The Relationship of CCL5 and CCR1 Variants with Response Rate and Survival Taking into Account Thalidomide/Bortezomib Treatment in Patients with Multiple Myeloma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. DNA Isolation
2.3. Genotyping
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Bortezomib In Vitro Treatment
2.6. Statistical Analysis
3. Results
3.1. Frequencies of Alleles and Genotypes and Their Association with MM Risk
3.2. CCL5 and CCR1 Variants as a Risk Factors of Death and MM Progression
3.3. Association of Studied Variants with Clinical/Laboratory Values
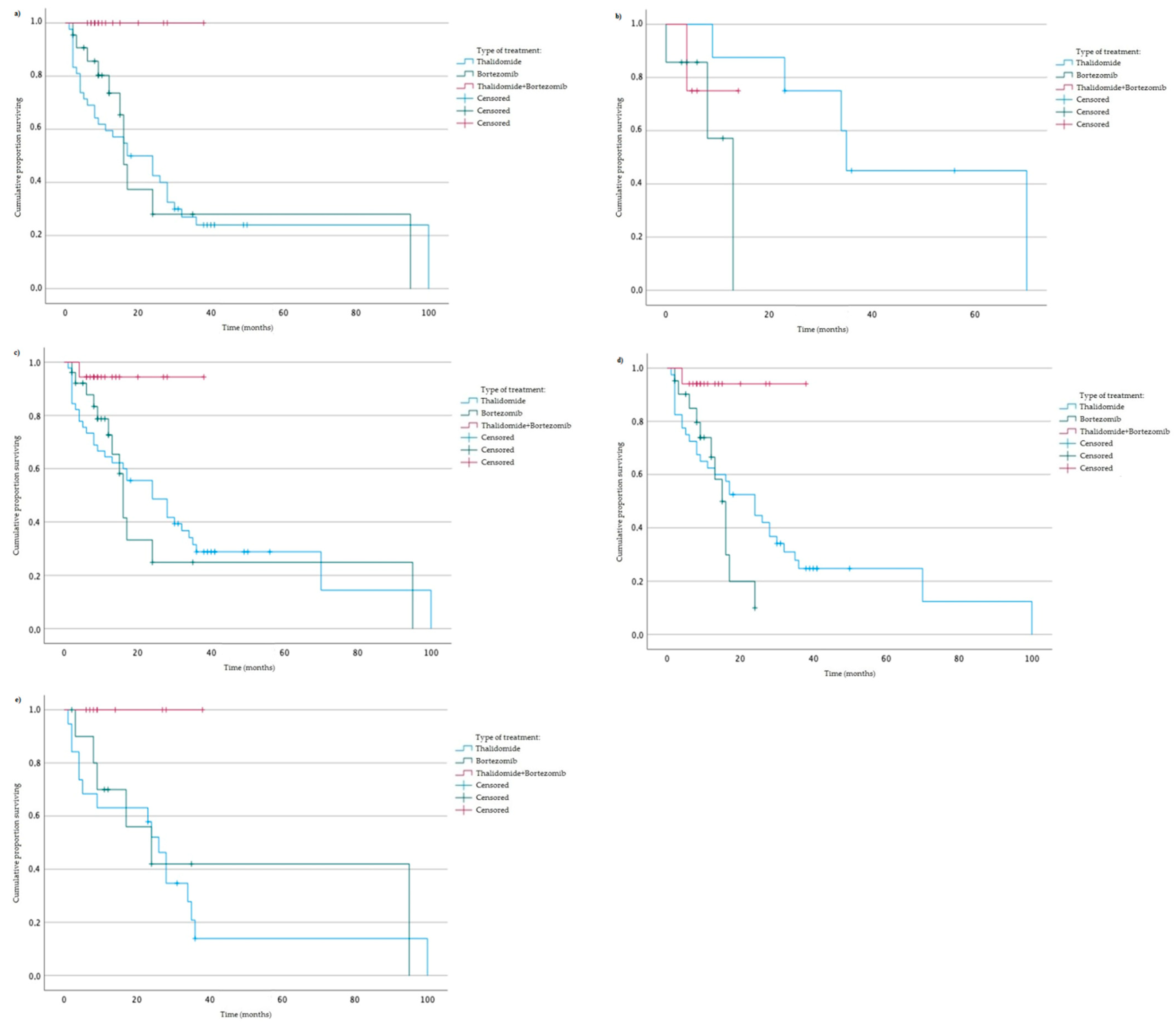
3.4. Survival of MM Patients Taking into Account Type of Tratment and Studied Variants
3.5. Levels of RANTES/CCL5 in Serum of MM Patients
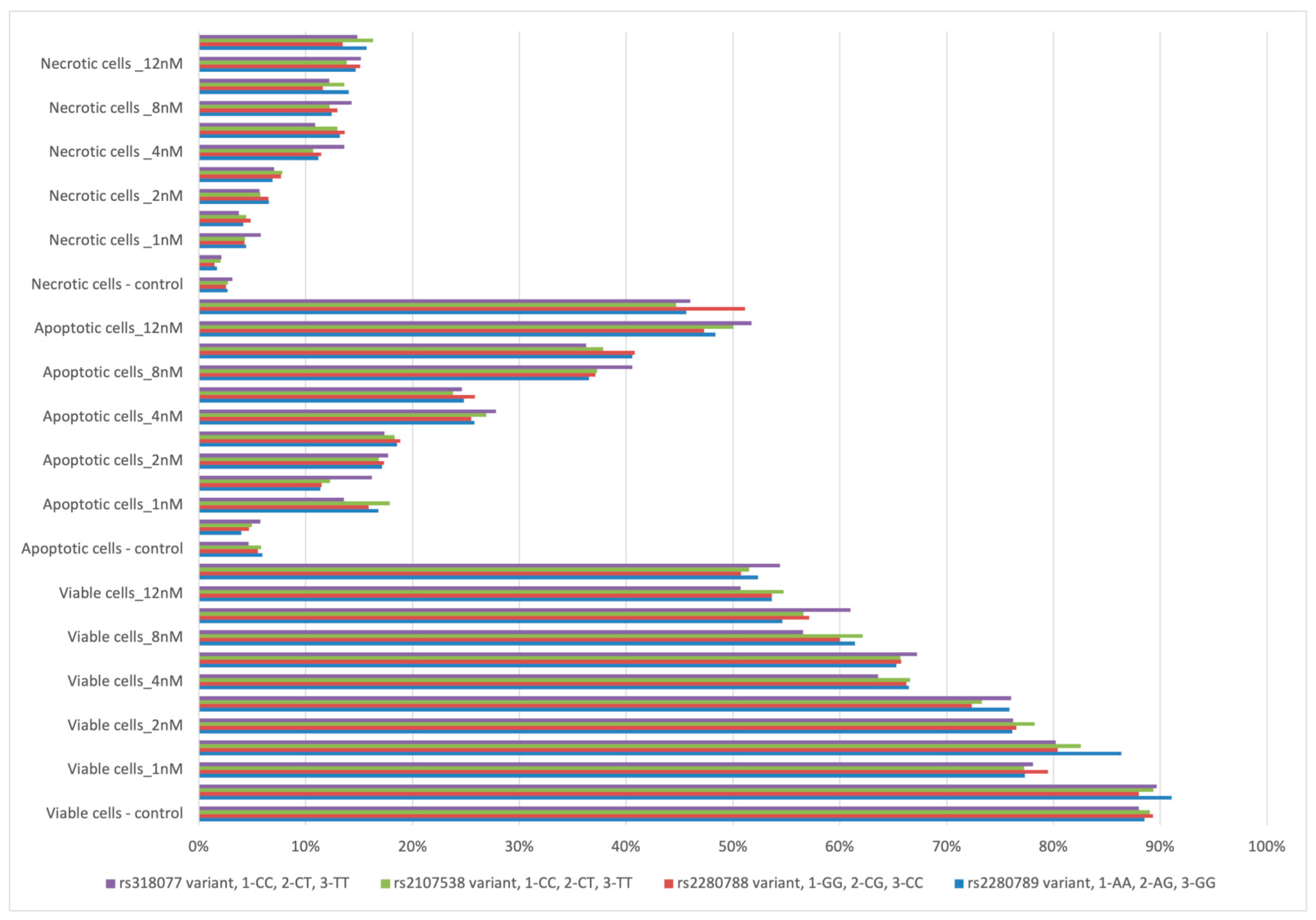
3.6. Bortezomib In Vitro Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma. JAMA 2022, 327, 464. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Botta, C.; Correale, P.; Tassone, P.; Tagliaferri, P. Immunologic microenvironment and personalized treatment in multiple myeloma. Expert Opin. Biol. Ther. 2013, 13, S83–S93. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Berardi, S.; Frassanito, M.A.; Ria, R.; De Re, V.; Cicco, S.; Battaglia, S.; Ditonno, P.; Dammacco, F.; Vacca, A.; et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood 2015, 126, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.; Caetano, J.; Ferreira, B.; Barahona, F.; Carneiro, E.; João, C. The Immune Microenvironment in Multiple Myeloma: Friend or Foe? Cancers 2021, 13, 625. [Google Scholar] [CrossRef]
- Minnie, S.A.; Kuns, R.D.; Gartlan, K.H.; Zhang, P.; Wilkinson, A.; Samson, L.; Guillerey, C.; Engwerda, C.; Macdonald, K.P.A.; Smyth, M.J.; et al. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood 2018, 132, 1675–1688. [Google Scholar] [CrossRef]
- De Magalhães, R.J.P.; Vidriales, M.-B.; Paiva, B.; Fernandez-Gimenez, C.; García-Sanz, R.; Mateos, M.-V.; Gutierrez, N.C.; Lecrevisse, Q.; Blanco, J.F.; Hernández, J.; et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica 2013, 98, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Cai, Z.; Wang, S.; Zhang, X.; Qian, J.; Hong, S.; Li, H.; Wang, M.; Yang, J.; Yi, Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug–induced apoptosis. Blood 2009, 114, 3625–3628. [Google Scholar] [CrossRef] [Green Version]
- Robak, P.; Węgłowska, E.; Dróżdż, I.; Mikulski, D.; Jarych, D.; Ferlińska, M.; Wawrzyniak, E.; Misiewicz, M.; Smolewski, P.; Fendler, W.; et al. Cytokine and Chemokine Profile in Patients with Multiple Myeloma Treated with Bortezomib. Mediat. Inflamm. 2020, 2020, 1835836. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and Anti-Inflammatory Equilibrium, Proliferative and Antiproliferative Balance: The Role of Cytokines in Multiple Myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.S.; Shi, Q.; Shah, N.D.; Heijnen, C.J.; Cohen, E.N.; Reuben, J.M.; Orlowski, R.Z.; Qazilbash, M.H.; Johnson, V.E.; Williams, L.A.; et al. Inflammatory Markers and Development of Symptom Burden in Patients with Multiple Myeloma during Autologous Stem Cell Transplantation. Clin. Cancer Res. 2014, 20, 1366–1374. