Rate and Predictors of Unforeseen PN1/PN2-Disease in Surgically Treated cN0 NSCLC-Patients with Primary Tumor > 3 cm: Nationwide Results from Italian VATS-Group Database
Abstract
1. Introduction
2. Materials and Methods
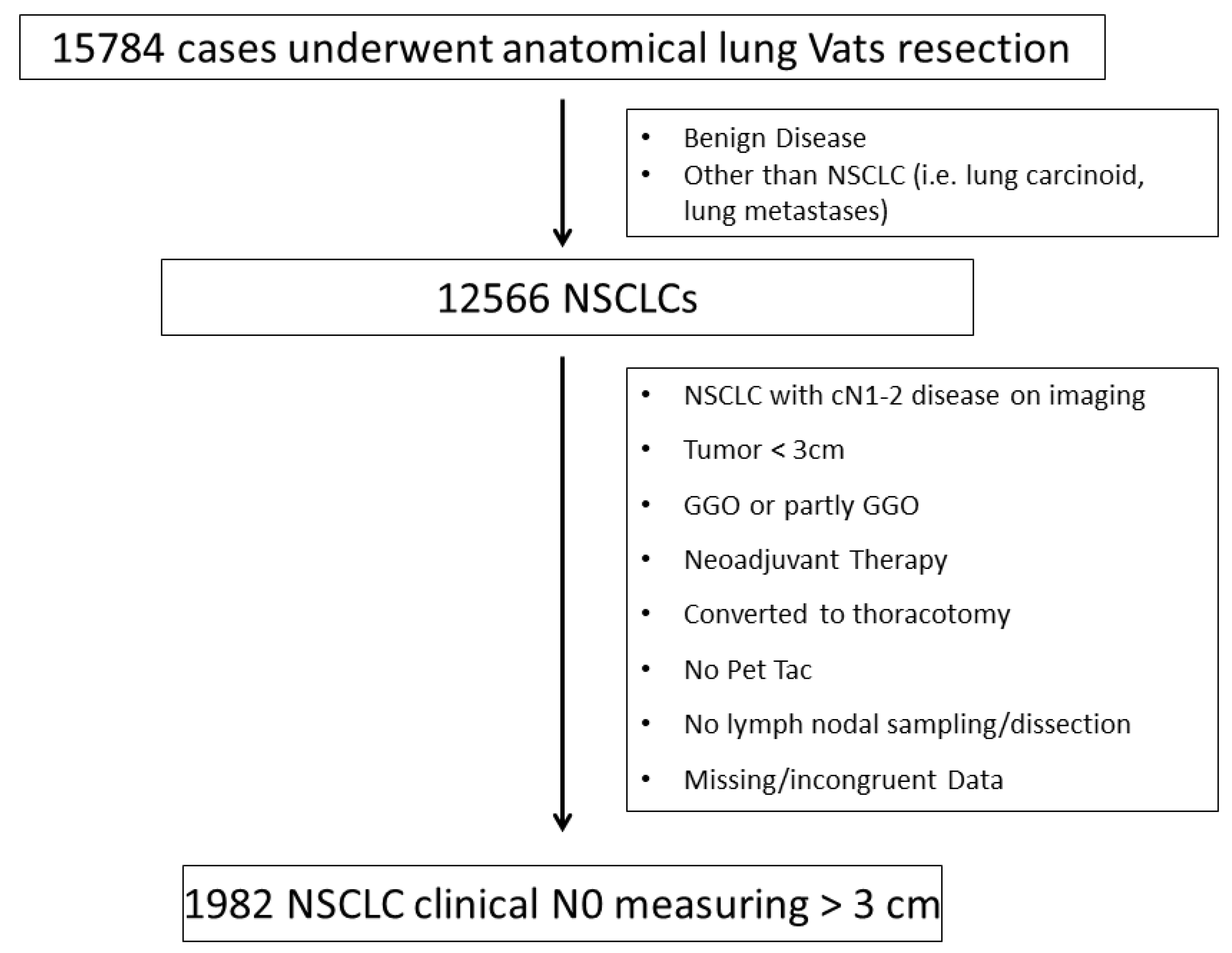
2.1. Patients
2.2. Statistical Methods
3. Results
3.1. Clinico-Pathological Characteristics in N0, uN1 and uN2 Disease
3.2. Predictive Factors for uN1 and uN2
3.3. Survival Results According to N-Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Licht, P.B.; Jørgensen, O.D.; Ladegaard, L.; Jakobsen, E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann. Thorac. Surg. 2013, 96, 943–949; discussion 949–950. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Özyurtkan, M.O.; Kaba, E. Nodal upstaging: Effects of instrumentation and three-dimensional view in clinical stage I lung cancer. J. Vis. Surg. 2017, 3, 76. [Google Scholar] [CrossRef]
- De Leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Zielinski, M. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in nonsmall cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Garelli, E.; Renaud, S.; Falcoz, P.E.; Weingertner, N.; Olland, A.; Santelmo, N.; Massard, G. Microscopic N2 disease exhibits a better prognosis in resected non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2016, 50, 322–328. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e211S–e250S. [Google Scholar] [CrossRef]
- Rami-Porta, R.; Call, S.; Dooms, C.; Obiols, C.; Sánchez, M.; Travis, W.D.; Vollmer, I. Lung cancer staging: A concise update. Eur. Respir. J. 2018, 51, 1800190. [Google Scholar] [CrossRef]
- Bertani, A.; Gonfiotti, A.; Nosotti, M.; Ferrari, P.A.; De Monte, L.; Russo, E.; Guerrera, F. Nodal management and upstaging of disease: Initial results from the Italian VATS Lobectomy Registry. J. Thorac. Dis. 2017, 9, 2061–2070. [Google Scholar] [CrossRef]
- Rocha, A.T.; McCormack, M.; Montana, G.; Schreiber, G. Association between lower lobe location and upstaging for early-stage non-small cell lung cancer. Chest 2004, 125, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Kil Park, J.; Lee, K.Y.; Namkoong, M.; Ahn, S. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J. Surg. 2018, 42, 2872–2878. [Google Scholar] [CrossRef]
- Marulli, G.; Verderi, E.; Comacchio, G.M.; Monaci, N.; Natale, G.; Nicotra, S.; Rea, F. Predictors of unexpected nodal upstaging in patients with cT1-3N0 non-small cell lung cancer (NSCLC) submitted to thoracoscopic lobectomy. J. Vis. Surg. 2018, 4, 15. [Google Scholar] [CrossRef]
