Approaching Artificial Intelligence in Orthopaedics: Predictive Analytics and Machine Learning to Prognosticate Arthroscopic Rotator Cuff Surgical Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
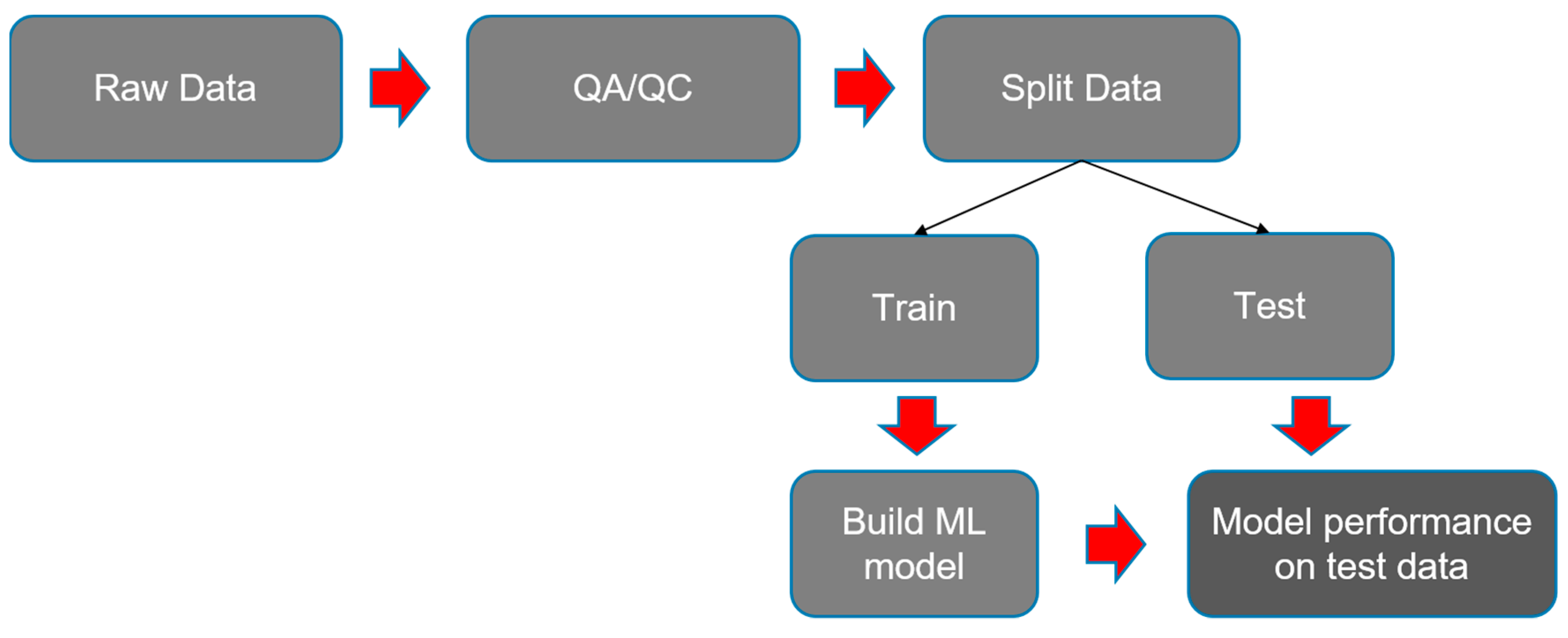
2.2. Data Preparation and Model Building
2.3. Data Analysis
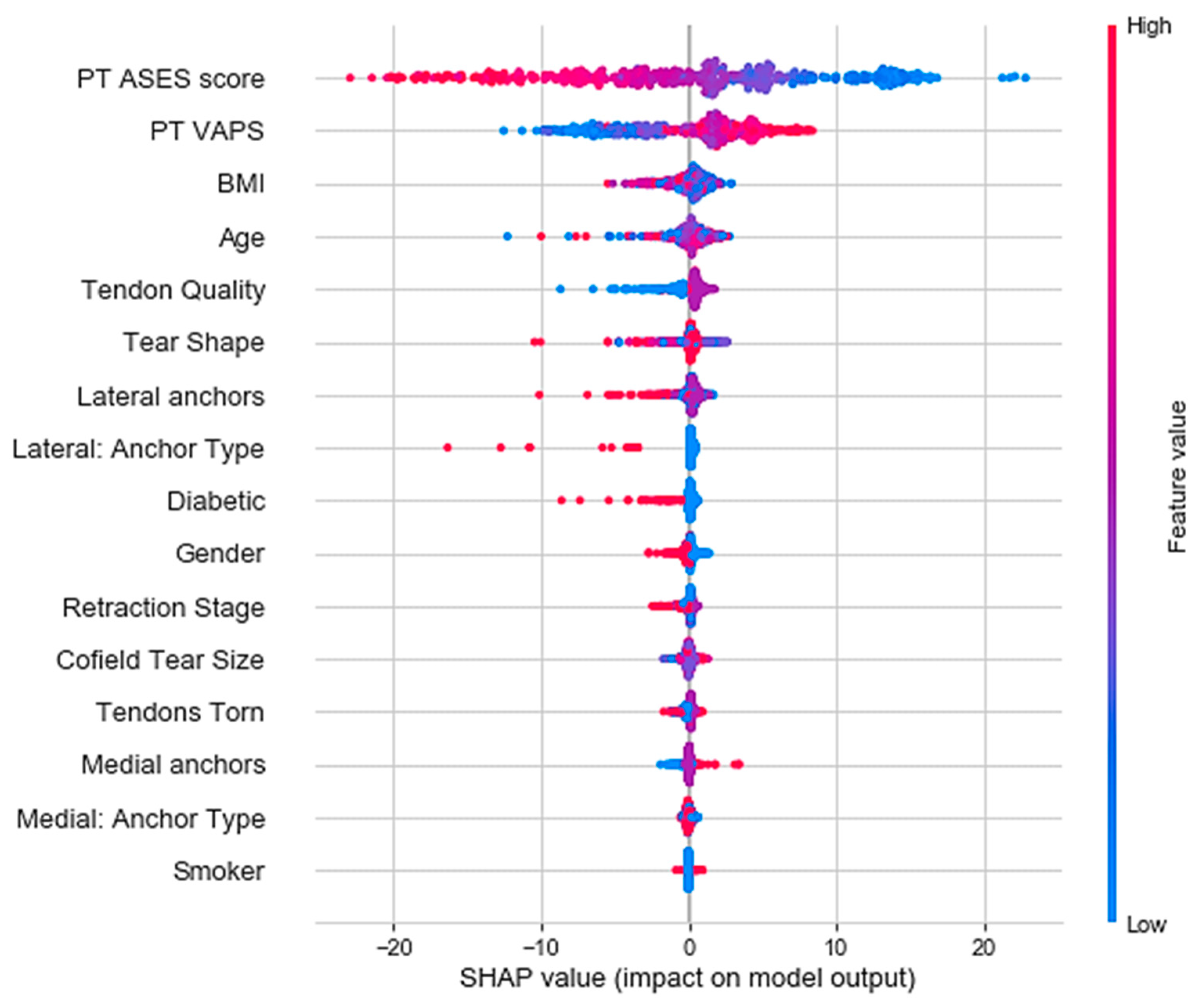
3. Results
Case Example
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michener, L.A.; Valier, A.R.S.; McClure, P.W. Defining substantial clinical benefit for patient-rated outcome tools for shoulder impingement syndrome. Arch. Phys. Med. Rehab. 2013, 94, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Potty, A.S.; Ganta, D.; Mistovich, R.J.; Penna, S.; Cady, C.; Potty, A.G. Streamlining the KOOS Activities of Daily Living Subscale Using Machine Learning. Orthop. J. Sports Med. 2020, 8, 2325967120910447. [Google Scholar] [CrossRef]
- Familiari, F.; Galasso, O.; Massazza, F.; Mercurio, M.; Fox, H.; Srikumaran, U.; Gasparini, G. Artificial Intelligence in the Management of Rotator Cuff Tears. Int. J. Environ. Res. Public Health 2022, 19, 16779. [Google Scholar] [CrossRef]
- Allaart, L.J.H.; Van Spanning, S.; Lafosse, L.; Lafosse, T.; Ladermann, A.; Athwal, G.S.; Hendrickx, L.A.M.; Doornberg, J.N.; Van Den Bekerom, M.P.J.; Buijze, G.A. Developing a machine learning algorithm to predict probability of retear and functional outcomes in patients undergoing rotator cuff repair surgery: Protocol for a retrospective, multicentre study. BMJ Open 2023, 13, e063673. [Google Scholar] [CrossRef]
- Gupta, P.; Haeberle, H.S.; Zimmer, Z.R.; Levine, W.N.; Williams, R.J.; Ramkumar, P.N. Artificial Intelligence-Based Applications in Shoulder Surgery Leaves Much to Be Desired: A Systematic Review. JSES Rev. Rep. Tech. 2023, 7, 158–161. [Google Scholar] [CrossRef]
- Michener, L.A.; McClure, P.W.; Sennett, B.J. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: Reliability, validity, and responsiveness. J. Shoulder Elb. Surg. 2002, 11, 587–594. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Deloach, J.; Green, A.; Porucznik, C.A.; Powell, A.P. Minimal clinically important differences in ASES and simple shoulder test scores after nonoperative treatment of rotator cuff disease. J. Bone Jt. Surg. 2010, 92, 296–303. [Google Scholar] [CrossRef]
