CD8+ Regulatory T Cell Deficiency in Elderly-Onset Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Selection of Patients
2.2. Clinical Evaluation
2.3. Measurement of Plasma Protein Levels
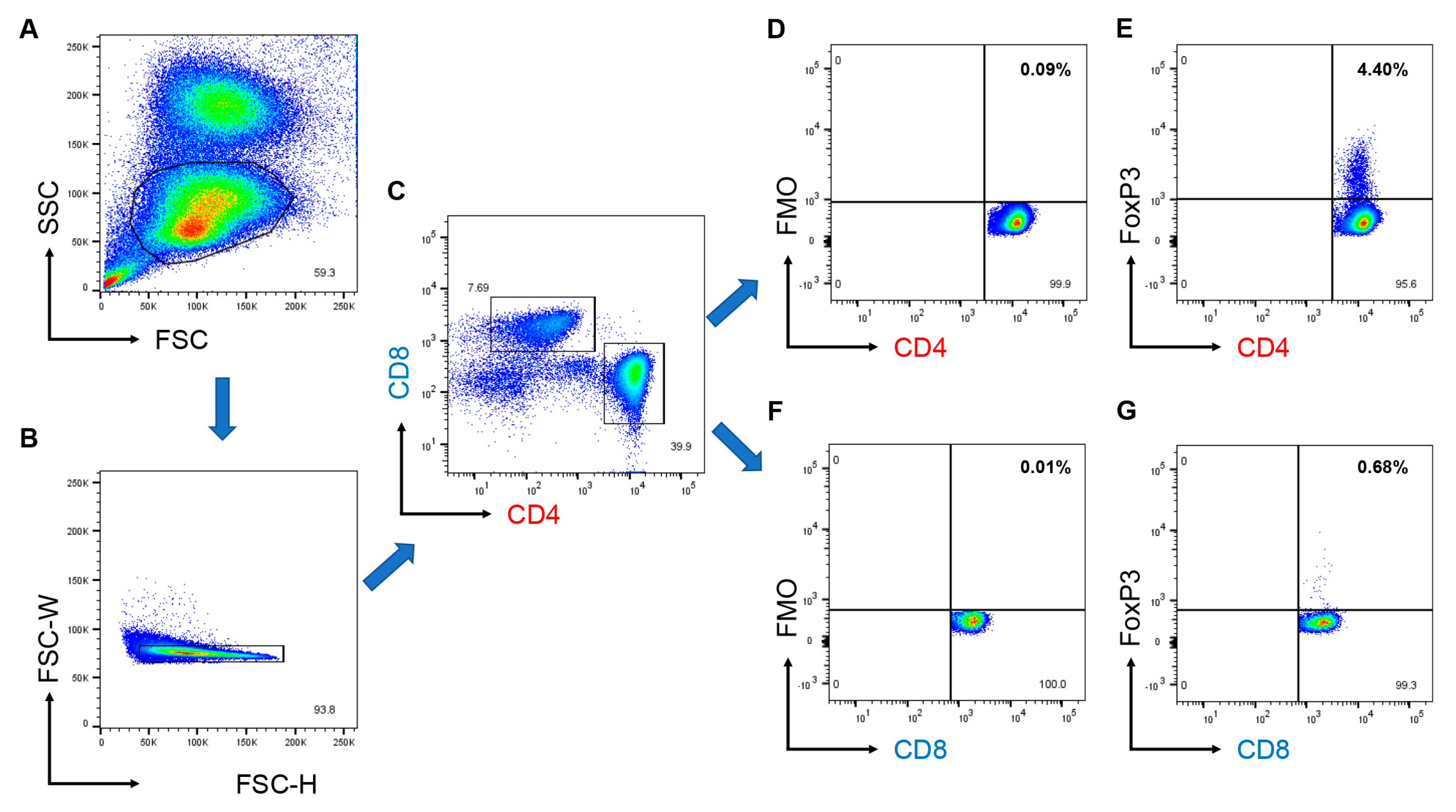
2.4. Flow Cytometry
2.5. Statistical Analysis
2.6. Ethics Approval and Consent to Participate
3. Results
3.1. Clinical Characteristics of Enrolled Patients
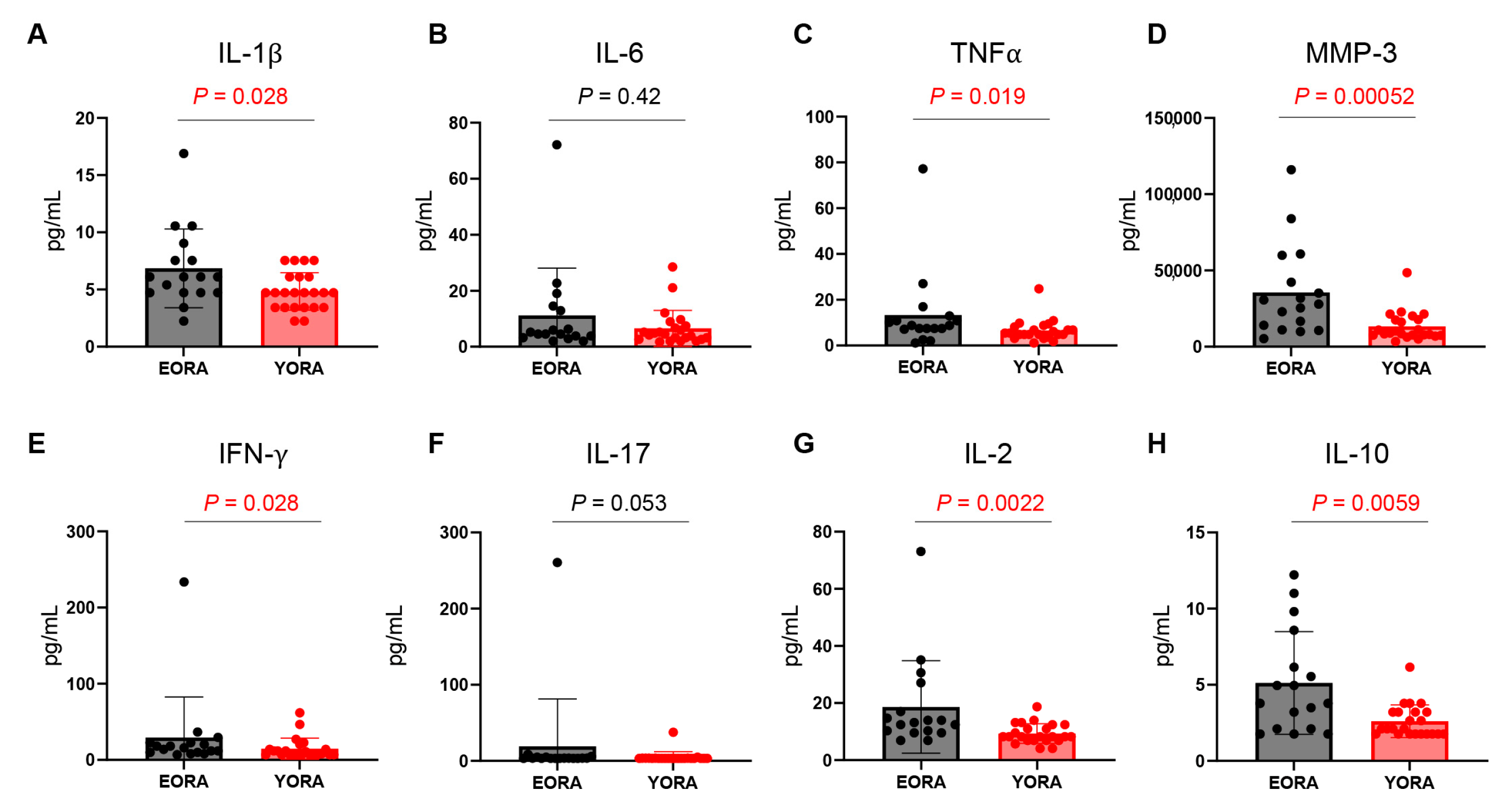
3.2. Inflammatory Milieu Persisted despite Treatment in EORA
3.3. CD8+ Tregs Are Deficient in EORA
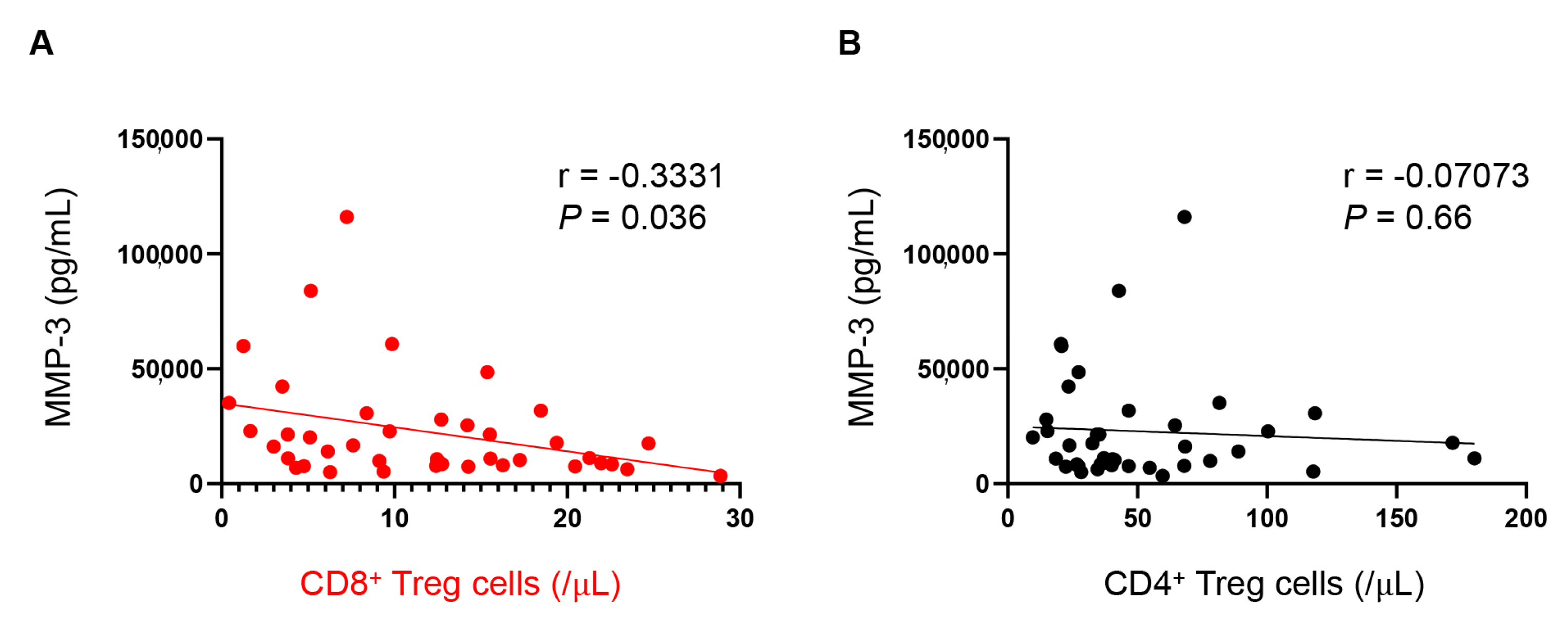
3.4. Number of CD8+ Tregs Are Associated with Plasma MMP-3 Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewe, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Serhal, L.; Lwin, M.N.; Holroyd, C.; Edwards, C.J. Rheumatoid arthritis in the elderly: Characteristics and treatment considerations. Autoimmun. Rev. 2020, 19, 102528. [Google Scholar] [CrossRef] [PubMed]
- Maassen, J.M.; Bergstra, S.A.; Chopra, A.; Govind, N.; Murphy, E.A.; Vega-Morales, D.; Huizinga, T.W.J.; Allaart, C.F. Phenotype and treatment of elderly onset compared with younger onset rheumatoid arthritis patients in international daily practice. Rheumatology 2021, 60, 4801–4810. [Google Scholar] [CrossRef]
- Sugihara, T. Treatment strategies for elderly-onset rheumatoid arthritis in the new era. Mod. Rheumatol. 2022, 32, 493–499. [Google Scholar] [CrossRef]
- Murata, K.; Ito, H.; Hashimoto, M.; Nishitani, K.; Murakami, K.; Tanaka, M.; Yamamoto, W.; Mimori, T.; Matsuda, S. Elderly onset of early rheumatoid arthritis is a risk factor for bone erosions, refractory to treatment: KURAMA cohort. Int. J. Rheum. Dis. 2019, 22, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Romao, V.C.; Humby, F.; Kelly, S.; Di Cicco, M.; Mahto, A.; Lazarou, I.; Hands, R.; Rocher-Ros, V.; van der Heijde, D.; Fonseca, J.E.; et al. Treatment-resistant synovitis and radiographic progression are increased in elderly-onset rheumatoid arthritis patients: Findings from a prospective observational longitudinal early arthritis cohort study. Semin. Arthritis Rheum. 