Relationship between Short- and Mid-Term Glucose Variability and Blood Pressure Profile Parameters: A Scoping Review
Abstract
1. Background
2. Methods
2.1. Objective and Research Question
2.2. Inclusion Criteria
2.3. Exclusion Criteria
- Long-term variability was defined by visit-to-visit office-determined glucose, HbA1c, and BP measurements;
- Use of SMBG for GV assessment or intra-arterial BP recordings for BPV. New technologies offer a wide range of new assessment tools and provide a broader picture of glucose concentrations throughout the day compared to the traditional methods (SMBG). In addition, intra-arterial BP recordings allow for the assessment of very-short-term BPV but their usefulness is mainly restricted to a given research field.
2.4. Search Strategy
3. Extraction of the Results
- Identify the main author and publication year;
- Report the aims of the study;
- Define the study population with respect to the following parameters:
- ▪
- Number of participants;
- ▪
- Participants’ main characteristics (age, sex, and body mass index);
- ▪
- Percentage of participants with cardiovascular risk factors (diabetes, hypertension, dyslipidemia, smoking);
- ▪
- Percentage of participants treated for cardiovascular risk factors;
- ▪
- Level of glycemic and BP control;
- ▪
- Duration of diabetes and hypertension.
- Determine the type of CGM device used:
- ▪
- CGM;
- ▪
- FGM.
- Determine the BP assessment method (office, ambulatory, or home monitoring);
- Determine the GV and BPV indices that were calculated;
- Ascertain the key findings related to the research question.
4. Presentation of the Results
5. Discussion
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
- (((“blood pressure”[Title/Abstract]) OR (bp[Title/Abstract])) OR (“Arterial Pressure”[Mesh])) OR (hypertension[Title/Abstract])
- ((“glucose alteration*”[Title/Abstract]) OR (“glycemic alteration*”[Title/Abstract])) OR (“glycaemic alteration*”[Title/Abstract])
- ((((“glucose fluctuation*”[Title/Abstract]) OR (“glycemic fluctuation*”[Title/Abstract])) OR (“glycaemic fluctuation*”[Title/Abstract])) OR (“blood sugar fluctuation*”[Title/Abstract])) OR (“blood-sugar fluctuation*”[Title/Abstract])
- (((((“glucose variability”[Title/Abstract]) OR (‘“gv”[Title/Abstract])) OR (“glycemic variability”[Title/Abstract])) OR (“glycaemic variability”[Title/Abstract])) OR (“blood sugar variability”[Title/Abstract])) OR (“blood-sugar variability”[Title/Abstract])
- (((cgm[Title/Abstract]) OR (“continuous glucose monitor*”[Title/Abstract])) OR (fgm[Title/Abstract])) OR (“flash glucose monitor*”[Title/Abstract])
- (((((((cgm[Title/Abstract]) OR (“continuous glucose monitoring”[Title/Abstract])) OR (fgm[Title/Abstract])) OR (“flash glucose monitoring”[Title/Abstract])) OR ((((((“glucose variability”[Title/Abstract]) OR (‘“gv”[Title/Abstract])) OR (“glycemic variability”[Title/Abstract])) OR (“glycaemic variability”[Title/Abstract])) OR (“blood sugar variability”[Title/Abstract])) OR (“blood-sugar variability”[Title/Abstract]))) OR (((((“glucose fluctuation*”[Title/Abstract]) OR (“glycemic fluctuation*”[Title/Abstract])) OR (“glycaemic fluctuation*”[Title/Abstract])) OR (“blood sugar fluctuation*”[Title/Abstract])) OR (“blood-sugar fluctuation*”[Title/Abstract]))) OR (((“glucose alteration*”[Title/Abstract]) OR (“glycemic alteration*”[Title/Abstract])) OR (“glycaemic alteration*”[Title/Abstract]))) AND ((((“blood pressure”[Title/Abstract]) OR (bp[Title/Abstract])) OR (“Arterial Pressure”[Mesh])) OR (hypertension[Title/Abstract]))
References