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Ali, J.; Munawar, K.; Arshad, F.; Badar, F.; Siddiqui, N. Characteristics and outcomes of patients with multiple myeloma: Data from a developing country. Med J. Islam. Repub. Iran 2018, 32, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, R.; Ghobrial, I.M.; Roodman, G.D. Chemokines in multiple myeloma. Exp. Hematol. 2006, 34, 1289–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Luetkens, T.; Kobold, S.; Hildebrandt, Y.; Gordic, M.; Lajmi, N.; Meyer, S.; Bartels, K.; Zander, A.R.; Bokemeyer, C.; et al. The cytokine/chemokine pattern in the bone marrow environment of multiple myeloma patients. Exp. Hematol. 2010, 38, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Derossi, D.R.; Amarante, M.K.; Guembarovski, R.L.; de Oliveira, C.E.C.; Suzuki, K.M.; Watanabe, M.A.E.; de Syllos Cólus, I.M. CCL5 protein level: Influence on breast cancer staging and lymph nodes commitment. Mol. Biol. Rep. 2019, 46, 6165–6170. [Google Scholar] [CrossRef]
- Tsukishiro, S.; Suzumori, N.; Nishikawa, H.; Arakawa, A.; Suzumori, K. Elevated serum RANTES levels in patients with ovarian cancer correlate with the extent of the disorder. Gynecol. Oncol. 2006, 102, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.R.; Sima, H.R.; Rafatpanah, H.; Hosseinnezhad, H.; Ghaffarzadehgan, K.; Valizadeh, N.; Bahar, M.M.; Hakimi, H.R.; Masoom, A.; Noorbakhsh, A.; et al. Serum Chemokine Ligand 5 (CCL5/RANTES) Level Might be Utilized as a Predictive Marker of Tumor Behavior and Disease Prognosis in Patients with Gastric Adenocarcinoma. J. Gastrointest. Cancer 2014, 45, 476–480. [Google Scholar] [CrossRef]
- Suenaga, M.; Mashima, T.; Kawata, N.; Wakatsuki, T.; Horiike, Y.; Matsusaka, S.; Dan, S.; Shinozaki, E.; Seimiya, H.; Mizunuma, N.; et al. Serum VEGF-A and CCL5 levels as candidate biomarkers for efficacy and toxicity of regorafenib in patients with metastatic colorectal cancer. Oncotarget 2016, 7, 34811–34823. [Google Scholar] [CrossRef]
- Suenaga, M.; Stintzing, S.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Schirripa, M.; et al. Role of CCL5 and CCR5 gene polymorphisms in epidermal growth factor receptor signalling blockade in metastatic colorectal cancer: Analysis of the FIRE-3 trial. Eur. J. Cancer 2019, 107, 100–114. [Google Scholar] [CrossRef]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Oba, Y.; Lee, J.W.; Ehrlich, L.A.; Chung, H.Y.; Jelinek, D.F.; Callander, N.S.; Horuk, R.; Choi, S.J.; Roodman, G.D. MIP-1α utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp. Hematol. 2005, 33, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Menu, E.; De Leenheer, E.; De Raeve, H.; Coulton, L.; Imanishi, T.; Miyashita, K.; Van Valckenborgh, E.; Van Riet, I.; Van Camp, B.; Horuk, R.; et al. