Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
3.1. Baseline Patients’ Characteristics
3.2. Remdesivir Therapy and Other Drugs in RDV-Treated Patients
3.3. Clinical Course and Outcomes of the Disease
3.3.1. Comparison between RDV and No AVT Populations
3.3.2. Comparison of RDV-Treated Patients in Both Waves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Tian, D. Bibliometric Analysis of Global Scientific Research on COVID-19. J. Biosaf. Biosecur. 2021, 3, 4–9. [Google Scholar] [CrossRef]
- Nowakowska, J.; Sobocińska, J.; Lewicki, M.; Lemańska, Ż.; Rzymski, P. When Science Goes Viral: The Research Response during Three Months of the COVID-19 Outbreak. Biomed. Pharmacother. 2020, 129, 110451. [Google Scholar] [CrossRef]
- Rahmah, L.; Abarikwu, S.O.; Arero, A.G.; Essouma, M.; Jibril, A.T.; Fal, A.; Flisiak, R.; Makuku, R.; Marquez, L.; Mohamed, K.; et al. Oral Antiviral Treatments for COVID-19: Opportunities and Challenges. Pharmacol. Rep. 2022, 74, 1255–1278. [Google Scholar] [CrossRef]
- Cusinato, J.; Cau, Y.; Calvani, A.M.; Mori, M. Repurposing Drugs for the Management of COVID-19. Expert Opin. Ther. Pat. 2021, 31, 295–307. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Saunders, O.L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J.E.; Feng, J.Y.; Ray, A.S.; Kim, C.U. Synthesis and Antiviral Activity of a Series of 1’-Substituted 4-Aza-7,9-Dideazaadenosine C-Nucleosides. Bioorg. Med. Chem. Lett. 2012, 22, 2705–2707. [Google Scholar] [CrossRef] [PubMed]
- EMA. Veklury. European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/veklur (accessed on 22 January 2023).
- Peck, K.M.; Lauring, A.S. Complexities of Viral Mutation Rates. J. Virol. 2018, 92, e01031-17. [Google Scholar] [CrossRef] [PubMed]
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 22 January 2023).
- O’Horo, J.C.; Challener, D.W.; Speicher, L.; Bosch, W.; Seville, M.T.; Bierle, D.M.; Ganesh, R.; Wilker, C.G.; Arndt, R.F.; Arndt, L.L.; et al. Effectiveness of Monoclonal Antibodies in Preventing Severe COVID-19 With Emergence of the Delta Variant. Mayo Clin. Proc. 2022, 97, 327–332. [Google Scholar] [CrossRef]
- Dryden-Peterson, S.; Kim, A.; Kim, A.Y.; Caniglia, E.C.; Lennes, I.T.; Patel, R.; Gainer, L.; Dutton, L.; Donahue, E.; Gandhi, R.T.; et al. Nirmatrelvir Plus Ritonavir for Early COVID-19 in a Large U.S. Health System: A Population-Based Cohort Study. Ann. Intern. Med. 2023, 176, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Maggi, F.; McConnell, S.; Casadevall, A. Very Low Levels of Remdesivir Resistance in SARS-COV-2 Genomes after 18 Months of Massive Usage during the COVID19 Pandemic: A GISAID Exploratory Analysis. Antivir. Res. 2022, 198, 105247. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, T.; Nemudryi, A.; Nemudraia, A.; McVey, A.; Little, A.; Taylor, D.N.; Walk, S.T.; Wiedenheft, B. The Rise and Fall of SARS-CoV-2 Variants and Ongoing Diversification of Omicron. Viruses 2022, 14, 2009. [Google Scholar] [CrossRef]
- Chand, G.B.; Banerjee, A.; Azad, G.K. Identification of Novel Mutations in RNA-Dependent RNA Polymerases of SARS-CoV-2 and Their Implications on Its Protein Structure. PeerJ 2020, 8, e9492. [Google Scholar] [CrossRef] [PubMed]
- Hakmaoui, A.; Khan, F.; Liacini, A.; Kaur, A.; Berka, Y.; Machraoui, S.; Soualhine, H.; Berka, N.; Rais, H.; Admou, B. Relevant SARS-CoV-2 Genome Variation through Six Months of Worldwide Monitoring. Biomed. Res. Int. 2021, 2021, 5553173. [Google Scholar] [CrossRef]
- Eskier, D.; Karakülah, G.; Suner, A.; Oktay, Y. RdRp Mutations Are Associated with SARS-CoV-2 Genome Evolution. PeerJ 2020, 8, e9587. [Google Scholar] [CrossRef]
- Stevens, L.J.; Pruijssers, A.J.; Lee, H.W.; Gordon, C.J.; Tchesnokov, E.P.; Gribble, J.; George, A.S.; Hughes, T.M.; Lu, X.; Li, J.; et al. Mutations in the SARS-CoV-2 RNA-Dependent RNA Polymerase Confer Resistance to Remdesivir by Distinct Mechanisms. Sci. Transl. Med. 2022, 14, eabo0718. [Google Scholar] [CrossRef]