2020, 50, 735–743. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [Green Version]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [Green Version]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef]
- Rapetti, L.; Chavele, K.M.; Evans, C.M.; Ehrenstein, M.R. B cell resistance to Fas-mediated apoptosis contributes to their ineffective control by regulatory T cells in rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, W.; Pan, W.; Zhang, Q.; Wang, G.; Feng, D.; Geng, X.; Yan, X.; Li, S. Tim3+ Foxp3 + Treg Cells Are Potent Inhibitors of Effector T Cells and Are Suppressed in Rheumatoid Arthritis. Inflammation 2017, 40, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.; Savitskaya, A.; von Dalwigk, K.; Steiner, C.W.; Aletaha, D.; Smolen, J.S.; Scheinecker, C. Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE). Int. Immunol. 2008, 20, 861–868. [Google Scholar] [CrossRef]
- Jacquemin, C.; Augusto, J.F.; Scherlinger, M.; Gensous, N.; Forcade, E.; Douchet, I.; Levionnois, E.; Richez, C.; Lazaro, E.; Duffau, P.; et al. OX40L/OX40 axis impairs follicular and natural Treg function in human SLE. JCI Insight 2018, 3, e122167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia, X.; Yarboro, C.; Illei, G.; Lipsky, P.E. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J. Immunol. 2007, 178, 2579–2588. [Google Scholar] [CrossRef] [Green Version]
- Adriawan, I.R.; Atschekzei, F.; Dittrich-Breiholz, O.; Garantziotis, P.; Hirsch, S.; Risser, L.M.; Kosanke, M.; Schmidt, R.E.; Witte, T.; Sogkas, G. Novel aspects of regulatory T cell dysfunction as a therapeutic target in giant cell arteritis. Ann. Rheum. Dis. 2022, 81, 124–131. [Google Scholar] [CrossRef]
