2023, 12, 2336. https://doi.org/10.3390/jcm12062336
Jiang Y-H, Jhang J-F, Kuo H-C. Urinary Oxidative Stress Biomarker Levels Might Be Useful in Identifying Functional Bladder Disorders in Women with Frequency and Urgency Syndrome. Journal of Clinical Medicine. 2023; 12(6):2336. https://doi.org/10.3390/jcm12062336
Chicago/Turabian StyleJiang, Yuan-Hong, Jia-Fong Jhang, and Hann-Chorng Kuo. 2023. "Urinary Oxidative Stress Biomarker Levels Might Be Useful in Identifying Functional Bladder Disorders in Women with Frequency and Urgency Syndrome" Journal of Clinical Medicine 12, no. 6: 2336. https://doi.org/10.3390/jcm12062336
APA StyleJiang, Y. -H., Jhang, J. -F., & Kuo, H. -C. (2023). Urinary Oxidative Stress Biomarker Levels Might Be Useful in Identifying Functional Bladder Disorders in Women with Frequency and Urgency Syndrome. Journal of Clinical Medicine, 12(6), 2336. https://doi.org/10.3390/jcm12062336