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Scheer, F.A.J.L. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-Y.; Guo, Q.-H.; An, D.-W.; Li, Y.; Wang, J.-G. A comparative meta-analysis of prospective observational studies on masked hypertension and masked uncontrolled hypertension defined by ambulatory and home blood pressure. J. Hypertens. 2019, 37, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Hoshide, S.; Mizuno, H.; Kabutoya, T.; Nishizawa, M.; Yoshida, T.; Abe, H.; Katsuya, T.; Fujita, Y.; Okazaki, O.; et al. Nighttime Blood Pressure Phenotype and Cardiovascular Prognosis. Circulation 2020, 142, 1810–1820. [Google Scholar] [CrossRef]
- Stevens, S.L.; Wood, S.; Koshiaris, C.; Law, K.; Glasziou, P.; Stevens, R.J.; McManus, R.J. Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ 2016, 354, i4098. [Google Scholar] [CrossRef]
- Chiriacò, M.; Pateras, K.; Virdis, A.; Charakida, M.; Kyriakopoulou, D.; Nannipieri, M.; Emdin, M.; Tsioufis, K.; Taddei, S.; Masi, S.; et al. Association between blood pressure variability, cardiovascular disease and mortality in type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2019, 21, 2587–2598. [Google Scholar] [CrossRef]
- Alfieri, V.; Myasoedova, V.A.; Vinci, M.C.; Rondinelli, M.; Songia, P.; Massaiu, I.; Cosentino, N.; Moschetta, D.; Valerio, V.; Ciccarelli, M.; et al. The Role of Glycemic Variability in Cardiovascular Disorders. Int. J. Mol. Sci. 2021, 22, 8393. [Google Scholar] [CrossRef]
- Yapanis, M.; James, S.; Craig, M.E.; O’Neal, D.; Ekinci, E.I. Complications of Diabetes and Metrics of Glycemic Management Derived From Continuous Glucose Monitoring. J. Clin. Endocrinol. Metab. 2022, 107, e2221–e2236. [Google Scholar] [CrossRef]
- Lurbe, E.; Redon, J.; Kesani, A.; Pascual, J.M.; Tacons, J.; Alvarez, V.; Batlle, D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N. Engl. J. Med. 2002, 347, 797–805. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S4–S7. [Google Scholar] [CrossRef]
- Battelino, T.; Alexander, C.M.; Amiel, S.A.; Arreaza-Rubin, G.; Beck, R.W.; Bergenstal, R.M.; Buckingham, B.A.; Carroll, J.; Ceriello, A.; Chow, E.; et al. Continuous glucose monitoring and metrics for clinical trials: An international consensus statement. Lancet Diabetes Endocrinol. 2023, 11, 42–57. [Google Scholar] [CrossRef]
- Parati, G.; Bilo, G.; Kollias, A.; Pengo, M.; Ochoa, J.E.; Castiglioni, P.; Stergiou, G.S.; Mancia, G.; Asayama, K.; Asmar, R.; et al. Blood pressure variability: Methodological aspects, clinical relevance and practical indications for management. J. Hypertens. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Beck, R.W.; Riddlesworth, T.; Ruedy, K.; Ahmann, A.; Bergenstal, R.; Haller, S.; Kollman, C.; Kruger, D.; McGill, J.B.; Polonsky, W.; et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults with Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 2017, 317, 371–378. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Konishi, K.; Tsunoda, T.; Koyama, H. Significance of Glycemic Variability in Diabetes Mellitus. Intern. Med. 2022, 61, 281–290. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Huang, S.; Zhu, C.; Bian, M. Glycemic variability: Adverse clinical outcomes and how to improve it? Cardiovasc. Diabetol. 2020, 19, 102. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Parati, G.; Vlachopoulos, C.; Achimastos, A.; Andreadis, E.; Asmar, R.; Avolio, A.; Benetos, A.; Bilo, G.; Boubouchairopoulou, N.; et al. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: Current status and future directions—Position statement of the European Society of Hypertension Working Group on blood pressure monitoring. J. Hypertens. 2016, 34, 1665–1677. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Kollias, A.; Ntineri, A. Assessment of drug effects on blood pressure variability: Which method and which index? J. Hypertens. 2014, 32, 1197–1200. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.M.; Mcinerney, P.; Soares, C.B. The Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI Scoping Reviews; The Joanna Briggs Institute, The University of Adelaide: Adelaide, Australia, 2015; pp. 1–24.