- Negru, P.A.; Radu, A.F.; Vesa, C.M.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Endres, L.; Stoicescu, M.; Pasca, B.; Tit, D.M.; et al. Therapeutic Dilemmas in Addressing SARS-CoV-2 Infection: Favipiravir versus Remdesivir. Biomed. Pharmacother. 2022, 147, 112700. [Google Scholar] [CrossRef]
- Gandhi, S.; Klein, J.; Robertson, A.J.; Peña-Hernández, M.A.; Lin, M.J.; Roychoudhury, P.; Lu, P.; Fournier, J.; Ferguson, D.; Mohamed Bakhash, S.A.K.; et al. De Novo Emergence of a Remdesivir Resistance Mutation during Treatment of Persistent SARS-CoV-2 Infection in an Immunocompromised Patient: A Case Report. Nat. Commun. 2022, 13, 1547. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.I.; Duerr, R.; Dimartino, D.; Marier, C.; Hochman, S.E.; Mehta, S.; Wang, G.; Heguy, A. Remdesivir Resistance in Transplant Recipients With Persistent Coronavirus Disease 2019. Clin. Infect. Dis. 2023, 76, 342–345. [Google Scholar] [CrossRef]
- Fitero, A.; Bungau, S.G.; Tit, D.M.; Endres, L.; Khan, S.A.; Bungau, A.F.; Romanul, I.; Vesa, C.M.; Radu, A.-F.; Tarce, A.G.; et al. Comorbidities, Associated Diseases, and Risk Assessment in COVID-19—A Systematic Review. Int. J. Clin. Pract. 2022, 2022, 1571826. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, NEJMoa2007016. [Google Scholar] [CrossRef]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.-P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus Standard of Care versus Standard of Care Alone for the Treatment of Patients Admitted to Hospital with COVID-19 (DisCoVeRy): A Phase 3, Randomised, Controlled, Open-Label Trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Lau, K.T.K.; Au, I.C.H.; Xiong, X.; Lau, E.H.Y.; Cowling, B.J. Clinical Improvement, Outcomes, Antiviral Activity, and Costs Associated With Early Treatment With Remdesivir for Patients With Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2022, 74, 1450–1458. [Google Scholar] [CrossRef]
- Vangeel, L.; Chiu, W.; De Jonghe, S.; Maes, P.; Slechten, B.; Raymenants, J.; André, E.; Leyssen, P.; Neyts, J.; Jochmans, D. Remdesivir, Molnupiravir and Nirmatrelvir Remain Active against SARS-CoV-2 Omicron and Other Variants of Concern. Antivir. Res. 2022, 198, 105252. [Google Scholar] [CrossRef]
- Pitts, J.; Li, J.; Perry, J.K.; Du Pont, V.; Riola, N.; Rodriguez, L.; Lu, X.; Kurhade, C.; Xie, X.; Camus, G.; et al. Remdesivir and GS-441524 Retain Antiviral Activity against Delta, Omicron, and Other Emergent SARS-CoV-2 Variants. Antimicrob. Agents Chemother. 2022, 66, e0022222. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Tan, S.T.; Kwan, A.T.; Rodríguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 Breakthrough Infections and Reinfections during the Omicron Wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Diagnosis and Therapy of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of November 12, 2021. Annex No. 1 to the Recommendations of April 26, 2021. Pol. Arch. Intern. Med. 2021, 131, 16140. [Google Scholar] [CrossRef]
- Flisiak, R.; Horban, A.; Jaroszewicz, J.; Kozielewicz, D.; Mastalerz-Migas, A.; Owczuk, R.; Parczewski, M.; Pawłowska, M.; Piekarska, A.; Simon, K.; et al. Management of SARS-CoV-2 Infection: Recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, 2022. Pol. Arch. Intern. Med. 2022, 132, 16230. [Google Scholar] [CrossRef]
- Flisiak, R.; Rzymski, P.; Zarębska-Michaluk, D.; Ciechanowski, P.; Dobrowolska, K.; Rogalska, M.; Jaroszewicz, J.; Szymanek-Pasternak, A.; Rorat, M.; Kozielewicz, D.; et al. Variability in the Clinical Course of COVID-19 in a Retrospective Analysis of a Large Real-World Database. Viruses 2023, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolska, K.; Brzdęk, M.; Zarębska-Michaluk, D.; Rzymski, P.; Rogalska, M.; Moniuszko-Malinowska, A.; Szymanek-Pasternak, A.; Jaroszewicz, J.; Dutkiewicz, E.; Kowalska, J.; et al. Differences between the Course of SARS-CoV-2 Infections in the Periods of the Delta and Omicron Variants Dominance in Poland. Pol. Arch. Intern. Med. 2023, 16403. [Google Scholar] [CrossRef]
- Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused on Europe Since Pandemic Start. Nextstrain. Available online: https://nextstrain.org/ncov/gisaid/europe/ (accessed on 22 January 2023).