- Li, L.; Bokshan, S.L.; Ready, L.V.; Owens, B.D. The primary cost drivers of arthroscopic rotator cuff repair surgery: A cost-minimization analysis of 40,618 cases. J. Shoulder Elb. Surg. 2019, 28, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Cabitza, F.; Locoro, A.; Banfi, G. Machine learning in orthopedics: A literature review. Front. Bioeng. Biotech. 2018, 6, 75. [Google Scholar] [CrossRef]
- Kumar, V.; Roche, C.; Overman, S.; Simovitch, R.; Flurin, P.H.; Wright, T.; Zuckerman, J.; Routman, H.; Teredesai, A. What Is the Accuracy of Three Different Machine Learning Techniques to Predict Clinical Outcomes after Shoulder Arthroplasty? Clin. Orthop. Relat. Res. 2020, 478, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Rodriguez, H.C.; Stone, I.W.; Nam, A.; Song, A.; Gupta, M.; Alvarado, R.; Ramon, D.; Gupta, A. Artificial intelligence and machine learning in orthopedic surgery: A systematic review protocol. J. Orthop. Surg. Res. 2020, 15, 478. [Google Scholar] [CrossRef]
- Kakavas, G.; Malliaropoulos, N.; Pruna, R.; Maffulli, N. Artificial intelligence: A tool for sports trauma prediction. Injury 2020, 51 (Suppl. S3), S63–S65. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Bayle, P.; Bayle, A.; Janson, L.; Mackey, L. Cross-Validation Confidence Intervals for Test Error. In Proceedings of the 34th Conference on Neural Information Processing Systems, Vancouver, BC, Canada, 6–12 December 2020. [Google Scholar]
- Dobbin, K.K.; Simon, R.M. Optimally splitting cases for training and testing high dimensional classifiers. BMC Genom. 2011, 4, 31. [Google Scholar] [CrossRef]
- Putatunda, S.; Rama, K. A Comparative Analysis of Hyperopt as against Other Approaches for Hyper-Parameter Optimization of XGBoost. In Proceedings of the 2018 International Conference on Signal Processing and Machine Learning, ACM, Shanghai China, 28 November 2018; pp. 6–10. [Google Scholar]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Pirracchio, R.; Petersen, M.L.; Carone, M.; Rigon, M.R.; Chevret, S.; van der Laan, M.J. Mortality Prediction in Intensive Care Units with the Super ICU Learner Algorithm (SICULA): A Population Based Study. Lancet Respir. Med. 2015, 3, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Cvetanovich, G.L.; Gowd, A.K.; Liu, J.N.; Nwachukwu, B.U.; Carbarcas, B.C.; Cole, B.J.; Forsythe, B.; Romeo, A.A.; Verma, N.N. Establishing clinically significant outcome after arthroscopic rotator cuff repair. J. Shoulder Elb. Surg. 2019, 28, 939–948. [Google Scholar] [CrossRef]