- Marulli, G.; Faccioli, E.; Mammana, M.; Nicotra, S.; Comacchio, G.; Verderi, E.; De Palma, A.; Rea, F.; Italian VATS Group. Predictors of nodal upstaging in patients with cT1-3N0 non-small cell lung cancer (NSCLC): Results from the Italian VATS Group Registry. Surg. Today 2020, 50, 711–718, Erratum in Surg. Today 2020, 50, 719–720. [Google Scholar] [CrossRef]
- Lee, P.C.; Port, J.L.; Korst, R.J.; Liss, Y.; Meherally, D.N.; Altorki, N.K. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann. Thorac. Surg. 2007, 84, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Nachira, D.; Swierzy, M.; Ferretti, G.M.; Englisch, J.P.; Saidy, R.R.O.; Li, F.; Badakhshi, H.; Rueckert, J.C. Lymph node upstaging for non-small cell lung cancer after uniportal video-assisted thoracoscopy. J. Thorac. Dis. 2018, 10 (Suppl. 31), S3648–S3654. [Google Scholar] [CrossRef]
- Nachira, D.; Meacci, E.; Congedo, M.T.; Chiappetta, M.; Petracca Ciavarella, L.; Vita, M.L.; Margaritora, S. Upstaging, centrality and survival in early stage non-small cell lung cancer video-assisted surgery: Lymph nodal upstaging in lung cancer surgery: Is it really a surgical technique problem? Lung Cancer 2020, 144, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Kapiris, M.; Podvez Nevajda, A.; McGrath, H.; Stavraka, C.; Ahmad, S.; Taylor, B.; Cook, G.J.R.; Ghosh, S.; Josephs, D.; et al. Non-Small Cell Lung Cancer (NSCLC) in Young Adults, Age < 50, Is Associated with Late Stage at Presentation and a Very Poor Prognosis in Patients That Do Not Have a Targeted Therapy Option: A Real-World Study. Cancers 2022, 14, 6056. [Google Scholar] [CrossRef] [PubMed]
- Cerfolio, R.J.; Bryant, A.S. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann. Thorac. Surg. 2008, 86, 362–366; discussion 366–367. [Google Scholar] [CrossRef]
- Boada, M.; Sánchez-Lorente, D.; Libreros, A.; Lucena, C.M.; Marrades, R.; Sánchez, M.; Paredes, P.; Serrano, M.; Guirao, A.; Guzmán, R.; et al. Is invasive mediastinal staging necessary in intermediate risk patients with negative PET/CT? J. Thorac. Dis. 2020, 12, 3976–3986. [Google Scholar] [CrossRef]
- Chiappetta, M.; Leuzzi, G.; Sperduti, I.; Bria, E.; Mucilli, F.; Lococo, F.; Filosso, P.L.; Ratto, G.B.; Spaggiari, L.; Facciolo, F. Mediastinal Up-Staging During Surgery in Non-Small-Cell Lung Cancer: Which Mediastinal Lymph Node Metastasis Patterns Better Predict the Outcome? A Multicenter Analysis. Clin. Lung Cancer 2020, 21, 464–471.e1. [Google Scholar] [CrossRef]