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Rogalska, M.; Martonik, D.; Pabjan, P.; Berkan-Kawińska, A.; Bolewska, B.; Oczko-Grzesik, B.; Kozielewicz, D.; Tudrujek-Zdunek, M.; et al. Effectiveness of Tocilizumab with and without Dexamethasone in Patients with Severe COVID-19: A Retrospective Study. J. Inflamm. Res. 2021, 14, 3359–3366. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Zarębska-Michaluk, D.; Rogalska, M.; Kryńska, J.A.; Kowalska, J.; Dutkiewicz, E.; Dobrowolska, K.; Jaroszewicz, J.; Moniuszko-Malinowska, A.; Rorat, M.; et al. Real-World Experience with Molnupiravir during the Period of SARS-CoV-2 Omicron Variant Dominance. Pharmacol. Rep. 2022, 74, 1279–1285. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Kasianchuk, N.; Sikora, D.; Poniedziałek, B. COVID-19 Vaccinations and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in Europe during SARS-CoV-2 Omicron Wave in the First Quarter of 2022. J. Med. Virol. 2023, 95, e28131. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid Epidemic Expansion of the SARS-CoV-2 Omicron Variant in Southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Ao, D.; Lan, T.; He, X.; Liu, J.; Chen, L.; Baptista-Hon, D.T.; Zhang, K.; Wei, X. SARS-CoV-2 Omicron Variant: Immune Escape and Vaccine Development. MedComm 2022, 3, e126. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable Escape of SARS-CoV-2 Omicron to Antibody Neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Dabrowska, A.; Szczepanski, A.; Botwina, P.; Mazur-Panasiuk, N.; Jiřincová, H.; Rabalski, L.; Zajic, T.; Popowicz, G.; Pyrc, K. Efficacy of Antiviral Drugs against the Omicron Variant of SARS-CoV-2. bioRxiv 2021. [Google Scholar] [CrossRef]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced Fusogenicity and Pathogenicity of SARS-CoV-2 Delta P681R Mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef]
- Auvigne, V.; Vaux, S.; Strat, Y.L.; Schaeffer, J.; Fournier, L.; Tamandjou, C.; Montagnat, C.; Coignard, B.; Levy-Bruhl, D.; Parent du Châtelet, I. Severe Hospital Events Following Symptomatic Infection with Sars-CoV-2 Omicron and Delta Variants in France, December 2021–January 2022: A Retrospective, Population-Based, Matched Cohort Study. eClinicalMedicine 2022, 48, 101455. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Van Goethem, N.; Chung, P.Y.J.; Meurisse, M.; Vandromme, M.; De Mot, L.; Brondeel, R.; Stouten, V.; Klamer, S.; Cuypers, L.; Braeye, T.; et al. Clinical Severity of SARS-CoV-2 Omicron Variant Compared with Delta among Hospitalized COVID-19 Patients in Belgium during Autumn and Winter Season 2021-2022. Viruses 2022, 14, 1297. [Google Scholar] [CrossRef]