- Linardatos, P.; Papastefanopoulos, V.; Kotsiantis, S. Explainable AI: A Review of Machine Learning Interpretability Methods. Entropy 2020, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.A.; Lyman, S.; Sarker, G.K.; Padgett, D.E.; MacLean, C.H. Can Machine Learning Algorithms Predict Which Patients Will Achieve Minimally Clinically Important Differences from Total Joint Arthroplasty? Clin. Orthop. Relat. Res. 2019, 477, 1267–1279. [Google Scholar] [CrossRef]
- Brynjolfsson, E.; Mitchell, T. What Can Machine Learning Do? Workforce Implications. Science 2017, 358, 1530–1534. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist Level Classification of Skin Cancer with Deep Neural Networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.J.; Eichinger, J.; Schoch, B.; Wright, T.; Zuckerman, J.; Flurin, P.-H.; Bolch, C.; Roche, C. Preoperative Parameters that Predict Postoperative Patient-Reported Outcome Measures and Range of Motion with Anatomic and Reverse Total Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2019, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, V.; Lee, M.; Ang, B.F.H.; Chen, J.Y.; Lie, D.T.T. Comparing the Predictors of Functional Outcomes after Arthroscopic Rotator Cuff Repair Modified Frailty Index, Clinical Frailty Scale, and Charlson Comorbidity Index. Orthop. J. Sport. Med. 2021, 9, 23259671211005091. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.T.; Bishop, M.; Lam, P.H.; Murrell, G.A.C. Factors Predicting Frequency and Severity of Postoperative Pain after Arthroscopic Rotator Cuff Repair Surgery. Am. J. Sport. Med. 2021, 49, 146–153. [Google Scholar] [CrossRef]
- Wong, S.E.; Zhang, A.L.; Berliner, J.L.; Ma, C.B.; Feeley, B.T. Preoperative Patient-Reported Scores Can Predict Postoperative Outcomes after Shoulder Arthroplasty. J. Shoulder Elb. Surg. 2016, 25, 913–919. [Google Scholar] [CrossRef]
- Jenssen, K.K.; Lundgreen, K.; Madsen, J.E.; Kvakestad, R.; Dimmen, S. Prognostic Factors for Functional Outcome after Rotator Cuff Repair: A Prospective Cohort Study with 2-Year Follow-Up. Am. J. Sport. Med. 2018, 46, 3463–3470. [Google Scholar] [CrossRef]
- Dhar, Y.; Anakwenze, O.A.; Steele, B.; Lozano Calderon, S.A.; Abboud, J.A. Arthroscopic Rotator Cuff Repair: Impact of Diabetes Mellitus on Patient Outcomes. Physician Sport. 2013, 41, 22–29. [Google Scholar] [CrossRef]
- Gambhir, N.; Shankar, D.; Alben, M.; Kwon, Y.; Rokito, A.; Virk, M.S. The Effects of Obesity on1-Year Functional Outcomes after Arthroscopic Rotator Cuff Tear Repair. J. Shoulder Elb. Surg. Int. 2022, 6, 631–637. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Jung, K.-H.; Kim, J.-W.; Kim, U.-S.; Hwang, D.-H. Factors Affecting Rotator Cuff Integrity after Arthroscopic Repair for Medium-Sized or Larger Cuff Tears: A Retrospective Cohort Study. J. Shoulder Elb. Surg. 2018, 27, 1012–1020. [Google Scholar] [CrossRef]