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: A study in the 5TMM model. Clin. Exp. Metastasis 2006, 23, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Gloghini, A.; Pinto, A.; Colombatti, A.; Carbone, A. The role of CD40/CD40L and interferon regulatory factor 4 in Hodgkin lymphoma microenvironment. Leuk. Lymphoma 2012, 53, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, D.; Casagrande, N. Inhibition of the CCL5/CCR5 Axis against the Progression of Gastric Cancer. Int. J. Mol. Sci. 2018, 19, 1477. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Nelson, G.W.; Wang, L.; Donfield, S.; Goedert, J.J.; Phair, J.; Vlahov, D.; Buchbinder, S.; Farrar, W.L.; Modi, W.; et al. Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proc. Natl. Acad. Sci. USA 2002, 99, 10002–10007. [Google Scholar] [CrossRef] [Green Version]
- Dossou-Yovo, O.P.; Zaccaria, I.; Benkerrou, M.; Hauchecorne, M.; Alberti, C.; Rahimy, M.C.; Elion, J.; Lapoumeroulie, C. Effects ofRANTESandMBL2gene polymorphisms in sickle cell disease clinical outcomes: Association of the g.In1.1T>CRANTESvariant with protection against infections. Am. J. Hematol. 2009, 84, 378–380. [Google Scholar] [CrossRef]
- Kalai, M.; Chaouch, L.; Ben Mansour, I.; Hafsia, R.; Ghanem, A.; Abbes, S. Frequency of three polymorphisms of the CCL5 gene (rs2107538, rs2280788 and rs2280789) and their implications for the phenotypic expression of sickle cell anemia in Tunisia. Pol. J. Pathol. 2013, 2, 84–89. [Google Scholar] [CrossRef]
- Liou, J.-M.; Lin, J.-T.; Huang, S.-P.; Wu, C.-Y.; Wang, H.-P.; Lee, Y.-C.; Chiu, H.-M.; Shun, C.-T.; Lin, M.-T.; Wu, M.-S. RANTES-403 polymorphism is associated with reduced risk of gastric cancer in women. J. Gastroenterol. 2008, 43, 115–123. [Google Scholar] [CrossRef]
- Sáenz-López, P.; Carretero, R.; Cózar, J.M.; Romero, J.M.; Canton, J.; Vilchez, J.R.; Tallada, M.; Garrido, F.; Ruiz-Cabello, F. Genetic polymorphisms of RANTES, IL1-A, MCP-1 and TNF-A genes in patients with prostate cancer. BMC Cancer 2008, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Shan, J.; Chouchane, A.; Mokrab, Y.; Saad, M.; Boujassoum, S.; Sayaman, R.W.; Ziv, E.; Bouaouina, N.; Remadi, Y.; Gabbouj, S.; et al. Genetic Variation in CCL5 Signaling Genes and Triple Negative Breast Cancer: Susceptibility and Prognosis Implications. Front. Oncol. 2019, 9, 1328. [Google Scholar] [CrossRef] [Green Version]
- Duell, E.J.; Casella, D.P.; Burk, R.D.; Kelsey, K.T.; Holly, E.A. Inflammation, Genetic Polymorphisms in Proinflammatory Genes TNF-A, RANTES, and CCR5, and Risk of Pancreatic Adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, A.; Rajkumar, S.V.; San Miguel, J.F.; Larocca, A.; Niesvizky, R.; Morgan, G.; Landgren, O.; Hajek, R.; Einsele, H.; Anderson, K.C.; et al. International Myeloma Working Group Consensus Statement for the Management, Treatment, and Supportive Care of Patients With Myeloma Not Eligible for Standard Autologous Stem-Cell Transplantation. J. Clin. Oncol. 2014, 32, 587–600. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Harousseau, J.-L.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federal Drug Administration. Guidance for Industry: Clinical Trial Endpoints for the Approval of Non-Small Cell Lung Cancer Drugs and Biologics. Available online: https://www.fda.gov/media/71195/download (accessed on 14 February 2021).