- Russo, P.; Tacconelli, E.; Olimpieri, P.P.; Celant, S.; Colatrella, A.; Tomassini, L.; Palù, G. Mortality in SARS-CoV-2 Hospitalized Patients Treated with Remdesivir: A Nationwide, Registry-Based Study in Italy. Viruses 2022, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Chokkalingam, A.P.; Hayden, J.; Goldman, J.D.; Li, H.; Asubonteng, J.; Mozaffari, E.; Bush, C.; Wang, J.R.; Kong, A.; Osinusi, A.O.; et al. Association of Remdesivir Treatment With Mortality Among Hospitalized Adults With COVID-19 in the United States. JAMA Netw. Open 2022, 5, e2244505. [Google Scholar] [CrossRef]
- Lakhanpal, M.; Sarkar, D.; Kumar, R.; Yadav, I. Reduction in the Rate of Mortality of Moderate to Severe COVID 19 Infected Patients with the Use of Remdesivir—A Tertiary Care Hospital-Based Retrospective Observational Study. Anesth. Essays Res. 2022, 16, 296–300. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Wang, K.; Robinson, M.L.; Betz, J.; Caleb Alexander, G.; Andersen, K.M.; Joseph, C.S.; Mehta, H.B.; Korwek, K.; Sands, K.E.; et al. Real-World Effectiveness of Remdesivir in Adults Hospitalized With Coronavirus Disease 2019 (COVID-19): A Retrospective, Multicenter Comparative Effectiveness Study. Clin. Infect. Dis. 2022, 75, e516–e524. [Google Scholar] [CrossRef] [PubMed]
- Karolyi, M.; Kaltenegger, L.; Pawelka, E.; Kuran, A.; Platzer, M.; Totschnig, D.; Koenig, F.; Hoepler, W.; Laferl, H.; Omid, S.; et al. Early Administration of Remdesivir May Reduce Mortality in Hospitalized COVID-19 Patients: A Propensity Score Matched Analysis. Wien. Klin. Wochenschr. 2022, 134, 883–891. [Google Scholar] [CrossRef]
- Slim, M.A.; Appelman, B.; Peters-Sengers, H.; Dongelmans, D.A.; de Keizer, N.F.; Schade, R.P.; de Boer, M.G.J.; Müller, M.C.A.; Vlaar, A.P.J.; Wiersinga, W.J.; et al. Real-World Evidence of the Effects of Novel Treatments for COVID-19 on Mortality: A Nationwide Comparative Cohort Study of Hospitalized Patients in the First, Second, Third, and Fourth Waves in the Netherlands. Open Forum. Infect. Dis. 2022, 9, ofac632. [Google Scholar] [CrossRef] [PubMed]
- Beckerman, R.; Gori, A.; Jeyakumar, S.; Malin, J.J.; Paredes, R.; Póvoa, P.; Smith, N.J.; Teixeira-Pinto, A. Remdesivir for the Treatment of Patients Hospitalized with COVID-19 Receiving Supplemental Oxygen: A Targeted Literature Review and Meta-Analysis. Sci. Rep. 2022, 12, 9622. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Azher, T.; Baqi, M.; Binnie, A.; Borgia, S.; Carrier, F.M.; Cavayas, Y.A.; Chagnon, N.; Cheng, M.P.; Conly, J.; et al. Remdesivir for the Treatment of Patients in Hospital with COVID-19 in Canada: A Randomized Controlled Trial. CMAJ 2022, 194, E242–E251. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium Remdesivir and Three Other Drugs for Hospitalised Patients with COVID-19: Final Results of the WHO Solidarity Randomised Trial and Updated Meta-Analyses. Lancet 2022, 399, 1941–1953. [CrossRef] [PubMed]
