Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of DPSCs
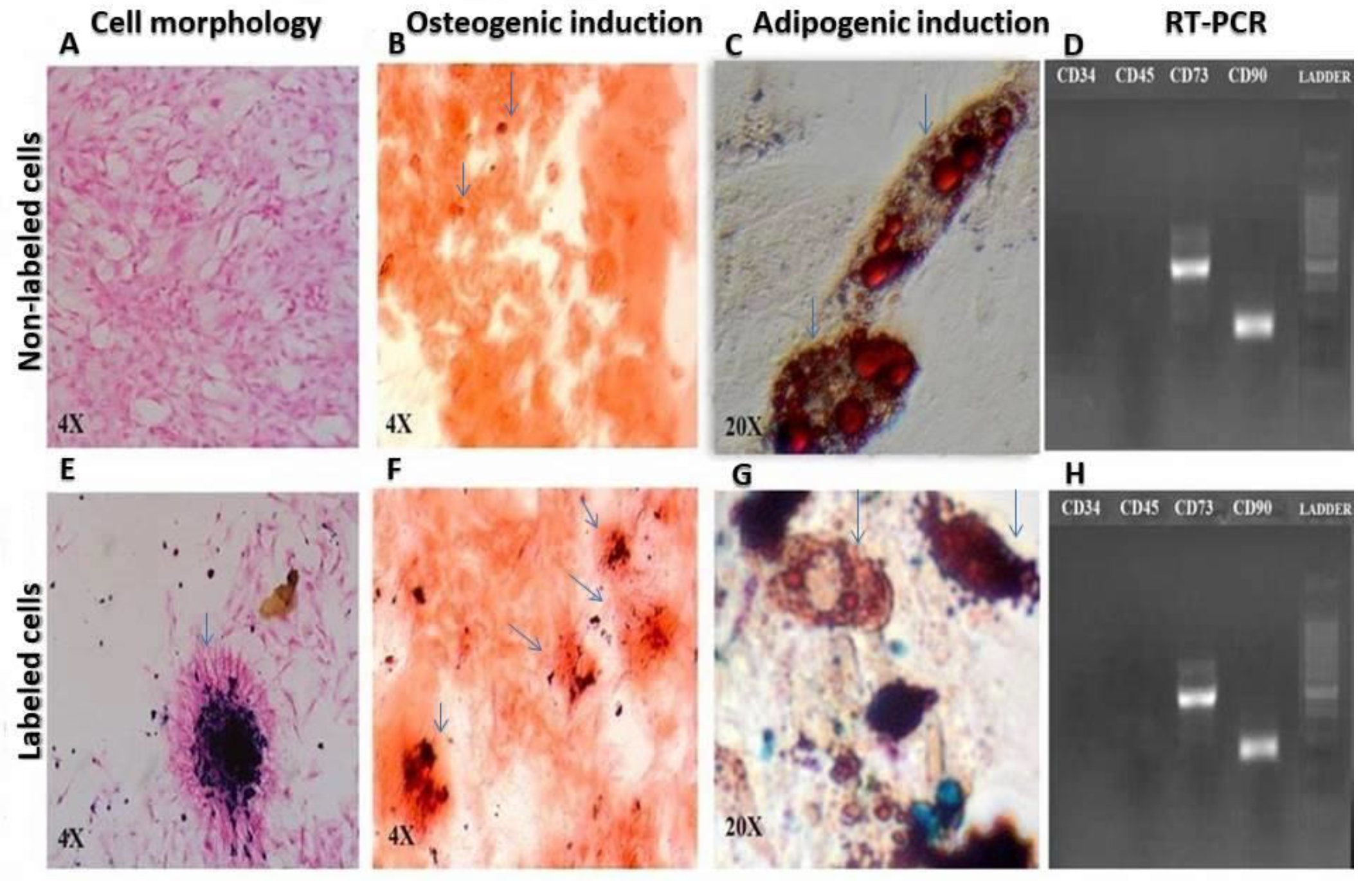
2.2. Cell Characterization
2.3. Growth Kinetics
2.4. Cell Labeling
2.5. The 3-4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT) Assay
2.6. In Vitro Tracking by MRI
2.7. In Vivo Tracking by MRI
2.8. Prussian Blue (PB) Staining
2.9. Bax and Bcl-2 Gene Expression Assay
2.10. Apoptosis Analysis by Flow Cytometry
2.11. Statistical Analysis
2.12. Ethics Approval
3. Results
3.1. Cell Characterization
3.2. MTT Assay
3.3. Growth Kinetics
3.4. In Vitro MRI
3.5. In Vivo MRI
3.6. Prussian Blue Staining
3.7. Bax and Bcl-2 Gene Expression
3.8. Apoptosis Analysis by Flow Cytometry
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kaboodkhani, R.; Mehrabani, D.; Karimi-Busheri, F. Achievements and Challenges in Transplantation of Mesenchymal Stem Cells in Otorhinolaryngology. J. Clin. Med. 2021, 10, 2940. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Asl, S.-K.; Mehrabani, D.; Karimi-Busheri, F. Therapeutic Effect of Mesenchymal Stem Cells in Ulcerative Colitis: A Review on Achievements and Challenges. J. Clin. Med. 2020, 9, 3922. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, D.; Arshi, S.; Sadeghi, L.; Khodabandeh, Z.; Zare, S.; Rabiee, M. The ameliorating effect of adipose tissue stem cells on liver function in experimental rats with liver fibrosis. Int. J. Nutr. Sci. 2022, 7, 225–232. [Google Scholar]
- Khodabandeh, Z.; Mehrabani, D.; Dehghani, F.; Gashmardi, N.; Erfanizadeh, M.; Zare, S.; Bozorg-Ghalati, F. Spinal cord injury repair using mesenchymal stem cells derived from bone marrow in mice: A stereological study. Acta Histochem. 2021, 123, 151720. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, D.; Rabiee, M.; Tamadon, A.; Zare, S.; Razeghian Jahromi, I.; Dianatpour, M.; Khodabandeh, Z. The growth kinetic, differentiation properties, karyotyping, and characterization of adipose tissue-derived stem cells in hamster. Comp. Clin. Pathol. 2016, 25, 1017–1022. [Google Scholar] [CrossRef]
- Borzou, B.; Mehrabani, D.; Zare, S.; Zamani-Pereshkaft, M.; Acker, J.P. The Effect of Age and Type of Media on Growth Kinetics of Human Amniotic Fluid Stem Cells. Biopreserv. Biobank. 2020, 18, 389–394. [Google Scholar] [CrossRef]
- Nazempour, M.; Mehrabani, D.; Mehdinavaz-Aghdam, R.; Hashemi, S.S.; Derakhshanfar, A.; Zare, S.; Zardosht, M.; Moayedi, J.; Vahedi, M. The effect of allogenic human Wharton’s jelly stem cells seeded onto acellular dermal matrix in healing of rat burn wounds. J. Cosmet. Dermatol. 2020, 19, 995–1001. [Google Scholar] [CrossRef]
- Mehrabani, D.; Mahdiyar, P.; Torabi, K.; Robati, R.; Zare, S.; Dianatpour, M.; Tamadon, A. Growth kinetics and characterization of human dental pulp stem cells: Comparison between third molar and first premolar teeth. J. Clin. Exp. Dent. 2017, 9, e172–e177. [Google Scholar] [CrossRef]
- Sholehvar, F.; Mehrabani, D.; Yaghmaei, P.; Vahdati, A. The effect of Aloe vera gel on viability of dental pulp stem cells. Dent. Traumatol. 2016, 32, 390–396. [Google Scholar] [CrossRef]