- Zmorzyński, S.; Popek-Marciniec, S.; Szudy-Szczyrek, A.; Wojcierowska-Litwin, M.; Korszeń-Pilecka, I.; Chocholska, S.; Styk, W.; Hus, M.; Filip, A.A. The Association of GSTT1, GSTM1, and TNF-α Polymorphisms With the Risk and Outcome in Multiple Myeloma. Front. Oncol. 2019, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Preacher, K.J. Calculation for the Chi-Square Test: An Interactive Calculation Tool for Chi-Square Tests of Goodness of Fit and Independence. 2019. Available online: http://quantpsy.org/ (accessed on 2 November 2022).
- Zmorzyński, S.; Popek-Marciniec, S.; Styk, W.; Wojcierowska-Litwin, M.; Korszeń-Pilecka, I.; Szudy-Szczyrek, A.; Chocholska, S.; Hus, M.; Filip, A.A. The Impact of the NOD2/CARD15 Variant (3020insC) and PSMA6 Polymorphism (-8C>G) on the Development and Outcome of Multiple Myeloma. BioMed Res. Int. 2020, 2020, 7629456. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Kastritis, E.; Dimopoulos, M.A.; Lonial, S. Treatment options for relapsed and refractory multiple myeloma. Blood 2015, 125, 3085–3099. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Harousseau, J.-L.; Durie, B.; Anderson, K.C.; Dimopoulos, M.; Kyle, R.; Blade, J.; Richardson, P.; Orlowski, R.; Siegel, D.; et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 2011, 117, 4691–4695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.-Y.; Lin, Y.-C.; Mahalingam, J.; Huang, C.-T.; Chen, T.-W.; Kang, C.-W.; Peng, H.-M.; Chu, Y.-Y.; Chiang, J.-M.; Dutta, A.; et al. Tumor-Derived Chemokine CCL5 Enhances TGF-β–Mediated Killing of CD8+ T Cells in Colon Cancer by T-Regulatory Cells. Cancer Res. 2012, 72, 1092–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Qin, J.; Zhong, L.; Gong, L.; Zhang, B.; Zhang, Y.; Gao, W.-Q. CCL5-Mediated Th2 Immune Polarization Promotes Metastasis in Luminal Breast Cancer. Cancer Res. 2015, 75, 4312–4321. [Google Scholar] [CrossRef] [Green Version]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget 2015, 6, 2981–2991. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, F.; Ping, Y.; Wang, L.; Chen, X.; Wang, D.; Cao, L.; Zhao, S.; Li, B.; Kalinski, P.; et al. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget 2015, 6, 24978–24989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskandari-Nasab, E.; Hashemi, M.; Ebrahimi, M.; Amininia, S.; Bahari, G.; Mashhadi, M.-A.; Taheri, M. Evaluation of CCL5-403 G>A and CCR5 Δ32 gene polymorphisms in patients with breast cancer. Cancer Biomark. 2014, 14, 343–351. [Google Scholar] [CrossRef]
- Wysocki, C.A.; Panoskaltsis-Mortari, A.; Blazar, B.R.; Serody, J.S. Leukocyte migration and graft-versus-host disease. Blood 2005, 105, 4191–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, G.R.; Krenger, W.; Ferrara, J.L.M. Cytokine Dysregulation in Acute Graft-versus-Host Disease. Hematology 1997, 2, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.W.; Hildebrandt, G.C.; Olkiewicz, K.M.; Hanauer, D.A.; Chaudhary, M.N.; Silva, I.A.; Rogers, C.E.; Deurloo, D.T.; Fisher, J.M.; Liu, C.; et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood 2007, 110, 3447–3455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Du Jung, H.; Lee, N.Y.; Sohn, S.K. Single Nucleotide Polymorphism of CC Chemokine Ligand 5 Promoter Gene in Recipients May Predict the Risk of Chronic Graft-Versus-Host Disease and its Severity After Allogeneic Transplantation. Transplantation 2007, 84, 917–925. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Kim, I.; Kim, J.H.; Lee, Y.-G.; Kang, E.J.; Cho, H.J.; Lee, K.-H.; Kim, H.J.; Park, E.-H.; Lee, J.-E.; et al. RANTES Polymorphisms and the Risk of Graft-versus-Host Disease in Human Leukocyte Antigen-Matched Sibling Allogeneic Hematopoietic Stem Cell Transplantation. Acta Haematol. 2013, 129, 137–145. [Google Scholar] [CrossRef]