- Miyabe, C.; Miyabe, Y.; Strle, K.; Kim, N.D.; Stone, J.H.; Luster, A.D.; Unizony, S. An expanded population of pathogenic regulatory T cells in giant cell arteritis is abrogated by IL-6 blockade therapy. Ann. Rheum. Dis. 2017, 76, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, R.; Hosgur, E.; Zhang, H.; Wen, Z.; Berry, G.; Goronzy, J.J.; Weyand, C.M. Pro-inflammatory and anti-inflammatory T cells in giant cell arteritis. Joint Bone Spine 2017, 84, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Samson, M.; Audia, S.; Fraszczak, J.; Trad, M.; Ornetti, P.; Lakomy, D.; Ciudad, M.; Leguy, V.; Berthier, S.; Vinit, J.; et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 2012, 64, 3788–3798. [Google Scholar] [CrossRef]
- Niederlova, V.; Tsyklauri, O.; Chadimova, T.; Stepanek, O. CD8+ Tregs revisited: A heterogeneous population with different phenotypes and properties. Eur. J. Immunol. 2021, 51, 512–530. [Google Scholar] [CrossRef]
- Li, J.; Zaslavsky, M.; Su, Y.; Guo, J.; Sikora, M.J.; van Unen, V.; Christophersen, A.; Chiou, S.H.; Chen, L.; Li, J.; et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 2022, 376, eabi9591. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, F.; Wang, Y.; Zeng, Q.; Liu, C.; Chen, Y.; Liang, C.L.; Zhang, Q.; Han, L.; Dai, Z. CD8+CD122+PD-1+ Tregs Synergize With Costimulatory Blockade of CD40/CD154, but Not B7/CD28, to Prolong Murine Allograft Survival. Front. Immunol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Menager-Marcq, I.; Pomie, C.; Romagnoli, P.; van Meerwijk, J.P. CD8+CD28− regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology 2006, 131, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Shimojima, Y.; Shirai, T.; Li, Y.; Ju, J.; Yang, Z.; Tian, L.; Goronzy, J.J.; Weyand, C.M. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J. Clin. Investig. 2016, 126, 1953–1967. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Watanabe, R.; Hashimoto, M.; Murata, K.; Murakami, K.; Tanaka, M.; Ohmura, K.; Ito, H.; Matsuda, S. Prevalence and predictive factors of difficult-to-treat rheumatoid arthritis: The KURAMA cohort. Immunol. Med. 2022, 45, 35–44. [Google Scholar] [CrossRef]