- Obiols, C.; Call, S.; Rami-Porta, R.; Trujillo-Reyes, J.C.; Saumench, R.; Iglesias, M.; Serra-Mitjans, M.; Gonzalez-Pont, G.; Belda-Sanchís, J. Survival of patients with unsuspected pN2 non-small cell lung cancer after an accurate preoperative mediastinal staging. Ann. Thorac. Surg. 2014, 97, 957–964. [Google Scholar] [CrossRef] [PubMed]


| Variables | |
|---|---|
| Gender | |
| M | 1315 (66.3%) |
| F | 667 (33.6%) |
| Age of Diagnosis | |
| Mean ± SD | 69.7 ± 8.9 |
| <70 years | 952 (48.0%) |
| ≥70 years | 1030 (51.9%) |
| Side | |
| Left | 828 (41.7%) |
| Right | 1154 (58.2%) |
| Tumour Location | |
| Upper | 785 (39.6%) |
| Middle | 77 (3.8%) |
| Lower | 1120 (56.5%) |
| Primary tumour PET SUVmax | |
| Mean ± SD | 8.7 ± 6.4 |
| SUVmax < 2.5 | 267 (13.4%) |
| SUVmax ≥ 2.5 | 1563 (78.8%) |
| Histology | |
| Adenocarcinoma | 1418 (71.5%) |
| Squamous cell carcinoma | 456 (23.0%) |
| Others | 108 (5.4%) |
| Tumour-size | |
| 3–5 cm | 1526 (76.9%) |
| 5–7 cm | 373 (18.8%) |
| >7 cm | 83 (4.1%) |
| Surgery | |
| (Bi)Lobar Resection | 1949 (98.3%) |
| Sublobar Resection | 33 (1.6 %) |
| Type of surgical approach | |
| Triportal | 1546 (78.0%) |
| Biportal | 236 (11.9%) |
| Uniportal | 200 (10.1%) |
| Lymph Node Assessment | |
| Radical Dissection | 1341 (67.6%) |
| Systematic Sampling or Sampling | 641 (32.3%) |
| Number of LFN dissected (Mean ± SD) | 13.7 ± 8.3 |
| All | 1982 |
| Variables | pN0 (n, %) | uN1 (n, %) | uN2 (n, %) |
|---|---|---|---|
| Population | 1584 (79.9%) | 229 (11.6%) | 169 (8.5%) |
| Gender | |||
| M | 1064 (80.9%) | 145 (11.0%) | 106 (8.1%) |
| F | 520 (78.0%) | 84 (12.6%) | 63 (9.4%) |
| Age of Diagnosis | |||
| Mean ± SD | 70.3 ± 5.4 | 69 ± 8.5 | 68 ± 11.0 |
| <70 years | 744 (78.2%) | 119 (12.5%) | 89 (9.3%) |
| ≥70 years | 840 (81.5%) | 110 (10.7%) | 80 (7.8%) |
| Side | |||
| Left | 642 (77.6%) | 109 (13.2%) | 77 (9.3%) |
| Right | 942 (81.6%) | 120 (10.4%) | 92 (8.0%) |
| Tumour Location | |||
| Upper | 610 (77.7%) | 103 (13.1%) | 72 (9.2%) |
| Middle | 63 (81.8%) | 7 (9.1%) | 7 (9.1%) |
| Lower | 1001 (89.4%) | 119 (10.6%) | 90 (8.0%) |
| Primary tumour PET SUVmax | |||
| Mean ± SD | 7.8 ± 5.9 | 10.1 ± 6.1 | 8.8 ± 5.5 |
| SUVmax < 2.5 | 235 (88.0% | 19 (7.1%) | 13 (4.9%) |
| SUVmax ≥ 2.5 | 1226 (78.5%) | 191 (12.2%) | 146 (9.3%) |
| Histology | |||
| Adenocarcinoma | 1109 (78.2%) | 170 (11.9%) | 139 (9.8%) |
| Squamous cell carcinoma | 386 (84.7%) | 49 (10.7%) | 21 (4.6%) |