- Van de Schoot, R.; de Bruin, J.; Schram, R.; Zahedi, P.; de Boer, J.; Weijdema, F.; Kramer, B.; Huijts, M.; Hoogerwerf, M.; Ferdinands, G.; et al. ASReview: Active Learning for Systematic Reviews (v1.0rc3). 2022. Available online: https://zenodo.org/record/6591802#.ZBVi2fZBzV9 (accessed on 14 March 2023).
- Golicka, D.; Lipka, M.; Szypowska, A.; Groele, L.; Biazik, M.; Golicki, D.; Pańkowska, E. Influence of daily glycemic fluctuations and poor glycemic control on arterial pressure in children and adolescents with type-1 diabetes. In Proceedings of the 68th Annual Meeting of the American-Diabetes-Association, San Francicso, CA, USA, 6–10 June 2008. [Google Scholar]
- Gordin, D.; Rönnback, M.; Forsblom, C.; Mäkinen, V.; Saraheimo, M.; Groop, P.H. Glucose variability, blood pressure and arterial stiffness in type 1 diabetes. Diabetes Res. Clin. Pract. 2008, 80, e4–e7. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, W.-P.; Ma, X.-J.; Bao, Y.-Q.; Lu, W.; Li, M.; Li, Q.; Hu, C.; Xiang, K.-S. Relationship between blood glucose variability and microalbuminuria in type 2 diabetic patients with well-controlled glycosylated hemoglobin. Natl. Med. J. China 2008, 88, 2977–2981. [Google Scholar]
- Borg, R.; Kuenen, J.C.; Carstensen, B.; Zheng, H.; Nathan, D.M.; Heine, R.J.; Nerup, J.; Borch-Johnsen, K.; Witte, D.R. HbA1c and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: The A1C-Derived Average Glucose (ADAG) study. Diabetologia 2011, 54, 69–72. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, H.; Iuchi, H.; Ohhashi, K.; Hayashi, T.; Nishimura, R.; Tojo, K.; Utsunomiya, K. Relationship between Glycemic Variability and Blood Pressure Variability in Diabetic Patients with Hypertension: Jikei Variability of ABPM and CGM Study. Diabetes 2013, 62, A219. [Google Scholar]
- Rosales, L.; Zuniga, L.; Hernandez, A.; Hernandez, D.; Cardona, E.; Ramos, C.; Ramos, J.; Gonzalez, M.; Martinez, E. Correlation between the Glycemic Variability and the Circadian Blood Pressure Variability in Individuals with Normal Weight and Glucose Tolerance. In Proceedings of the 76th Scientific Sessions of the American-Diabetes-Association, New Orleans, LA, USA, 10–14 June 2016. [Google Scholar]
- Rezki, A.; Chiheb, S.; Merioud, B.; Fysekidis, M.; Cosson, E.; Valensi, P. In Patients with Impaired Glucose Tolerance or Type 2 Diabetes, Greater Glycemic Variability Is Associated with Lower Cutaneous Blood Flow, Microcirculatory Endothelium Dysfunction, and Higher Blood Pressure. In Proceedings of the 77th Scientific Sessions of the American-Diabetes-Association, San Diego, CA, USA, 9–13 June 2017. [Google Scholar]