- Mattei, V.; Martellucci, S.; Pulcini, F.; Santilli, F.; Sorice, M.; Delle Monache, S. Regenerative Potential of DPSCs and Revascularization: Direct, Paracrine or Autocrine Effect? Stem Cell Rev. Rep. 2021, 17, 1635–1646. [Google Scholar] [CrossRef]
- Zare, S.; Mehrabani, D.; Jalli, R.; Saeedi Moghadam, M.; Manafi, N.; Mehrabani, G.; Jamhiri, I.; Ahadian, S. MRI-Tracking of Dental Pulp Stem Cells In Vitro and In Vivo Using Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles. J. Clin. Med. 2019, 8, 1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.; Hu, K.; Zhang, M.; Sheng, J.; Xu, X.; Tang, S.; Li, Y.; Yang, S.; Si, G.; Mao, Y.; et al. Extracellular magnetic labeling of biomimetic hydrogel-induced human mesenchymal stem cell spheroids with ferumoxytol for MRI tracking. Bioact. Mater. 2022, 19, 418–428. [Google Scholar] [CrossRef] [PubMed]
- García-Belda, P.; Prima-García, H.; Aliena-Valero, A.; Castelló-Ruiz, M.; Ulloa-Navas, M.J.; Ten-Esteve, A.; Martí-Bonmatí, L.; Salom, J.B.; García-Verdugo, J.M.; Gil-Perotín, S. Intravenous SPION-labeled adipocyte-derived stem cells targeted to the brain by magnetic attraction in a rat stroke model: An ultrastructural insight into cell fate within the brain. Nanomedicine 2022, 39, 102464. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Fung, K.L.B.; Zhou, X.Y.; Cui, W.; Colson, C.; Mai, D.; Jeffris, K.; Huynh, Q.; Saayujya, C.; Kabuli, L.; et al. Non-radioactive and sensitive tracking of neutrophils towards inflammation using antibody functionalized magnetic particle imaging tracers. Nanotheranostics 2021, 5, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Z.; Chen, R.; Xue, P.-P.; Luo, L.-Z.; Zhong, B.; Tong, M.-Q.; Chen, B.; Yao, Q.; Yuan, J.-D.; Xu, H.-L. Magnetic PLGA microspheres loaded with SPIONs promoted the reconstruction of bone defects through regulating the bone mesenchymal stem cells under an external magnetic field. Mater. Sci. Eng. C 2021, 122, 111877. [Google Scholar] [CrossRef] [PubMed]
- Iravani, K.; Mehrabani, D.; Doostkam, A.; Azarpira, N.; Iranpour, P.; Bahador, M.; Mehravar, S. Use of magnetic resonance imaging to assess the regenerative effects of adipose tissue-derived mesenchymal stem cells in a rabbit cartilaginous laryngeal defect model. Curr. Ther. Res. Clin. Exp. 2022, 97, 100682. [Google Scholar] [CrossRef]
- Arbab, A.S.; Bashaw, L.A.; Miller, B.R.; Jordan, E.K.; Bulte, J.W.; Frank, J.A. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: Methods and techniques. Transplantation 2003, 76, 1123–1130. [Google Scholar] [CrossRef]
- Mishra, S.K.; Khushu, S.; Gangenahalli, G. Effects of iron oxide contrast agent in combination with various transfection agents during mesenchymal stem cells labelling: An in vitro toxicological evaluation. Toxicol. Vitr. 2018, 50, 179–189. [Google Scholar] [CrossRef]
- Christiaens, O.; Petek, M.; Smagghe, G.; Taning, C.N.T. The use of nanocarriers to improve the efficiency of RNAi-based pesticides in agriculture. In Nanopesticides: From Research and Development to Mechanisms of Action and Sustainable Use in Agriculture; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–68. [Google Scholar]