- Singh, B.; Anbalagan, S.; Selvaraj, P. Regulatory role of CCL5 (rs2280789) and CXCL10 (rs56061981) gene polymorphisms on intracellular CCL5 and CXCL10 expression in pulmonary tuberculosis. Hum. Immunol. 2017, 78, 430–434. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, L.; Zeng, X.; Wu, H.; Liang, F.; Lv, Q.; Du, Z. CCL5 mediates breast cancer metastasis and prognosis through CCR5/Treg cells. Front. Oncol. 2022, 12, 972383. [Google Scholar] [CrossRef]
- Verheye, E.; Melgar, J.B.; Deschoemaeker, S.; Raes, G.; Maes, A.; De Bruyne, E.; Menu, E.; Vanderkerken, K.; Laoui, D.; De Veirman, K. Dendritic Cell-Based Immunotherapy in Multiple Myeloma: Challenges, Opportunities, and Future Directions. Int. J. Mol. Sci. 2022, 23, 904. [Google Scholar] [CrossRef]
- Nencioni, A.; Schwarzenberg, K.; Brauer, K.M.; Schmidt, S.M.; Ballestrero, A.; Grünebach, F.; Brossart, P. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood 2006, 108, 551–558. [Google Scholar] [CrossRef]
- Sallusto, F.; Mackay, C.R.; Lanzavecchia, A. The Role of Chemokine Receptors in Primary, Effector, and Memory Immune Responses. Annu. Rev. Immunol. 2000, 18, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara-Ota, S.; Shimura, Y.; Steinebach, C.; Isa, R.; Yamaguchi, J.; Nishiyama, D.; Fujibayashi, Y.; Takimoto-Shimomura, T.; Mizuno, Y.; Matsumura-Kimoto, Y.; et al. Lenalidomide and pomalidomide potently interfere with induction of myeloid-derived suppressor cells in multiple myeloma. Br. J. Haematol. 2020, 191, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Lokensgard, J.R.; Hu, S.; Van Fenema, E.M.; Sheng, W.S.; Peterson, P.K. Effect of Thalidomide on Chemokine Production by Human Microglia. J. Infect. Dis. 2000, 182, 983–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.C. Lenalidomide and Thalidomide: Mechanisms of Action—Similarities and Differences. Semin. Hematol. 2005, 42, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- van Veen, T.; Nielsen, J.; Berkhof, J.; Barkhof, F.; Kamphorst, W.; Bö, L.; Ravid, R.; Verweij, C.L.; Huitinga, I.; Polman, C.H.; et al. CCL5 and CCR5 genotypes modify clinical, radiological and pathological features of multiple sclerosis. J. Neuroimmunol. 2007, 190, 157–164. [Google Scholar] [CrossRef]
- Zhernakova, A.; Alizadeh, B.Z.; Eerligh, P.; Hanifi-Moghaddam, P.; Schloot, N.C.; Diosdado, B.; Wijmenga, C.; Roep, B.O.; Koeleman, B.P.C. Genetic variants of RANTES are associated with serum RANTES level and protection for type 1 diabetes. Genes Immun. 2006, 7, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Schaller, M.A.; Kallal, L.E.; Lukacs, N.W. A Key Role for CC Chemokine Receptor 1 in T-Cell-Mediated Respiratory Inflammation. Am. J. Pathol. 2008, 172, 386–394. [Google Scholar] [CrossRef] [Green Version]
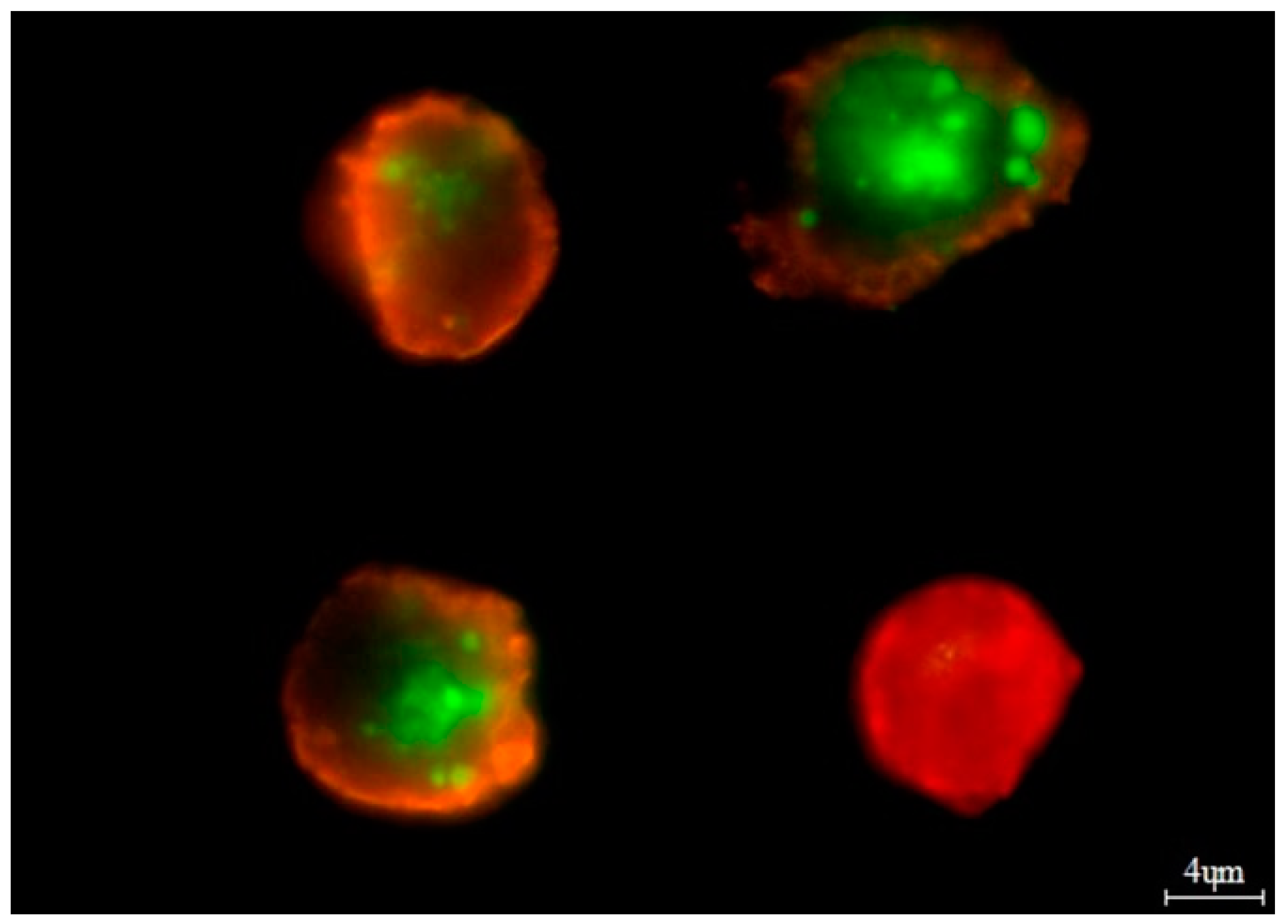

| Variables | MM Patients, n = 101 |
|---|---|
| Age (years) | 65.46 |
| Sex | |
| Male | 53 |
| Female | 48 |
| Type of MM * | |
| IgG | 56 |
| IgA | 26 |
| Light chain | 19 |
| Stage according to the International Staging System * | |
| I | 28 |
| II | 30 |
| III | 43 |
| Smoking | |
| Yes | 20 |
| No: Non-smokers | 69 |
| No: Ex-smokers | 12 |
| Exposure to carcinogenic factors | |
| Yes | 28 |
| No | 73 |
| Renal failure * | |
| No | 82 |
| Yes | 19 |
| The stage of chronic kidney disease (grade) | |
| G1 | 30 |
| G2 | 28 |
| G3A | 16 |
| G3B | 12 |
| G4 | 7 |
| G5 | 8 |