- Jaiswal, M.; Ang, L.; Mizokami-Stout, K.; Pop-Busui, R. Is there an association between non-dipping blood pressure and measures of glucose variability in type 1 diabetes? J. Diabetes Its Complicat. 2018, 32, 947–950. [Google Scholar] [CrossRef] [PubMed]
- De Backer, T.; Deruyter, S.; Deschuytere, L.; Shadid, S.; T’Sjoen, G.; Lapauw, B. Association of Measures of Short- and Long-Term Glycaemic Variability and Glycaemic Control with Ambulatory Blood Pressure Pattern in Type 1 Diabetes Mellitus. In Proceedings of the 28th European Meeting of Hypertension and Cardiovascular Protection of the European Society of Hypertension (ESH), Barcelona, Spain, 8–11 June 2018. [Google Scholar]
- Karnebeek, K.; Rijks, J.M.; Dorenbos, E.; Gerver, W.-J.M.; Plat, J.; Vreugdenhil, A.C.E. Changes in free-living glycemic profiles after 12 months of lifestyle intervention in children with overweight and with obesity. Nutrients 2020, 12, 1228. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Uzui, H.; Sato, Y.; Miyoshi, M.; Shiomi, Y.; Hasegawa, K.; Ikeda, H.; Tama, N.; Fukuoka, Y.; Morishita, T.; et al. Association between changes in the systolic blood pressure from evening to the next morning and night glucose variability in heart disease patients. Intern. Med. 2021, 60, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Homhuan, W.; Poomthavorn, P.; Paksi, W.; Khlairit, P.; Nongnuch, A.; Pirojsakul, K. Masked hypertension and its associations with glycemic variability metrics in children and adolescents with type 1 diabetes. Pediatr. Nephrol. 2021, 36, 379–386. [Google Scholar] [CrossRef]
- Sezer, H.; Yazici, D.; Copur, S.; Dagel, T.; Deyneli, O.; Kanbay, M. The relationship between glycemic variability and blood pressure variability in normoglycemic normotensive individuals. Blood Press. Monit. 2021, 26, 102–107. [Google Scholar] [CrossRef]
- Medical Advisory Secretariat. Continuous Glucose Monitoring for Patients with Diabetes: An Evidence-Based Analysis. Ont. Health Technol. Assess. Ser. 2011, 11, 1–29. [Google Scholar]
- Kovatchev, B. Glycemic Variability: Risk Factors, Assessment, and Control. J. Diabetes Sci. Technol. 2019, 13, 627–635. [Google Scholar] [CrossRef]