- Yang, W.; Wang, B.; Lei, G.; Chen, G.; Liu, D. Advances in nanocarriers to improve the stability of dsRNA in the environment. Front. Bioeng. Biotechnol. 2022, 10, 974646. [Google Scholar] [CrossRef]
- Rui, Y.; Wilson, D.R.; Tzeng, S.Y.; Yamagata, H.M.; Sudhakar, D.; Conge, M.; Berlinicke, C.A.; Zack, D.J.; Tuesca, A.; Green, J.J. High-throughput and high-content bioassay enables tuning of polyester nanoparticles for cellular uptake, endosomal escape, and systemic in vivo delivery of mRNA. Sci. Adv. 2022, 8, eabk2855. [Google Scholar] [CrossRef]
- Ohkura, N.; Edanami, N.; Takeuchi, R.; Tohma, A.; Ohkura, M.; Yoshiba, N.; Yoshiba, K.; Ida-Yonemochi, H.; Ohshima, H.; Okiji, T.; et al. Effects of pulpotomy using mineral trioxide aggregate on prostaglandin transporter and receptors in rat molars. Sci. Rep. 2017, 7, 6870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkura, N.; Yoshiba, K.; Yoshiba, N.; Edanami, N.; Ohshima, H.; Takenaka, S.; Noiri, Y. SVCT2-GLUT1-mediated ascorbic acid transport pathway in rat dental pulp and its effects during wound healing. Sci. Rep. 2023, 13, 1251. [Google Scholar] [CrossRef]
- Bertassoli, B.M.; Costa, E.S.; Sousa, C.A.; Albergaria, J.D.S.; Maltos, K.L.M.; Goes, A.M.; Matins, T.M.D.M.; Silva, G.A.B.; Jorge, E.C. Rat dental pulp stem cells: Isolation and phenotypic characterization method aiming bone tissue bioengineering. Braz. Arch. Biol. Technol. 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Walboomers, X.F.; Wolke, J.G.; Bian, Z.; Fan, M.W.; Jansen, J.A. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005, 11, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Nagendrababu, V.; Kishen, A.; Murray, P.E.; Nekoofar, M.H.; de Figueiredo, J.A.P.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; Silva, R.M.; et al. PRIASE 2021 guidelines for reporting animal studies in Endodontology: A consensus-based development. Int. Endod. J. 2021, 54, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Santacroce, C.; Tasciotti, V.; Martellucci, S.; Santilli, F.; Manganelli, V.; Piccoli, L.; Misasi, R.; Sorice, M.; Garofalo, T. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp. Cell Res. 2015, 339, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Delle Monache, S.; Martellucci, S.; Clementi, L.; Pulcini, F.; Santilli, F.; Mei, C.; Piccoli, L.; Angelucci, A.; Mattei, V. In Vitro Conditioning Determines the Capacity of Dental Pulp Stem Cells to Function as Pericyte-Like Cells. Stem Cells Dev. 2019, 28, 695–706. [Google Scholar] [CrossRef]
- Masuda, K.; Han, X.; Kato, H.; Sato, H.; Zhang, Y.; Sun, X.; Hirofuji, Y.; Yamaza, H.; Yamada, A.; Fukumoto, S. Dental Pulp-Derived Mesenchymal Stem Cells for Modeling Genetic Disorders. Int. J. Mol. Sci. 2021, 22, 2269. [Google Scholar] [CrossRef]
- Malekzadeh, S.; Edalatmanesh, M.A.; Mehrabani, D.; Shariati, M. Dental Pulp Stem Cells Transplantation Improves Passive Avoidance Memory and Neuroinflammation in Trimethyltin-Induced Alzheimer’s Disease Rat Model. Galen Med. J. 2021, 10, e2254. [Google Scholar] [CrossRef]