- Warrender, W.J.; Brown, O.L.; Abboud, J.A. Outcomes of Arthroscopic Rotator Cuff Repairs in Obese Patients. J. Shoulder Elb. Surg. 2011, 20, 961–967. [Google Scholar] [CrossRef]
- Fermont, A.J.; Wolterbeek, N.; Wessel, R.N.; Baeyens, J.-P.; de Bie, R.A. Prognostic Factors for Recovery after Arthroscopic Rotator Cuff Repair: A Prognostic Study. J. Shoulder Elb. Surg. 2015, 24, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, A.M. Factors Affecting Healing after Arthroscopic Rotator Cuff Repair. WJO 2015, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Fermont, A.J.M.; Wolterbeek, N.; Wessel, R.N.; Baeyens, J.-P.; de Bie, R.A. Prognostic Factors for Successful Recovery after Arthroscopic Rotator Cuff Repair: A Systematic Literature Review. J. Orthop. Sport. Phys. Ther. 2014, 44, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Brassart, N.; Watkinson, D.J.; Carles, M.; Hatzidakis, A.M.; Krishnan, S.G. Arthroscopic repair of full thickness tears of the supraspinatus: Does the tendon really heal? J. Bone Jt. Surg. Am. 2005, 87, 1229–1240. [Google Scholar] [CrossRef]
- Guo, A.A.; Stitz, D.J.; Lam, P.; Murrell, G.A.C. Tear Size and Stiffness Are Important Predictors of Retear: An Assessment of Factors Associated with Repair Integrity at 6 Months in 1526 Rotator Cuff Repairs. J. Bone Jt. Surg. 2022, 7, e22.00006. [Google Scholar] [CrossRef]
- Manaka, T.; Ito, Y.; Matsumoto, I.; Takaoka, K.; Nakamura, H. Functional Recovery Period after Arthroscopic Rotator Cuff Repair: Is It Predictable Before Surgery? Clin. Orthop. Relat. Res. 2011, 469, 1660–1666. [Google Scholar] [CrossRef]
- Chung, S.W.; Kim, J.Y.; Kim, M.H.; Kim, S.H.; Oh, J.H. Arthroscopic Repair of Massive Rotator Cuff Tears: Outcome and Analysis of Factors Associated with Healing Failure or Poor Postoperative Function. Am. J. Sport. Med. 2013, 41, 1674–1683. [Google Scholar] [CrossRef]
- Chung, S.W.; Oh, J.H.; Gong, H.S.; Kim, J.Y.; Kim, S.H. Factors Affecting Rotator Cuff Healing after Arthroscopic Repair: Osteoporosis as One of the Independent Risk Factors. Am. J. Sport. Med. 2011, 39, 2099–2107. [Google Scholar] [CrossRef]
- Han Oh, J.; Hoon Kim, S.; Kang, J.Y.; Hee Oh, C.; Gong, H.S. Effect of Age on Functional and Structural Outcome after Rotator Cuff Repair. Am. J. Sport. Med. 2010, 38, 672–678. [Google Scholar] [CrossRef]