- Rama Chandran, S.; Tay, W.L.; Lye, W.K.; Lim, L.L.; Ratnasingam, J.; Tan, A.T.B.; Gardner, D.S. Beyond HbA1c: Comparing Glycemic Variability and Glycemic Indices in Predicting Hypoglycemia in Type 1 and Type 2 Diabetes. Diabetes Technol. Ther. 2018, 20, 353–362. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, M.K.; Rhee, E.-J. Effects of Cardiovascular Risk Factor Variability on Health Outcomes. Endocrinol. Metab. 2020, 35, 217–226. [Google Scholar] [CrossRef]
- Matsutani, D.; Sakamoto, M.; Iuchi, H.; Minato, S.; Suzuki, H.; Kayama, Y.; Takeda, N.; Horiuchi, R.; Utsunomiya, K. Glycemic variability in continuous glucose monitoring is inversely associated with baroreflex sensitivity in type 2 diabetes: A preliminary report. Cardiovasc. Diabetol. 2018, 17, 36. [Google Scholar] [CrossRef]
- Ohara, M.; Kohata, Y.; Nagaike, H.; Koshibu, M.; Gima, H.; Hiromura, M.; Yamamoto, T.; Mori, Y.; Hayashi, T.; Fukui, T.; et al. Association of glucose and blood pressure variability on oxidative stress in patients with type 2 diabetes mellitus and hypertension: A cross-sectional study. Diabetol. Metab. Syndr. 2019, 11, 29. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Okumura, K.; Cheng, X.W. Blood Pressure Variability and Vascular Dysfunction in Essential Hypertension. J. Korean Soc. Hypertens. 2012, 18, 75–87. [Google Scholar] [CrossRef]
- Stergiou, G.S.; Mukkamala, R.; Avolio, A.; Kyriakoulis, K.G.; Mieke, S.; Murray, A.; Parati, G.; Schutte, A.E.; Sharman, J.E.; Asmar, R.; et al. Cuffless blood pressure measuring devices: Review and statement by the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. J. Hypertens. 2022, 40, 1449–1460. [Google Scholar] [CrossRef]
- Vakali, E. Diabetes: Time to Use the Technology We Already Have. Curr. Diabetes Rev. 2023, 19. [Google Scholar] [CrossRef]
- Lehmann, V.; Föll, S.; Maritsch, M.; van Weenen, E.; Kraus, M.; Lagger, S.; Odermatt, K.; Albrecht, C.; Fleisch, E.; Zueger, T.; et al. Noninvasive Hypoglycemia Detection in People with Diabetes Using Smartwatch Data. Diabetes Care 2023. Epub Ahead of Print. [Google Scholar] [CrossRef]
| Index | Definition |
|---|---|
| Indices of both glucose and blood pressure variability | |
| Standard Deviation (SD) | Dispersion of the raw values (square root of variance) |
| Coefficient of variation (CV) | Extent of dispersion in relation to mean value (SD/mean value) × 100 |
| Average real variability (ARV) | Average of absolute differences between consecutive values |
| Indices of blood pressure variability | |
| Nighttime dipping | Percentage of decrease in nighttime blood pressure |
| Indices of glucose variability | |
| Mean amplitude of glycemic excursions (MAGE) | Mean differences from peaks to nadirs |