- Adjei, S.; Hong, K.; Molinari, N.-A.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods—United States, April 2020-June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Hussain Alsayed, H.A.; Saheb Sharif-Askari, F.; Saheb Sharif-Askari, N.; Hussain, A.A.S.; Hamid, Q.; Halwani, R. Early Administration of Remdesivir to COVID-19 Patients Associates with Higher Recovery Rate and Lower Need for ICU Admission: A Retrospective Cohort Study. PLoS ONE 2021, 16, e0258643. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Hayakawa, K.; Uemura, Y.; Shinozaki, T.; Matsunaga, N.; Terada, M.; Suzuki, S.; Asai, Y.; Kitajima, K.; Saito, S.; et al. Effectiveness of Remdesivir in Hospitalized Nonsevere Patients with COVID-19 in Japan: A Large Observational Study Using the COVID-19 Registry Japan. Int. J. Infect. Dis. 2022, 118, 119–125. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Kowalska, J.; Pawłowska, M.; Rogalska, M.; Zarębska-Michaluk, D.; Rorat, M.; Lorenc, B.; Czupryna, P.; Sikorska, K.; Piekarska, A.; et al. Remdesivir Decreases Mortality in COVID-19 Patients with Active Malignancy. Cancers 2022, 14, 4720. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B.; Rosińska, J.; Rogalska, M.; Zarębska-Michaluk, D.; Rorat, M.; Moniuszko-Malinowska, A.; Lorenc, B.; Kozielewicz, D.; Piekarska, A.; et al. The Association of Airborne Particulate Matter and Benzo[a]Pyrene with the Clinical Course of COVID-19 in Patients Hospitalized in Poland. Environ. Pollut. 2022, 306, 119469. [Google Scholar] [CrossRef]
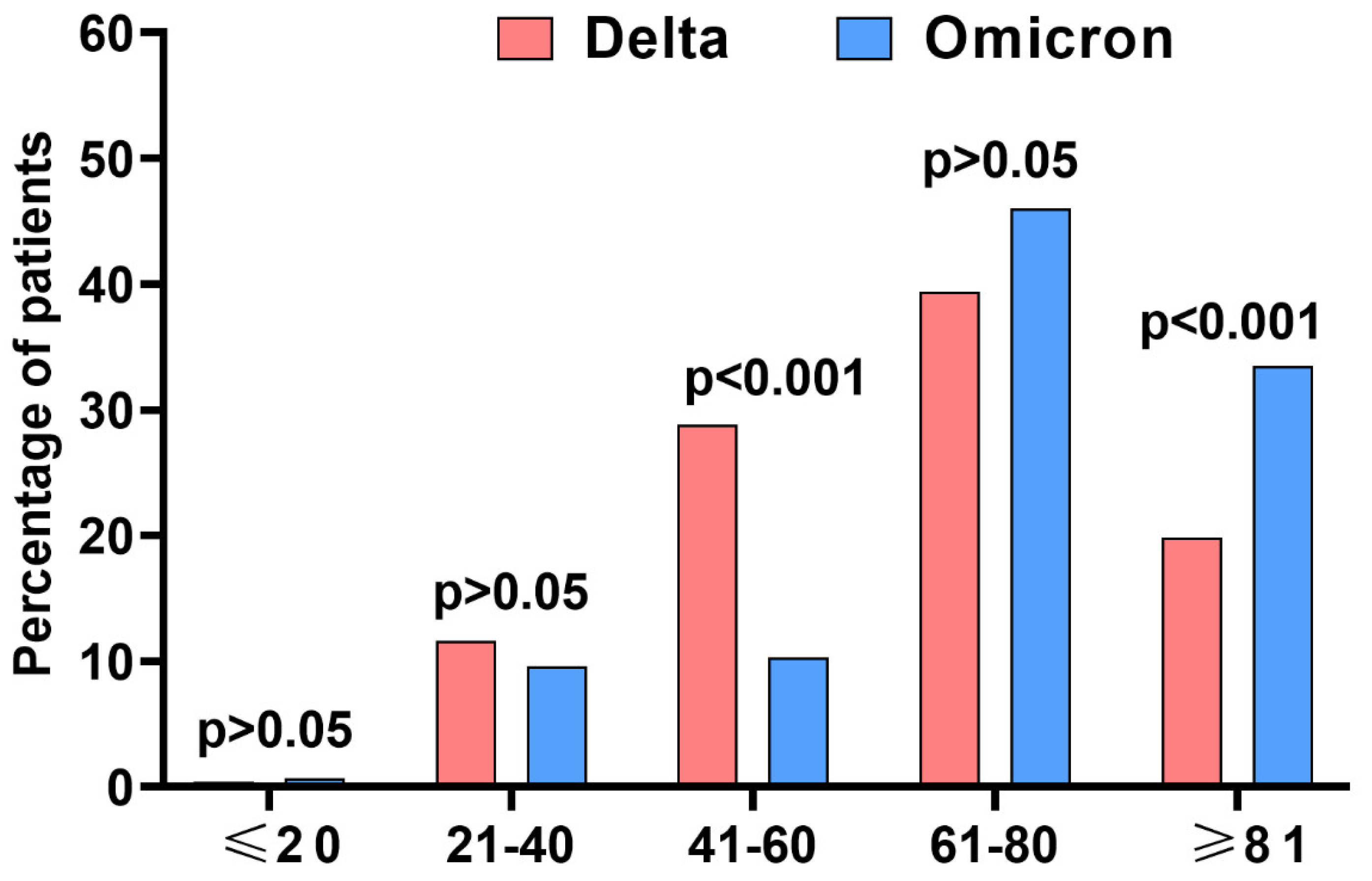

| Delta | Omicron | p (between RDV-Treated Patients in Delta and Omicron Wave) | |||||
|---|---|---|---|---|---|---|---|
| RDV (n = 490) | NO AVT (n = 680) | p | RDV (n = 272) | NO AVT (n = 380) | p | ||
| Gender. females/males, % | 47.1/52.9 | 46.5/53.5 | >0.05 | 43.8/56.3 | 48.4/51.6 | >0.05 | >0.05 |
| Age (years), mean ± SD | 63.3 ± 17.1 | 65.1 ± 16.8 | >0.05 | 70.6 ± 17.2 | 68.4 ± 18.8 | >0.05 | <0.001 |
| BMI (kg/m2), mean ± SD | 28.7 ± 5.1 | 28.2 ± 5.1 | >0.05 | 27.2 ± 5.9 | 27.0 ± 5.3 | >0.05 | <0.001 |
| SpO2 | 89.0 (6.7) | 88.1 (8.1) | >0.05 | 91.7 (4.8) | 91.6 (6.3) | >0.05 | <0.001 |
| Comorbidities, % (n) | |||||||
| Any Comorbidity | 75.5 (370) | 77.2 (525) | >0.05 | 93.0 (253) | 92.1 (350) | >0.05 | <0.001 |