- Watanabe, R.; Murakami, K.; Fujisaki, T.; Ito, H.; Murata, K.; Yamamoto, W.; Fujii, T.; Onizawa, H.; Onishi, A.; Tanaka, M.; et al. Baseline erythrocyte sedimentation rate level predicts long-term inhibition of radiographic progression by tocilizumab: The KURAMA cohort. Immunol. Med. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.; Breedveld, F.C.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; de Wit, M.; Dougados, M.; et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2010, 69, 631–637. [Google Scholar] [CrossRef]
- Watanabe, R.; Shirai, T.; Namkoong, H.; Zhang, H.; Berry, G.J.; Wallis, B.B.; Schaefgen, B.; Harrison, D.G.; Tremmel, J.A.; Giacomini, J.C.; et al. Pyruvate controls the checkpoint inhibitor PD-L1 and suppresses T cell immunity. J. Clin. Investig. 2017, 127, 2725–2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Vaglio, A.; Liao, Y.J.; Warrington, K.J.; Goronzy, J.J.; Weyand, C.M. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc. Natl. Acad. Sci. USA 2017, 114, E970–E979. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Kolios, A.G.A.; Tsokos, G.C.; Klatzmann, D. Interleukin-2 and regulatory T cells in rheumatic diseases. Nat. Rev. Rheumatol. 2021, 17, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.R.; Dambuza, I.M.; Lee, Y.J.; Frank, G.M.; Egwuagu, C.E. STAT3 regulates proliferation and survival of CD8+ T cells: Enhances effector responses to HSV-1 infection, and inhibits IL-10+ regulatory CD8+ T cells in autoimmune uveitis. Mediat. Inflamm. 2013, 2013, 359674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assenmacher, M.; Schmitz, J.; Radbruch, A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: Expression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur. J. Immunol. 1994, 24, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bieber, K.; Manz, R.A. IL-10 revisited in systemic lupus erythematosus. Front. Immunol. 2022, 13, 970906. [Google Scholar] [CrossRef]