| Others | 89 (82.4%) | 10 (9.3%) | 9 (8.3%) |
| Tumour-size | |||
| 3–5 cm | 1238 (81.1%) | 157 (10.2%) | 131 (8.7%) |
| 5–7 cm | 284 (76.1%) | 58 (15.5%) | 31 (8.3%) |
| >7 cm | 62 (74.7%) | 14 (16.9%) | 7 (8.4%) |
| Surgery | |||
| (Bi)Lobar Resection | 1553 (79.7%) | 228 (11.7%) | 168 (8.6%) |
| Sublobar Resection | 31 (94.0%) | 1 (3.0%) | 1 (3.0%) |
| Lymph Node Assessment | |||
| Radical Dissection | 1061 (79.1%) | 163 (12.2%) | 117 (8.7%) |
| Sampling | 523 (81.6%) | 66 (10.3%) | 52 (8.1%) |
| Number of nodes dissected (Mean ± SD) | 12.4 ± 6.6 | 15.7 ± 9.8 | 15.7 ± 8.6 |
| N1 Upstaging | ||
|---|---|---|
| Variable | OR (CI95%) | p Value |
| SUVmax ≥ 6 | 1.98(1.44–2.73) | <0.0001 |
| Tumour-size ≥ 5 cm | 1.52 (1.11–2.10) | 0.01 |
| N2 Upstaging | ||
| Variable | OR (CI95%) | pValue |
| Age | 0.98 (0.96–0.99) | 0.039 |
| Histology (Ref:SCC) | 0.48 (0.30–0.78) | 0.003 |
| SUVmax ≥ 6 | 1.807 (1.27–2.58) | 0.001 |
| Resected-LN ≥ 6 | 2.37 (1.26–4.45) | 0.007 |
| Study | Patients | Inclusion Criteria | pN1/N2-Upstaging (%) | Upstaging Risk Factors | Survival pN0 | Survival u-pN1 | Survival u-pN2 |
|---|---|---|---|---|---|---|---|
| Rocha, 2004 [10] (prospective; thoracotomy) | 109 | c-stage: I/II (cN0, cN1) NSCLC | upN1:5.5% upN2: 8.3% | -lower lobe location (p < 0.006) | NA | NA | NA |
| Lee, 2007 [14] (retrospective; thoracotomy) | 224 | c-stage: I NSCLC | upN1: 9.8% upN2:6.5% (T1)-8.7% (T2) | -central tumours (p < 0.001) -larger cT size (p < 0.001) -adeno-carcinoma histology (p: 0.082) -higher tumour PET-SUVmax (p: 0.017) | NA | NA | NA |
| Licht, 2013 [1] (retrospective on a National registry; Thoracotomy vs. VATS) | 1513 | c-stage: I NSCLC | upN1: 13.1% vs. 8.1% (p < 0.001) upN2: 11.5 vs. 3.8% (p < 0.001) | -cT stage (p = 0.01) -invasive mediastinal staging (p < 0.001) -number of nodal stations dissected (p = 0.02) -surgical approach (p < 0.001) -lower lobe (p = 0.045). | HR: 1 | HR:1.84 | HR: 2.79 (p < 0.001) |
| Marulli, 2018 [12] (retrospective; VATS) | 231 | cT1-T3N0, I-IIB NSCLC | upN1: 9.1% upN2: 7.4% | -T size (p: 0.027) -adenocarcinoma histology (p: 0.0382) | NA | NA | NA |
| Ismail, 2018 [15] (retrospective; VATS) | 136 | c-stage: I-IIB | upN1: 7.4% upN2: 5.2% | -positive nodes in stations 2–4 (0.009) and 5–6 (0.027) | NA | NA | NA |
| Moon, 2018 [11] (retrospective; Thoracotomy) | 486 | Peripheral cT1N0 | upN1: 4.7% upN2: 3.9% | -tumour diameter (p: 0.039) -consolidation/tumour ratio (p = 0.001) | NA | NA | NA |