| Anemia grade before treatment (WHO) | |
| Absent | 28 |
| I—mild | 33 |
| II—moderate | 30 |
| III—severe | 10 |
| Cytogenetic changes * | |
| del(17p13.1) | 14 |
| t(4;14) | 15 |
| Other types | 2 |
| Chemotherapy | |
| Cyclophosphamide, Thalidomide, and Dexamethasone (CTD) | 50 |
| Velcade, Cyclophosphamide, Dexamethasone (VCD) | 29 |
| Velcade, Thalidomide, and Dexamethasone (VTD) | 20 |
| Died before chemotherapy | 2 |
| Inclusion Criteria for MM Patients |
|---|
| -Newly diagnosed MM patients. -Signed informed consent -18 years of age or older -Measurable disease, defined as follows:
-Life expectancy more than 3 months -Successful genotyping |
| Inclusion criteria for control group |
| -18 years of age or older -Signed informed consent -Successful genotyping |
| Exclusion criteria for MM patients |
| -Active smoldering MM -Active plasma cell leukemia -Documented systemic amyloid light chain amyloidosis -Active central nervous system involvement with MM -Other active hematologic malignancy or solid tumor |
| Exclusion criteria for Control group |
| -Known to be infected with HIV, syphilis, tuberculosis, hepatitis B, or hepatitis C -A condition in which repeated blood draws or injections pose more than minimal risk for the subject such as hemophilia, other severe coagulation disorders, or significantly impaired venous access -A condition that requires active medical intervention or monitoring to avert serious danger to the participant’s health or well-being |
| GROUPS | GENOTYPES | Total | HWE p Value and χ2 * | ||
|---|---|---|---|---|---|
| CCL5 gene rs2280789 | |||||
| - | AA | AG | GG | - | - |
| CONTROL | |||||
| E | 85.56 | 13.87 | 0.56 | 100 | p = 0.51, χ2 = 0.42 |
| O | 86 | 13 | 1 | 100 | |
| CASE | |||||
| E | 80.19 | 19.6 | 1.19 | 101 | p = 0.09 χ2 = 2.84 |
| O | 82 | 16 | 3 | 101 | |
| CCL5 gene rs2280788 | |||||
| - | GG | CG | CC | - | - |
| CONTROL | |||||
| E | 89.3 | 10.39 | 0.3 | 100 | p = 0.18, χ2 = 1.73 |
| O | 90 | 9 | 1 | 100 | |
| CASE | |||||
| E | 91.24 | 9.5 | 0.24 | 101 | p = 0.55, χ2 = 0.34 |
| O | 91 | 10 | 0 | 101 | |
| CCL5 gene rs2107538 | |||||
| - | CC | CT | TT | - | - |
| CONTROL | |||||
| E | 66.42 | 30.15 | 3.42 | 100 | p = 0.71, χ2 = 0.13 |
| O | 67 | 29 | 4 | 100 | |
| CASE | |||||
| E | 78.42 | 21.14 | 1.42 | 101 | p = 0.17, χ2 = 1.83 |
| O | 80 | 18 | 3 | 101 | |
| CCR1 gene rs318077 | |||||
| - | TT | CT | CC | - | - |
| CONTROL | |||||
| E | 44.89 | 44.22 | 10.89 | 100 | p = 0.68, χ2 = 0.16 |
| O | 46 | 42 | 12 | 100 | |
| CASE | |||||
| E | 43.12 | 45.74 | 12.12 | 101 | p = 0.3, χ2 = 1.05 |
| O | 40 | 52 | 9 | 101 | |
| CCL5 Variants | Individuals | D Value | Dmax Value | D’Value | r2 Value |
|---|---|---|---|---|---|
| rs2280789 and rs2280788 | MM patients | 0.026 | 0.045 | 0.57 | 0.18 |
| Control group | 0.023 | 0.036 | 0.64 | 0.25 | |
| rs2280789 and rs2107538 | MM patients | 0.050 | 0.090 | 0.55 | 0.31 |
| Control group | 0.035 | 0.065 | 0.54 | 0.09 | |
| rs2280788 and rs2107538 | MM patients | 0.040 | 0.044 | 0.90 | 0.20 |
| Control group | 0.029 | 0.032 | 0.91 | 0.13 |
| Gene Variants and Alleles | MM n (%) | Controls n (%) | Odds Ratio | 95% CI | p Values |
|---|---|---|---|---|---|
| CCL5 rs2280789 | |||||
| AA | 82 (81.18%) | 86 (86%) | 1 | - | - |
| AG | 16 (15.84%) | 13 (13%) | 0.77 | 0.35–1.71 | 0.52 |
| GG | 3 (2.97%) | 1 (1%) | 3.14 | 0.32–30.86 | 0.59 |
| AG + GG | 19 (18.81%) | 14 (14%) | 0.70 | 0.33–1.49 | 0.35 |
| Total: | 101 (100%) | 100 (100%) | |||
| A | 180 (89.1%) | 185 (92.5%) | 1 | - | - |
| G | 22 (10.9%) | 15 (7.5%) | 0.66 | 0.33–1.31 | 0.24 |
| Total: | 202 (100%) | 200 (100%) | |||