- Yang, L.; Xia, Y.; Zhao, H.; Zhao, J.; Zhu, X. Magnetic resonance imaging of transplanted neural stem cells in Parkinson disease rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 489–492. [Google Scholar] [CrossRef]
- Liu, Y.; Gan, L.; Cui, D.-X.; Yu, S.-H.; Pan, Y.; Zheng, L.-W.; Wan, M. Epigenetic regulation of dental pulp stem cells and its potential in regenerative endodontics. World J. Stem Cells 2021, 13, 1647–1666. [Google Scholar] [CrossRef] [PubMed]
- Kwack, K.H.; Lee, H.-W. Clinical Potential of Dental Pulp Stem Cells in Pulp Regeneration: Current Endodontic Progress and Future Perspectives. Front. Cell Dev. Biol. 2022, 10, 857066. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Ma, Y.; Tan, S.; Ren, B.; Liu, S.; Dai, H.; Xu, Z. Application of dental pulp stem cells in oral maxillofacial tissue engineering. Int. J. Med. Sci. 2022, 19, 310–320. [Google Scholar] [CrossRef]
- Staniowski, T.; Zawadzka-Knefel, A.; Skośkiewicz-Malinowska, K. Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types. Molecules 2021, 26, 7423. [Google Scholar] [CrossRef]
- Moayeri, A.; Darvishi, M.; Amraei, M. Homing of Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) Labeled Adipose-Derived Stem Cells by Magnetic Attraction in a Rat Model of Parkinson’s Disease. Int. J. Nanomed. 2020, 15, 1297–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Feng, L.; Chen, Z.; Lan, Y.; Liu, Y.; Li, D.; Yan, C.; Xu, Y. Ultrasmall Superparamagnetic Iron Oxide Labeled Silk Fibroin/Hydroxyapatite Multifunctional Scaffold Loaded with Bone Marrow-Derived Mesenchymal Stem Cells for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 697. [Google Scholar] [CrossRef]
- Naito, E.; Kudo, D.; Sekine, S.; Watanabe, K.; Kobatake, Y.; Tamaoki, N.; Inden, M.; Iida, K.; Ito, Y.; Hozumi, I.; et al. Characterization of canine dental pulp cells and their neuroregenerative potential. Vitr. Cell. Dev. Biol.-Anim. 2015, 51, 1012–1022. [Google Scholar] [CrossRef]
- Billotey, C.; Wilhelm, C.; Devaud, M.; Bacri, J.C.; Bittoun, J.; Gazeau, F. Cell internalization of anionic maghemite nanoparticles: Quantitative effect on magnetic resonance imaging. Magn. Reson. Med. 2003, 49, 646–654. [Google Scholar] [CrossRef]
- Cardarelli, F.; Digiacomo, L.; Marchini, C.; Amici, A.; Salomone, F.; Fiume, G.; Rossetta, A.; Gratton, E.; Pozzi, D.; Caracciolo, G. The intracellular trafficking mechanism of Lipofectamine-based transfection reagents and its implication for gene delivery. Sci. Rep. 2016, 6, 25879. [Google Scholar] [CrossRef] [Green Version]
- Caglayan, S.; Hansen, J.B.; Snir, O. Optimized workflow to modify microRNA expression in primary human intravascular cells. BMC Immunol. 2023, 24, 5. [Google Scholar] [CrossRef]