- Iwasaki, T.; Watanabe, R.; Ito, H.; Fujii, T.; Okuma, K.; Oku, T.; Hirayama, Y.; Ohmura, K.; Murata, K.; Murakami, K.; et al. Dynamics of Type I and Type II Interferon Signature Determines Responsiveness to Anti-TNF Therapy in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 901437. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Suzuki, M.; Jagger, A.L.; Konya, C.; Shimojima, Y.; Pryshchep, S.; Goronzy, J.J.; Weyand, C.M. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: A novel subset of inducible human regulatory T cells. J. Immunol. 2012, 189, 2118–2130. [Google Scholar] [CrossRef] [Green Version]
- Tulunay, A.; Yavuz, S.; Direskeneli, H.; Eksioglu-Demiralp, E. CD8+CD28−, suppressive T cells in systemic lupus erythematosus. Lupus 2008, 17, 630–637. [Google Scholar] [CrossRef]
- Pellegrino, M.; Crino, A.; Rosado, M.M.; Fierabracci, A. Identification and functional characterization of CD8+ T regulatory cells in type 1 diabetes patients. PLoS ONE 2019, 14, e0210839. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, R.; Berry, G.J.; Liang, D.H.; Goronzy, J.J.; Weyand, C.M. Cellular Signaling Pathways in Medium and Large Vessel Vasculitis. Front. Immunol. 2020, 11, 587089. [Google Scholar] [CrossRef]
- He, J.; Zhang, R.; Shao, M.; Zhao, X.; Miao, M.; Chen, J.; Liu, J.; Zhang, X.; Zhang, X.; Jin, Y.; et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: A randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2020, 79, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Wittekind, P.S.; Kotschenreuther, K.; Schiller, J.; von Tresckow, J.; Haak, T.H.; Kofler, D.M. Regulatory T cell frequencies in patients with rheumatoid arthritis are increased by conventional and biological DMARDs but not by JAK inhibitors. Ann. Rheum. Dis. 2021, 80, e196. [Google Scholar] [CrossRef] [Green Version]