| Hypertension | 53.1 (260) | 50.9 (346) | >0.05 | 60.7 (165) | 52.9 (201) | 0.049 | 0.04 |
| Diabetes | 21.4 (105) | 19.7 (134) | >0.05 | 25.0 (68) | 25.3 (96) | >0.05 | >0.05 |
| Stroke | 3.1 (15) | 6.2 (42) | 0.015 | 12.5 (34) | 9.5 (36) | >0.05 | <0.001 |
| COPD | 4.5 (22) | 5.3 (36) | >0.05 | 7.7 (21) | 7.1 (27) | >0.05 | >0.05 |
| Neoplastic diseases | 7.6 (37) | 6.5 (44) | >0.05 | 13.2 (36) | 16.3 (62) | >0.05 | 0.0106 |
| Ischemic heart diseases | 9.4 (46) | 12.6 (86) | >0.05 | 23.5 (64) | 18.9 (72) | >0.05 | <0.001 |
| Other CVD | 19.2 (94) | 20.7 (141) | >0.05 | 33.1 (90) | 31.8 (121) | >0.05 | <0.001 |
| Other respiratory diseases | 8.2 (40) | 7.5 (51) | >0.05 | 11.8 (32) | 9.5 (36) | >0.05 | >0.05 |
| Other metabolic diseases | 10.6 (52) | 11.3 (77) | >0.05 | 16.2 (44) | 13.7 (52) | >0.05 | 0.03 |
| Others | 44.5 (218) | 47.6 (324) | >0.05 | 72.8 (198) | 70.8 (269) | >0.05 | <0.001 |
| Delta | Omicron | p (between RDV-Treated Patients in Delta and Omicron Wave) | |||||
|---|---|---|---|---|---|---|---|
| RDV (n = 490) | NO AVT (n = 680) | p | RDV (n = 272) | NO AVT (n = 380) | p | ||
| CRP, mg/L | 82.5 ± 71.7 | 94.0 ± 84.5 | >0.05 | 67.6 ± 67.6 | 69.9 ± 78.9 | >0.05 | <0.001 |
| PCT, ng/mL | 0.4 ± 1.3 | 1.1 ± 8.3 | >0.05 | 0.9 ± 3.1 | 1.8 ± 9.2 | >0.05 | >0.05 |
| WBC, ×103/µL | 7.0 ± 6.5 | 7.1 ± 3.9 | 0.006 | 7.8 ± 8.0 | 8.0 ± 4.9 | >0.05 | 0.003 |
| Lymphocytes, ×103/µL | 1.4 ± 4.7 | 1.1 ± 1.4 | >0.05 | 1.3 ± 1.8 | 1.2 ± 1.5 | >0.05 | 0.01 |
| Neutrophils, ×103/µL | 5 ± 3.4 | 5.3 ± 3.2 | 0.008 | 5.5 ± 4.7 | 6.1 ± 6.0 | >0.05 | >0.05 |
| Platelets, ×103/µL | 185.5 ± 79.5 | 213.0 ± 96.0 | <0.001 | 202.4 ± 93.2 | 212.8 ± 101.5 | 0.04 | 0.006 |
| IL-6, pg/mL | 102.9 ± 329.1 | 122.4 ± 383.1 | >0.05 | 160.3 ± 611.8 | 218.2 ± 1399.5 | 0.006 | >0.05 |
| d-dimer, ng/mL | 1904.9 ± 5909.8 | 2165.4 ± 4917.6 | 0.004 | 2282.4 ± 4918.7 | 2809.6 ± 8179.5 | >0.05 | 0.03 |
| ALT, IU/L | 42.5 ± 40.6 | 47.8 ± 51.1 | >0.05 | 34.9 ± 31.6 | 45.3 ± 96.3 | >0.05 | <0.001 |
| Stable symptomatic, SpO2 > 95% or asymptomatic | 12.0 (59) | 14.3 (97) | >0.05 | 27.9 (76) | 34.7 (132) | >0.05 | <0.001 |
| Unstable symptomatic, SpO2 ≤ 95% or ARDS | 87.9 (431) | 85.9 (579) | >0.05 | 72.1 (196) | 65.5 (248) | >0.05 | <0.001 |
| Parameters | Delta (n = 490) | Omicron (n = 272) | p |
|---|---|---|---|
| Time between onset of symptoms and start of the antiviral treatment, mean ± SD (min–max) | 5.1 ± 3.0 (0–21) n = 479 | 3.5 ± 2.2 (0–14) n = 266 | <0.001 |
| Patient treated within 5 days of symptoms, % (n) | 57.2 (274/479) | 86.8 (231/266) | <0.001 |
| Patient treated within 3 days of symptoms, % (n) | 27.8 (133/479) | 53.4 (142/266) | <0.001 |