| Marulli, 2019 [13] (retrospective on a National registry; VATS) | 3276 | cT1-T3N0, I-IIB NSCLC | upN1: 6.2% upN2: 2.4% | -adenocarcinoma histology (p < 0.001) -higher tumour grade (p < 0.001) -higher pathologicT status (p < 0.001) -tumour size > 3 cm (p < 0.001) -upper lobe tumours (p = 0.049) ->12 nodes resected (p < 0.001) | NA | NA | NA |
| Present series 2021 (retrospective on a National registry; VATS) | 1982 | cN0 peripheral solid-type NSCLC > 3 cm | upN1: 11.6% upN2: 8.5% | uN1: -SUVmax (OR: 1.98; CI95: 1.44–2.73, p = 0.0001), -tumour-size (OR: 1.52; CI: 1.11–2.10, p = 0.01); uN2: -age, p = 0.039), -histology (p = 0.003), -SUVmax (p = 0.015), -number of resected nodes (p = 0.002). | 5 y: 73% | 5 y: 35% | 5 y: 31% (p < 0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lococo, F.; Nachira, D.; Chiappetta, M.; Sperduti, I.; Congedo, M.T.; Meacci, E.; Leoncini, F.; Trisolini, R.; Crisci, R.; Curcio, C.; et al. Rate and Predictors of Unforeseen PN1/PN2-Disease in Surgically Treated cN0 NSCLC-Patients with Primary Tumor > 3 cm: Nationwide Results from Italian VATS-Group Database. J. Clin. Med. 2023, 12, 2345. https://doi.org/10.3390/jcm12062345
Lococo F, Nachira D, Chiappetta M, Sperduti I, Congedo MT, Meacci E, Leoncini F, Trisolini R, Crisci R, Curcio C, et al. Rate and Predictors of Unforeseen PN1/PN2-Disease in Surgically Treated cN0 NSCLC-Patients with Primary Tumor > 3 cm: Nationwide Results from Italian VATS-Group Database. Journal of Clinical Medicine. 2023; 12(6):2345. https://doi.org/10.3390/jcm12062345
Chicago/Turabian StyleLococo, Filippo, Dania Nachira, Marco Chiappetta, Isabella Sperduti, Maria Teresa Congedo, Elisa Meacci, Fausto Leoncini, Rocco Trisolini, Roberto Crisci, Carlo Curcio, and et al. 2023. "Rate and Predictors of Unforeseen PN1/PN2-Disease in Surgically Treated cN0 NSCLC-Patients with Primary Tumor > 3 cm: Nationwide Results from Italian VATS-Group Database" Journal of Clinical Medicine 12, no. 6: 2345. https://doi.org/10.3390/jcm12062345
APA StyleLococo, F., Nachira, D., Chiappetta, M., Sperduti, I., Congedo, M. T., Meacci, E., Leoncini, F., Trisolini, R., Crisci, R., Curcio, C., Casiraghi, M., Margaritora, S., & on the behalf of the Italian VATS Group. (2023). Rate and Predictors of Unforeseen PN1/PN2-Disease in Surgically Treated cN0 NSCLC-Patients with Primary Tumor > 3 cm: Nationwide Results from Italian VATS-Group Database. Journal of Clinical Medicine, 12(6), 2345. https://doi.org/10.3390/jcm12062345