| CCL5 rs2280788 | |||||
| GG | 91 (90.1%) | 90 (90%) | 1 | - | - |
| CG | 10 (9.9%) | 9 (9%) | 0.91 | 0.35–2.34 | 0.84 |
| CC * | 0 (0%) | 1 (1%) | |||
| CG + CC | 10 (9.9%) | 10 (10%) | 1.01 | 0.40–2.54 | 1.0 |
| Total: | 101 (100%) | 100 (100%) | |||
| G | 192 (95%) | 193 (96,5%) | 1 | - | - |
| C | 10 (5%) | 7 (3.5%) | 0.69 | 0.25–1.86 | 0.47 |
| Total: | 202 (100%) | 200 (100%) | |||
| CCL5 rs2107538 | |||||
| CC | 80 (79.2%) | 67 (67%) | 1 | - | - |
| CT | 18 (17.8%) | 29 (29%) | 1.92 | 0.98–3.76 | 0.05 |
| TT | 3 (2.97%) | 4 (4%) | 1.59 | 0.34–7.36 | 0.83 |
| CT + TT | 21 (20.77%) | 33 (33%) | 1.87 | 0.99–3.64 | 0.05 |
| Total: | 101 (100%) | 100 (100%) | |||
| C | 178 (88.1%) | 163 (81.5%) | 1 | - | - |
| T | 24 (11.8%) | 37 (18.5%) | 1.68 | 0.96–2.93 | 0.06 |
| Total: | 202 (100%) | 200 (100%) | |||
| CCR1 rs318077 | |||||
| TT | 40 (39.6%) | 46 (46%) | 1 | - | - |
| CT | 52 (51.5%) | 42 (42%) | 0.70 | 0.39–1.26 | 0.23 |
| CC | 9 (8.9%) | 12 (12%) | 1.16 | 0.44–3.03 | 0.76 |
| CT + CC | 61 (60.4%) | 54 (54%) | 0.76 | 0.43–1.34 | 0.35 |
| Total: | 101 (100%) | 100 (100%) | |||
| T | 131 (65%) | 134 (67%) | 1 | - | - |
| C | 71 (35%) | 66 (33%) | 0.90 | 0.60–1.37 | 0.64 |
| Total: | 202 (100%) | 200 (100%) | |||
| Variable | Univariate Cox Analysis for OS | Univariate Cox Analysis for PFS | ||||
|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |
| ISS | ||||||
| I + II | - | R | - | R | - | |
| III | 0.004 | 2.78 | 1.40–5.38 | <0.001 | 2.79 | 1.61–4.84 |
| Auto-HSCT | ||||||
| yes | <0.001 | 0.18 | 0.07–0.46 | 0.03 | 0.39 | 0.21–0.72 |
| no | - | R | - | - | R | - |
| CCL5 rs2280789 | ||||||
| AA | - | R | - | - | R | - |
| AG + GG | 0.21 | 0.51 | 0.17–1.43 | 0.73 | 0.88 | 0.43–1.81 |
| CCL5 rs2280788 | ||||||
| GG | - | R | - | - | R | - |
| CG + CC | 0.64 | 0.75 | 0.22–2.50 | 0.31 | 1.61 | 0.63–4.12 |
| CCL5 rs2107538 | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.12 | 0.46 | 0.14–1.20 | 0.17 | 0.59 | 0.27–1.26 |
| CCR1 rs318077 | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.90 | 0.98 | 0.70–1.38 | 0.58 | 0.92 | 0.70–1.22 |
| Variable | Multivariate Cox Analysis for OS | Multivariate Cox Analysis for PFS | ||||
|---|---|---|---|---|---|---|
| p Value | HR | 95% CI | p Value | HR | 95% CI | |
| ISS | ||||||
| I + II | - | R | - | - | R | - |
| III | 0.049 | 2.16 | 1.0–4.66 | 0.001 | 2.80 | 1.50–5.20 |
| Auto-HSCT | ||||||
| yes | - | R | - | - | R | - |
| no | 0.03 | 0.19 | 0.07–0.56 | 0.046 | 0.49 | 0.24–0.99 |
| CCL5 rs2280789 | ||||||
| AA | - | R | - | - | R | - |
| AG + GG | 0.74 | 0.80 | 0.20–3.17 | 0.82 | 1.10 | 0.45–2.70 |
| CCL5 rs2280788 | ||||||
| GG | - | R | - | - | R | - |
| CG + CC | 0.15 | 3.77 | 0.61–23.34 | 0.01 | 4.77 | 1.42–15.99 |
| CCL5 rs2107538 | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.028 | 0.18 | 0.04–0.83 | 0.01 | 0.26 | 0.09–0.73 |
| CCR1 rs318077 | ||||||
| CC | - | R | - | - | R | - |
| CT + TT | 0.99 | 0.99 | 0.70–1.44 | 0.41 | 0.88 | 0.65–1.20 |
| Variable | Response Rate | |
|---|---|---|
| CR + VGPR + PR + SD | PD | |
| p Value | OR (95% CI) | |
| ISS | ||
| I + II | - | reference |
| III | 0.07 | 3.06 (0.65–14.39) |
| Auto-HSCT | ||
| yes | - | reference |
| no | 0.02 | 4.02 (1.26–12.87) |
| CCL5 rs2280789 | ||
| AA | - | reference |
| AG + GG | 0.01 | 1.10 (0.35–3.45) |
| CCL5 rs2280788 | ||
| GG | - | reference |
| CG + CC | 0.41 | 1.17 (0.28–4.82) |
| CCL5 rs2107538 | ||
| CC | - | reference |
| CT + TT | 0.92 | 0.93 (0.30–2.88) |
| CCR1 rs318077 | ||
| TT | - | reference |
| CT + CC | 0.86 | 0.92 (0.36–2.33) |