| Immunomodulators, % (n) | 56.3 (276) | 55.9 (152) | >0.05 |
| Tocilizumab | 14.9 (73) | 9.6 (26) | 0.04 |
| Dexamethason | 49.6 (243) | 54.4 (148) | >0.05 |
| Baricitinib | 1.2 (6) | 3.3 (9) | 0.04 |
| Antibiotics, % (n) | 34.1 (167) | 41.2 (112) | 0.05 |
| Low molecular weight heparin in prophylactic dose, % (n) | 73.1 (358) | 73.5 (200) | >0.05 |
| Low molecular weight heparin in a therapeutic dose, % (n) | 16.9 (83) | 23.5 (64) | 0.03 |
| Delta | Omicron | |||||
|---|---|---|---|---|---|---|
| RDV (n = 490) | NO AVT (n = 680) | p | RDV (n = 272) | NO AVT (n = 380) | p | |
| Need for oxygen therapy, % (n) | 75.9 (372) | 77.1 (524) | >0.05 | 59.6 (162) | 50.3 (191) | 0.01 |
| Need for mechanical ventilation, % (n) | 7.8 (38) | 9.4 (63) | >0.05 | 2.2 (6) | 4.0 (15) | >0.05 |
| Mortality, % (n) | 10.8 (53) | 23.2 (158) | <0.001 | 11.4 (31) | 16.8 (64) | 0.05 |
| Age of patients who died (years), mean ± SD (min–max) | 75.6 ± 13.9 (34–95) | 76.5 ± 12.9 (30–99) | >0.05 | 77.7 ± 12.4 (37–95) | 79.0 ± 14.2 (25–99) | >0.05 |
| Variable | Delta | Omicron |
|---|---|---|
| Odds Ratio (95% Confidence Interval) | ||
| Age >70 years | 4.99 (3.55–7.01) p < 0.0001 | 2.2 (1.9–5.8) p < 0.0001 |
| Male sex | 0.82 (0.66–1.28) p > 0.05 | 0.71 (0.44–1.13) p > 0.05 |
| Obesity (BMI > 30 kg/m2) | 0.82 (0.56–1.20) p > 0.03 | 1.40 (0.92–1.89) p = 0.02 |
| SpO2 ≤ 90% at admission | 1.37 (0.89–4.63) p < 0.0001 | 2.40 (1.47–3.91) p = 0.0005 |
| Dexamethasone treatment | 1.81 (1.23–2.67) p = 0.003 | 2.97 (1.81–4.89) p < 0.0001 |
| RDV treatment | 0.42 (0.29–0.60) p < 0.0001 | 0.56 (0.35–0.92) p = 0.02 |
| Patients Subpopulations | Mortality | Mechanical Ventilation | ||||
|---|---|---|---|---|---|---|
| Delta | Omicron | p | Delta | Omicron | p | |
| All patients, % (n/N) | 10.8 (53/490) | 11.4 (31/272) | >0.05 | 7.8 (38/490) | 2.2 (6/272) | 0.002 |
| SpO2 ≤ 95%, % (n/N) | 11.8 (51/431) | 14.3 (28/196) | >0.05 | 7.9 (34/431) | 3.1 (6/196) | 0.02 |
| SpO2 ≤ 95%, 0–5 days, % (n/N) | 13.3 (31/233) | 14.3 (23/161) | >0.05 | 7.7 (18/233) | 2.5 (4/161) | 0.03 |
| SpO2 ≤ 95%, 0–5 days, >60 years, % (n/N) | 19.5 (30/154) | 15.4 (22/143) | >0.05 | 9.1 (14/154) | 2.1 (3/143) | 0.01 |
| SpO2 ≤ 95%, 0–5 days, >80 years, % (n/N) | 35.3 (18/51) | 21.4 (12/56) | >0.05 | 7.8 (4/51) | 1.8 (1/56) | >0.05 |
| Parameter | Delta | Omicron | ||||
|---|---|---|---|---|---|---|
| ≤5 | >5 | p | ≤5 | >5 | p | |
| All | ||||||
| n | 274 | 205 | 231 | 35 | ||
| Mortality, % (n) | 13.5 (37/274) | 7.8 (16/205) | >0.05 | 10.8 (25/231) | 14.3 (5/35) | >0.05 |
| Need for oxygen therapy, % (n) | 73 (200/274) | 83.9 (172/205) | <0.001 | 56.7 (131/231) | 77.1 (27/35) | 0.0218 |