- Desai, V.N.; Cheung, E.V. Postoperative Pain Associated with Orthopedic Shoulder and Elbow Surgery: A Prospective Study. J. Shoulder Elb. Surg. 2012, 21, 441–450. [Google Scholar] [CrossRef]
- Bishop, J.; Klepps, S.; Lo, I.K.; Bird, J.; Gladstone, J.N.; Flatow, E.L. Cuff Integrity after Arthroscopic versus Open Rotator Cuff Repair: A Prospective Study. J. Shoulder Elb. Surg. 2006, 15, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.S.; Rhee, Y.G. The Factors Affecting the Clinical Outcome and Integrity of Arthroscopically Repaired Rotator Cuff Tears of the Shoulder. Clin. Orthop. Surg. 2009, 1, 96. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Gilbart, M.K.; Hodler, J.; Gerber, C. Clinical and Structural Results of Open Repair of an Isolated One-Tendon Tear of the Rotator Cuff. J. Bone Jt. Surg. 2006, 88, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Nho, S.J.; Dodson, C.C.; Adler, R.S.; Altchek, D.W.; MacGillivray, J.D. Prospective Evaluation of Arthroscopic Rotator Cuff Repairs at 5 Years: Part II—Prognostic Factors for Clinical and Radiographic Outcomes. J. Shoulder Elb. Surg. 2011, 20, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Audigé, L.; Aghlmandi, S.; Grobet, C.; Stojanov, T.; Müller, A.M.; Felsch, Q.; Gleich, J.; Flury, M.; Scheibel, M. Prediction of Shoulder Stiffness after Arthroscopic Rotator Cuff Repair. Am. J. Sport. Med. 2021, 49, 3030–3039. [Google Scholar] [CrossRef]
- Chung, S.W.; Park, J.S.; Kim, S.H.; Shin, S.H.; Oh, J.H. Quality of Life After Arthroscopic Rotator Cuff Repair: Evaluation Using SF-36 and an Analysis of Affecting Clinical Factors. Am. J. Sport. Med. 2012, 40, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Frangiamore, S.; Dornan, G.J.; Horan, M.P.; Mannava, S.; Fritz, E.M.; Hussain, Z.B.; Moatshe, G.; Godin, J.A.; Pogorzelski, J.; Millett, P.J. Predictive Modeling to Determine Functional Outcomes after Arthroscopic Rotator Cuff Repair. Am. J. Sport. Med. 2020, 48, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Razmjou, H.; Holtby, R.; Myhr, T. Gender Differences in Quality of Life and Extent of Rotator Cuff Pathology. Arthroscopy 2006, 22, 57–62. [Google Scholar] [CrossRef]
- Gasbarro, G.; Ye, J.; Newsome, H.; Jiang, K.; Wright, V.; Vyas, D.; Irrgang, J.J.; Musahl, V. Morphologic Risk Factors in Predicting Symptomatic Structural Failure of Arthroscopic Rotator Cuff Repairs: Tear Size, Location, and Atrophy Matter. Arthroscopy 2016, 32, 1947–1952. [Google Scholar] [CrossRef]