- Samson, M.; Greigert, H.; Ciudad, M.; Gerard, C.; Ghesquiere, T.; Trad, M.; Corbera-Bellalta, M.; Genet, C.; Ouandji, S.; Cladiere, C.; et al. Improvement of Treg immune response after treatment with tocilizumab in giant cell arteritis. Clin. Transl. Immunol. 2021, 10, e1332. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hashimoto, M. Vasculitogenic T Cells in Large Vessel Vasculitis. Front. Immunol. 2022, 13, 923582. [Google Scholar] [CrossRef] [PubMed]
- Ceeraz, S.; Thompson, C.R.; Beatson, R.; Choy, E.H. Harnessing CD8+CD28− Regulatory T Cells as a Tool to Treat Autoimmune Disease. Cells 2021, 10, 2973. [Google Scholar] [CrossRef]
- Mishra, S.; Srinivasan, S.; Ma, C.; Zhang, N. CD8+ Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021, 12, 708874. [Google Scholar] [CrossRef] [PubMed]

| EORA | YORA | p-Value | ||
|---|---|---|---|---|
| N | 17 | 23 | ||
| Current age (y) | 75 [71, 79] | 61 [51, 65] | <0.001 | |
| Female (n, %) | 10 (58.8%) | 21 (91.3%) | 0.023 | |
| Disease duration (mo) | 106 [80, 220] | 93 [38, 197] | 0.23 | |
| RF-positive (n, %) | 8 (47.1%) | 21 (91.3%) | 0.003 | |
| RF titers | 9.3 [8.0, 32.6] | 54.7 [26.1, 112.6] | 0.003 | |
| ACPA-positive (n, %) | 5 (29.4%) | 21 (91.3%) | <0.001 | |
| ACPA titers | 0.6 [0.5, 12.9] | 91.6 [21.1, 164.5] | 0.002 | |
| CRP (mg/dL) | 0.10 [0.10, 0.20] | 0.10 [0.10, 0.10] | 0.33 | |
| ESR (mm/h) | 17.0 [11.0, 43.0] | 14.0 [7.0, 29.5] | 0.36 | |
| DAS28-CRP | 1.46 [1.28, 2.23] | 1.52 [1.36, 2.37] | 0.40 | |
| DAS28-ESR | 2.72 [1.98, 3.10] | 2.58 [1.94, 3.63] | 0.61 | |
| SDAI | 1.90 [0.90, 5.10] | 1.90 [1.15, 6.50] | 0.38 | |
| CDAI | 1.30 [0.70, 3.60] | 1.80 [1.05, 6.35] | 0.20 | |
| MTX use (n, %) | 11 (64.7%) | 18 (78.3%) | 0.48 | |
| PSL use (n, %) | 7 (41.2%) | 6 (26.1%) | 0.50 | |
| MTX dose (mg/week) | 6.0 [0, 10.0] | 4.0 [0, 8.0] | 0.28 | |
| PSL dose (mg/day) | 0 [0, 1.0] | 0 [0, 4.25] | 0.19 | |
| Biologics use (n, %) | 9 (52.9%) | 15 (65.2%) | 0.52 | |
| IFX | 1 (5.9%) | 2 (8.7%) | 1 | |
| ADA | 0 (0.0%) | 2 (8.7%) | 0.50 | |
| ETN | 0 (0.0%) | 1 (4.3%) | 1 | |
| GLM | 4 (23.5%) | 1 (4.3%) | 0.14 | |
| TCZ | 1 (5.9%) | 5 (21.7%) | 0.22 | |
| SAR | 0 (0.0%) | 1 (4.3%) | 1 | |
| ABT | 3 (17.6%) | 3 (13.0%) | 1 | |