| Continuous overlapping net glycemic action (CONGA) | Difference between a current blood glucose reading and a reading taken hours earlier |
| Mean of daily differences (MODD) | Absolute differences between two glucose values measured at the same time with a 24 h interval |
| Time in range (TIR) | Percentage of time per day within target glucose range (70–180 mg/dL) |
| Characteristics | Number (n = 13) | Percentage (%) |
|---|---|---|
| Publication year | ||
| 2008 | 3 | 23 |
| 2011–2013 | 2 | 15 |
| 2016–2018 | 3 | 23 |
| 2020–2021 | 5 | 39 |
| Publication type | ||
| Journal article | 7 | 54 |
| Abstract publication | 6 | 46 |
| Population size | ||
| <30 | 3 | 23 |
| 31–70 | 8 | 60 |
| >200 | 2 | 15 |
| GV assessment methodology | ||
| CGM | 12 | 92 |
| FGM | 1 | 8 |
| BP assessment methodology | ||
| Ambulatory BP monitoring | 7 | 54 |
| Office BP measurements | 5 | 38 |
| Self-monitoring | 1 | 8 |
| Diabetes status * | ||
| T1D | 6 | 46 |
| T2D | 5 | 39 |
| NGT | 4 | 30 |
| IGT/IFG | 2 | 15 |
| Hypertension status * | ||
| HTN | 2 | 15 |
| Masked HTN | 1 | 8 |
| Normal BP | 10 | 67 |
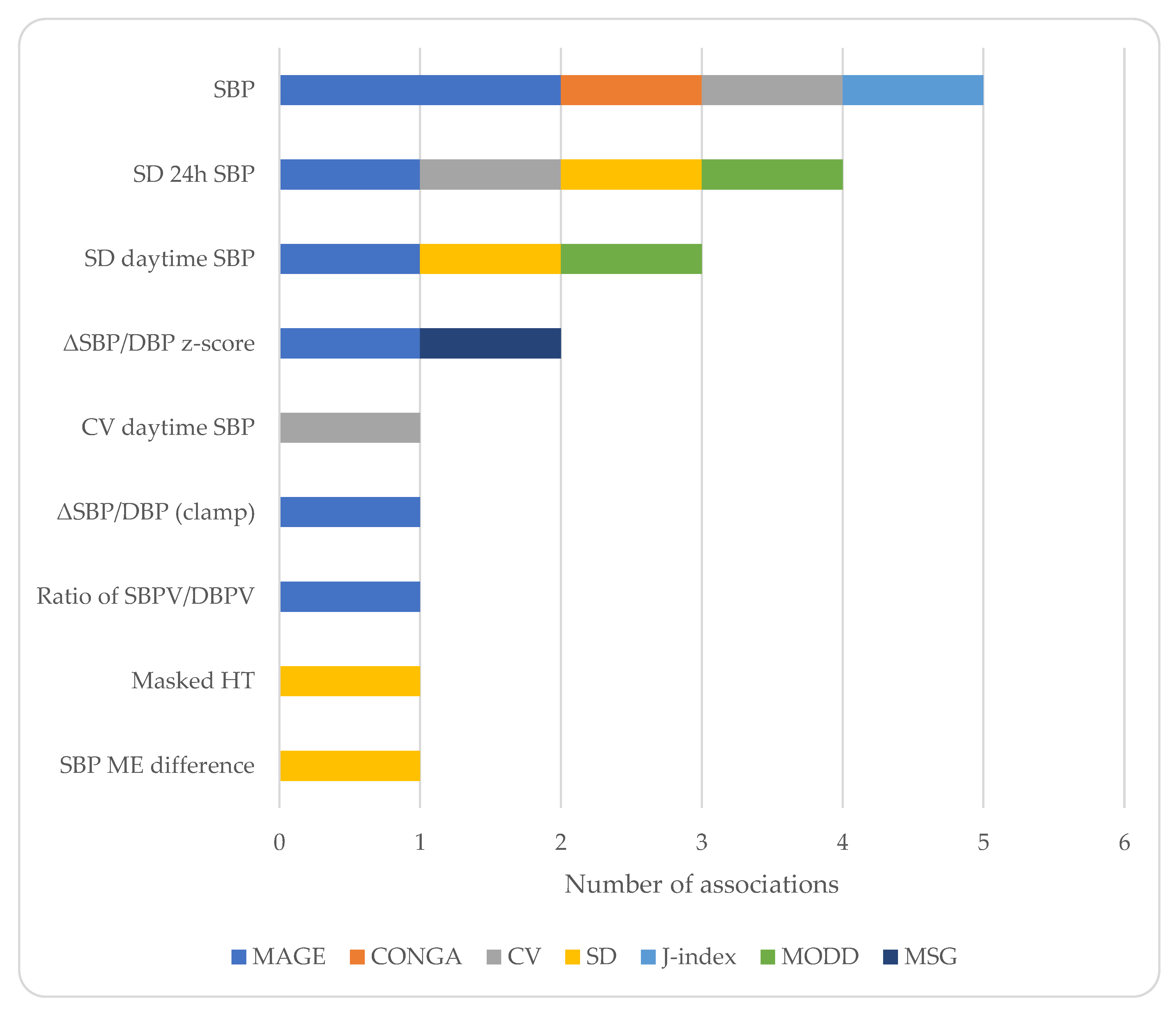
| Study | Population | Age ± SD (Years) | Males (%) | Methodology for GV | BP Assessment | Main Findings |
|---|---|---|---|---|---|---|
| Golicka 2008 [20] | n = 44 T1D normotensives | Range: 11–18 | NR | CGM | 24 h ABPM | MAGE associated with nocturnal SBP |
| Gordin 2008 [21] | n = 22 T1D | 25.9 ± 5.6 | 100 | 72 h CGM | Office BP and aortic BP (applanation tonometry) during a 2 h hyperglycemic clamp | MAGE associated with aortic BP difference between acute hyperglycemia and baseline values at 0′ |
| Zhou 2008 [22] | n = 176 T2D n = 48 NGT | NR | NR | CGM | Office BP | MAGE correlated with SBP |
| Borg 2011 [23] | n = 268 T1D n= 159 T2D | 48.7 ± 13.2 | 48 | 48 h CGM performed at baseline and at 4-week intervals | Self-monitoring of BP | Glucose variability indices (SD, CONGA, MAGE) not associated with SBP/DBP |
| Sakamoto 2013 [24] | n = 64 DM hypertensives | 53 ± 12 | NR | 48 h CGM | 48 h ABPM | CV of glucose associated with 24 h SBP and CV of awake SBP |
| Rosales 2016 [25] | n = 11 NGT | Range: 30–40 | NR | 72 h CGM | 24 h ABPM | MAGE inversely associated with indices of BP variability |
| Rezki 2017 [26] | n = 19 IGT normotensives n = 15 T2D normotensives | NR | NR | CGM 3 h after breakfast | Office aortic BP (applanation tonometry) 1 h after breakfast | CONGA and J-index associated with peripheral and aortic SBP |