- Carbone, S.; Gumina, S.; Arceri, V.; Campagna, V.; Fagnani, C.; Postacchini, F. The Impact of Preoperative Smoking Habit on Rotator Cuff Tear: Cigarette Smoking Influences Rotator Cuff Tear Sizes. J. Shoulder Elb. Surg. 2012, 21, 56–60. [Google Scholar] [CrossRef]







| Algorithm | 10-Fold CV RMSE for 3-Month Post-Operative ASES | 95% CI |
|---|---|---|
| LASSO | 15.25 | 14.36–16.14 |
| Ridge Regression | 15.31 | 14.47–16.16 |
| Linear Regression | 15.32 | 14.47–16.16 |
| XGBoost | 15.90 | 14.80–17.00 |
| Random Forest | 16.35 | 15.40–17.31 |
| K-Nearest Neighbour (KNN) | 17.02 | 16.04–18.01 |
| Support Vector Regression | 17.20 | 16.00–18.40 |
| Post-Operative Time | Train RMSE (10-Fold CV) in ASES | Test RMSE in ASES |
|---|---|---|
| 3 months | 15.90 | 16.50 |
| 6 months | 16.36 | 14.75 |
| 12 months | 14.60 | 12.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potty, A.G.; Potty, A.S.R.; Maffulli, N.; Blumenschein, L.A.; Ganta, D.; Mistovich, R.J.; Fuentes, M.; Denard, P.J.; Sethi, P.M.; Shah, A.A.; et al. Approaching Artificial Intelligence in Orthopaedics: Predictive Analytics and Machine Learning to Prognosticate Arthroscopic Rotator Cuff Surgical Outcomes. J. Clin. Med. 2023, 12, 2369. https://doi.org/10.3390/jcm12062369
Potty AG, Potty ASR, Maffulli N, Blumenschein LA, Ganta D, Mistovich RJ, Fuentes M, Denard PJ, Sethi PM, Shah AA, et al. Approaching Artificial Intelligence in Orthopaedics: Predictive Analytics and Machine Learning to Prognosticate Arthroscopic Rotator Cuff Surgical Outcomes. Journal of Clinical Medicine. 2023; 12(6):2369. https://doi.org/10.3390/jcm12062369
Chicago/Turabian StylePotty, Anish G., Ajish S. R. Potty, Nicola Maffulli, Lucas A. Blumenschein, Deepak Ganta, R. Justin Mistovich, Mario Fuentes, Patrick J. Denard, Paul M. Sethi, Anup A. Shah, and et al. 2023. "Approaching Artificial Intelligence in Orthopaedics: Predictive Analytics and Machine Learning to Prognosticate Arthroscopic Rotator Cuff Surgical Outcomes" Journal of Clinical Medicine 12, no. 6: 2369. https://doi.org/10.3390/jcm12062369
APA StylePotty, A. G., Potty, A. S. R., Maffulli, N., Blumenschein, L. A., Ganta, D., Mistovich, R. J., Fuentes, M., Denard, P. J., Sethi, P. M., Shah, A. A., & Gupta, A. (2023). Approaching Artificial Intelligence in Orthopaedics: Predictive Analytics and Machine Learning to Prognosticate Arthroscopic Rotator Cuff Surgical Outcomes. Journal of Clinical Medicine, 12(6), 2369. https://doi.org/10.3390/jcm12062369