| Variables | MM Patients | CCL5 rs2280789 | CCL5 rs2280788 | CCL5 r rs2107538 | CCR1 rs318077 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG + GG | p-Value | GG | CG + CC | p-Value | CC | CT + TT | p-Value | TT | CT+ CC | p-Value | ||
| Mean age (years) * | 65.46 | 65.80 | 64.0 | 0.48 | 65.84 | 62.10 | 0.26 | 65.72 | 64.48 | 0.61 | 62.20 | 65.64 | 0.83 |
| Free light chain ratio * | 303.05 | 332.9 | 178.6 | 0.44 | 309.3 | 248.0 | 0.81 | 302.9 | 303.7 | 0.98 | 433.0 | 224.2 | 0.20 |
| % of plasma cells in bone marrow * | 30.80 | 31.28 | 37.32 | 0.44 | 30.73 | 28.70 | 0.76 | 29.97 | 32.62 | 0.58 | 35.07 | 27.62 | 0.07 |
| Albumins (g/dL) * | 3.57 | 3.55 | 3.64 | 0.62 | 3.58 | 3.45 | 0.56 | 3.57 | 3.56 | 0.97 | 3.72 | 3.46 | 0.05 |
| β2-microglobulin * (mg/L) | 6.12 | 6.06 | 6.37 | 0.77 | 6.02 | 7.05 | 0.46 | 5.73 | 7.55 | 0.17 | 6.37 | 5.96 | 0.65 |
| Calcium * (mM/L) | 2.45 | 2.45 | 2.44 | 0.96 | 2.46 | 2.37 | 0.41 | 2.46 | 2.41 | 0.54 | 2.40 | 2.48 | 0.21 |
| Hemoglobin * (g/dL) | 10.40 | 10.41 | 10.33 | 0.87 | 10.45 | 9.90 | 0.38 | 10.44 | 10.21 | 0.62 | 10.06 | 10.61 | 0.15 |
| Creatinine * (mg/dL) | 1.60 | 1.67 | 1.15 | 0.05 | 1.60 | 1.31 | 0.60 | 1.58 | 1.54 | 0.93 | 1.74 | 1.47 | 0.43 |
| Platelets (K/μL) | 212.75 | 214.2 | 206.3 | 0.74 | 216.0 | 183.1 | 0.29 | 217.4 | 195.0 | 0.32 | 198.5 | 222.1 | 0.21 |
| C-reactive protein * (mg/L) | 15.53 | 17.54 | 6.56 | 0.02 | 16.54 | 6.11 | 0.39 | 18.47 | 4.82 | 0.05 | 10.50 | 18.86 | 0.25 |
| Estimated glomerular filtration rate * mL/min/1.73 m2 | 60.92 | 65.84 | 70.19 | 0.58 | 66.51 | 68.02 | 0.88 | 66.97 | 65.47 | 0.84 | 56.24 | 73.50 | 0.05 |
| Gene Variant | Genotypes | Number of Individuals | Mean Concentration (ng/mL) | Standard Deviation | p Value |
|---|---|---|---|---|---|
| rs2280789 | AA | 56 | 11.15 | 1.37 | <0.01 |
| GA + GG | 14 | 6.43 | 3.20 | ||
| rs2280788 | GG | 64 | 10.80 | 2.10 | <0.01 |
| CG + CC | 6 | 3.85 | 3.44 | ||
| rs2107538 | CC | 56 | 10.95 | 1.76 | <0.01 |
| CT + TT | 14 | 7.22 | 3.70 | ||
| rs3181077 | TT | 33 | 11.27 | 3.05 | 0.014 |
| CT + CC | 37 | 9.25 | 2.99 |
| CCL5 and CCR1 Variants | Frequency | Mean Concentration (ng/mL) | Standard Deviation | p Value | |||
|---|---|---|---|---|---|---|---|
| rs2280789 | rs2280788 | rs2107538 | rs3181077 | ||||
| AA | GG | CC | TT | 0.37 | 11.73 | 1.25 | 0.03 |
| AA | GG | CC | CT | 0.30 | 10.96 | 1.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popek-Marciniec, S.; Styk, W.; Wojcierowska-Litwin, M.; Szudy-Szczyrek, A.; Dudek, P.; Swiderska-Kolacz, G.; Czerwik-Marcinkowska, J.; Zmorzynski, S. The Relationship of CCL5 and CCR1 Variants with Response Rate and Survival Taking into Account Thalidomide/Bortezomib Treatment in Patients with Multiple Myeloma. J. Clin. Med. 2023, 12, 2384. https://doi.org/10.3390/jcm12062384
Popek-Marciniec S, Styk W, Wojcierowska-Litwin M, Szudy-Szczyrek A, Dudek P, Swiderska-Kolacz G, Czerwik-Marcinkowska J, Zmorzynski S. The Relationship of CCL5 and CCR1 Variants with Response Rate and Survival Taking into Account Thalidomide/Bortezomib Treatment in Patients with Multiple Myeloma. Journal of Clinical Medicine. 2023; 12(6):2384. https://doi.org/10.3390/jcm12062384
Chicago/Turabian StylePopek-Marciniec, Sylwia, Wojciech Styk, Magdalena Wojcierowska-Litwin, Aneta Szudy-Szczyrek, Paul Dudek, Grazyna Swiderska-Kolacz, Joanna Czerwik-Marcinkowska, and Szymon Zmorzynski. 2023. "The Relationship of CCL5 and CCR1 Variants with Response Rate and Survival Taking into Account Thalidomide/Bortezomib Treatment in Patients with Multiple Myeloma" Journal of Clinical Medicine 12, no. 6: 2384. https://doi.org/10.3390/jcm12062384