| Jaiswal 2018 [27] | n = 41 T1D normotensives | 34 ± 13 | 39 | 5-day CGM | 24 h ABPM | CV of glucose and MAGE not associated with BP dipping |
| De Backer 2018 [28] | n = 68 T1D | NR | NR | 7-day CGM | 24 h ABPM | Mean but not SD of glucose associated with nocturnal DBP |
| Karnebeek 2020 [29] | n = 33 overweight (32 NGT, 1 IGT) | 12.5 ± 3.2 | 39 | 48 h CGM | BP measured every 3 min for 1.5 h | Lifestyle-induced changes in CONGA and CV not correlated with changes in SBP/DBP z-scores |
| Shimizu 2021 [30] | n = 40 hospitalized with CVD (47.5% DM, 67.5% hypertension) | 70.0 ± 11.0 | 78 | Up to 14 days FGM | BP measured twice daily | SD of nighttime glucose correlated with morning minus evening SBP in DM patients |
| Homhuan 2021 [31] | n = 28 T1D normal office BP (27% masked HT) | 13.8 ± 3.8 | 33 | 7-day CGM | 24 h ABPM | Higher SD of glucose in masked HT vs. normotensionCV of glucose > 36% predicted masked HT |
| Sezer 2021 [32] | n = 27 NGT normotensives | 23.8 ± 2.7 | 33 | 48 h CGM | 24 h ABPM | SD of 24 h ambulatory SBP correlated with MAGE, MODD, SD of glucose. SD of daytime ambulatory SBP correlated with MAGE and MODD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakali, E.; Rigopoulos, D.; Dinas, P.C.; Drosatos, I.-A.; Theodosiadi, A.G.; Vazeou, A.; Stergiou, G.; Kollias, A. Relationship between Short- and Mid-Term Glucose Variability and Blood Pressure Profile Parameters: A Scoping Review. J. Clin. Med. 2023, 12, 2362. https://doi.org/10.3390/jcm12062362
Vakali E, Rigopoulos D, Dinas PC, Drosatos I-A, Theodosiadi AG, Vazeou A, Stergiou G, Kollias A. Relationship between Short- and Mid-Term Glucose Variability and Blood Pressure Profile Parameters: A Scoping Review. Journal of Clinical Medicine. 2023; 12(6):2362. https://doi.org/10.3390/jcm12062362
Chicago/Turabian StyleVakali, Elena, Dimitrios Rigopoulos, Petros C. Dinas, Ioannis-Alexandros Drosatos, Aikaterini G. Theodosiadi, Andriani Vazeou, George Stergiou, and Anastasios Kollias. 2023. "Relationship between Short- and Mid-Term Glucose Variability and Blood Pressure Profile Parameters: A Scoping Review" Journal of Clinical Medicine 12, no. 6: 2362. https://doi.org/10.3390/jcm12062362
APA StyleVakali, E., Rigopoulos, D., Dinas, P. C., Drosatos, I.-A., Theodosiadi, A. G., Vazeou, A., Stergiou, G., & Kollias, A. (2023). Relationship between Short- and Mid-Term Glucose Variability and Blood Pressure Profile Parameters: A Scoping Review. Journal of Clinical Medicine, 12(6), 2362. https://doi.org/10.3390/jcm12062362