| First Biologics (n, %) | 4 (23.5%) | 10 (43.5%) | 0.32 | |
| Pearson r | 95% CI | p Value | |
|---|---|---|---|
| Age | −0.2847 | −0.5476 to 0.02942 | 0.075 |
| DAS28-ESR | −0.08311 | −0.3847 to 0.2345 | 0.61 |
| DAS28−CRP | −0.0261 | −0.3349 to 0.2877 | 0.87 |
| SDAI | 0.01433 | −0.2985 to 0.3244 | 0.93 |
| CDAI | 0.02271 | −0.2909 to 0.3319 | 0.89 |
| IL-1β | −0.2481 | −0.5195 to 0.06872 | 0.12 |
| IL-6 | −0.1732 | −0.4599 to 0.1462 | 0.29 |
| TNFα | −0.01798 | −0.3277 to 0.2952 | 0.91 |
| MMP-3 | −0.3331 | −0.5840 to −0.02404 | 0.036 |
| IFN-γ | −0.1332 | −0.4270 to 0.1861 | 0.41 |
| IL-17 | −0.04573 | −0.3522 to 0.2696 | 0.78 |
| IL-2 | −0.2363 | −0.5103 to 0.08118 | 0.14 |
| IL-10 | −0.2569 | −0.5263 to 0.05937 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, R.; Kadoba, K.; Tamamoto, A.; Murata, K.; Murakami, K.; Onizawa, H.; Fujii, T.; Onishi, A.; Tanaka, M.; Ito, H.; et al. CD8+ Regulatory T Cell Deficiency in Elderly-Onset Rheumatoid Arthritis. J. Clin. Med. 2023, 12, 2342. https://doi.org/10.3390/jcm12062342
Watanabe R, Kadoba K, Tamamoto A, Murata K, Murakami K, Onizawa H, Fujii T, Onishi A, Tanaka M, Ito H, et al. CD8+ Regulatory T Cell Deficiency in Elderly-Onset Rheumatoid Arthritis. Journal of Clinical Medicine. 2023; 12(6):2342. https://doi.org/10.3390/jcm12062342
Chicago/Turabian StyleWatanabe, Ryu, Keiichiro Kadoba, Atsuko Tamamoto, Koichi Murata, Kosaku Murakami, Hideo Onizawa, Takayuki Fujii, Akira Onishi, Masao Tanaka, Hiromu Ito, and et al. 2023. "CD8+ Regulatory T Cell Deficiency in Elderly-Onset Rheumatoid Arthritis" Journal of Clinical Medicine 12, no. 6: 2342. https://doi.org/10.3390/jcm12062342
APA StyleWatanabe, R., Kadoba, K., Tamamoto, A., Murata, K., Murakami, K., Onizawa, H., Fujii, T., Onishi, A., Tanaka, M., Ito, H., Morinobu, A., & Hashimoto, M. (2023). CD8+ Regulatory T Cell Deficiency in Elderly-Onset Rheumatoid Arthritis. Journal of Clinical Medicine, 12(6), 2342. https://doi.org/10.3390/jcm12062342