| Time of oxygen therapy, mean ± SD | 8.4 ± 9.4 | 10.1 ± 8.7 | >0.05 | 9.4 ± 7.0 | 11.2 ± 6.8 | >0.05 |
| Need for mechanical ventilation, % (n) | 8.8 (24/274) | 6.8 (14/205) | >0.05 | 1.7 (4/231) | 5.7 (2/231) | >0.05 |
| >60 years | ||||||
| n | 172 | 110 | 182 | 29 | ||
| Mortality, % (n) | 20.3 (35/172) | 10 (11/110) | >0.05 | 12.6 (23/182) | 17.2 (5/29) | >0.05 |
| Need for oxygen therapy, % (n) | 79.1 (136/172) | 27.3 (30/110) | >0.05 | 63.7 (116/182) | 75.9 (22/29) | >0.05 |
| Time of oxygen therapy, mean ± SD | 9.6 ± 9.9 | 10.7 ± 9.1 | >0.05 | 9.3 ± 7 | 10.3 ± 6.7 | >0.05 |
| Need for mechanical ventilation, % (n) | 9.9 (17/172) | 7.3 (8/110) | >0.05 | 1.6 (3/182) | 0 (0/29) | >0.05 |
| >80 years | ||||||
| n | 56 | 36 | 75 | 13 | ||
| Mortality, % (n) | 33.9 (19/56) | 16.7 (6/36) | >0.05 | 16 (12/75) | 30.8 (4/13) | >0.05 |
| Need for oxygen therapy, % (n) | 89.3 (50/56) | 83.3 (30/36) | >0.05 | 62.7 (47/75) | 84.6 (11/13) | >0.05 |
| Time of oxygen therapy, mean ± SD | 10.1 ± 8.9 | 10.9 ± 8.1 | >0.05 | 8.4 ± 5.6 | 11.7 ± 7.4 | >0.05 |
| Need for mechanical ventilation, % (n) | 8.9 (5/56) | 8.3 (3/36) | >0.05 | 1.3 (1/75) | 0 (0/13) | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrowolska, K.; Zarębska-Michaluk, D.; Brzdęk, M.; Rzymski, P.; Rogalska, M.; Moniuszko-Malinowska, A.; Kozielewicz, D.; Hawro, M.; Rorat, M.; Sikorska, K.; et al. Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings. J. Clin. Med. 2023, 12, 2371. https://doi.org/10.3390/jcm12062371
Dobrowolska K, Zarębska-Michaluk D, Brzdęk M, Rzymski P, Rogalska M, Moniuszko-Malinowska A, Kozielewicz D, Hawro M, Rorat M, Sikorska K, et al. Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings. Journal of Clinical Medicine. 2023; 12(6):2371. https://doi.org/10.3390/jcm12062371
Chicago/Turabian StyleDobrowolska, Krystyna, Dorota Zarębska-Michaluk, Michał Brzdęk, Piotr Rzymski, Magdalena Rogalska, Anna Moniuszko-Malinowska, Dorota Kozielewicz, Marcin Hawro, Marta Rorat, Katarzyna Sikorska, and et al. 2023. "Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings" Journal of Clinical Medicine 12, no. 6: 2371. https://doi.org/10.3390/jcm12062371
APA StyleDobrowolska, K., Zarębska-Michaluk, D., Brzdęk, M., Rzymski, P., Rogalska, M., Moniuszko-Malinowska, A., Kozielewicz, D., Hawro, M., Rorat, M., Sikorska, K., Jaroszewicz, J., Kowalska, J., & Flisiak, R. (2023). Retrospective Analysis of the Effectiveness of Remdesivir in COVID-19 Treatment during Periods Dominated by Delta and Omicron SARS-CoV-2 Variants in Clinical Settings. Journal of Clinical Medicine, 12(6), 2371. https://doi.org/10.3390/jcm12062371