- Magro, M.; Martinello, T.; Bonaiuto, E.; Gomiero, C.; Baratella, D.; Zoppellaro, G.; Cozza, G.; Patruno, M.; Zboril, R.; Vianello, F. Covalently bound DNA on naked iron oxide nanoparticles: Intelligent colloidal nano-vector for cell transfection. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861 Pt A, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Oxygen Species and the Central Nervous System. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Jasmin de Souza, G.T.; Louzada, R.A.; Rosado-de-Castro, P.H.; Mendez-Otero, R.; Campos de Carvalho, A.C. Tracking stem cells with superparamagnetic iron oxide nanoparticles: Perspectives and considerations. Int. J. Nanomed. 2017, 12, 779–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrabani, D.; Nazempour, M.; Mehdinavaz-Aghdam, R.; Hashemi, S.S.; Jalli, R.; Moghadam, M.S.; Zare, S.; Jamhiri, I.; Mo-ayedi, J.; Karimi-Busheri, F. MRI tracking of human Wharton’s jelly stem cells seeded onto acellular dermal matrix labeled with superparamagnetic iron oxide nanoparticles in burn wounds. Burn. Trauma 2022, 10, tkac018. [Google Scholar] [CrossRef]
- Mohanty, S.; Jain, K.G.; Nandy, S.B.; Kakkar, A.; Kumar, M.; Dinda, A.K.; Singh, H.; Ray, A. Iron oxide labeling does not affect differentiation potential of human bone marrow mesenchymal stem cells exhibited by their differentiation into cardiac and neuronal cells. Mol. Cell. Biochem. 2018, 448, 17–26. [Google Scholar] [CrossRef]
- Omidkhoda, A.; Mozdarani, H.; Movasaghpoor, A.; Fatholah, A.A. Study of apoptosis in labeled mesenchymal stem cells with superparamagnetic iron oxide using neutral comet assay. Toxicol. Vitr. 2007, 21, 1191–1196. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, T.J.; Kim, Y.J.; Kang, L.; Kim, J.; Lee, N.; Hyeon, T.; Lim, M.S.; Mo, H.J.; Shin, J.H.; et al. Targeted Delivery of Iron Oxide Nanoparticle-Loaded Human Embryonic Stem Cell-Derived Spherical Neural Masses for Treating Intracerebral Hemorrhage. Int. J. Mol. Sci. 2020, 21, 3658. [Google Scholar] [CrossRef]
- Saleh, A.; Schroeter, M.; Jonkmanns, C.; Hartung, H.P.; Mödder, U.; Jander, S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain 2004, 127, 1670–1677. [Google Scholar] [CrossRef]
- Lin, M.M.; Kim, D.K.; El Haj, A.J.; Dobson, J. Development of Superparamagnetic Iron Oxide Nanoparticles (SPIONS) for Translation to Clinical Applications. IEEE Trans. Nanobioscience 2008, 7, 298–305. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Chen, Z.; Lan, M. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging Contrast Agent. J. Nanosci. Nanotechnol. 2014, 14, 210–220. [Google Scholar] [CrossRef]
- Czarniecki, M.; Pesapane, F.; Wood, B.J.; Choyke, P.L.; Turkbey, B. Ultra-small superparamagnetic iron oxide contrast agents for lymph node staging of high-risk prostate cancer. Transl. Androl. Urol. 2018, 7 (Suppl. 4), S453–S461. [Google Scholar] [CrossRef] [PubMed]
- Rice, H.E.; Hsu, E.W.; Sheng, H.; Evenson, D.A.; Freemerman, A.J.; Safford, K.M.; Provenzale, J.M.; Warner, D.S.; Johnson, G.A. Superparamagnetic Iron Oxide Labeling and Transplantation of Adipose-Derived Stem Cells in Middle Cerebral Artery Occlusion-Injured Mice. Am. J. Roentgenol. 2007, 188, 1101–1108. [Google Scholar] [CrossRef]
- Rogers, W.J.; Basu, P. Factors regulating macrophage endocytosis of nanoparticles: Implications for targeted magnetic resonance plaque imaging. Atherosclerosis 2005, 178, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oh, S.Y.; Joo, H.J.; Son, K.R.; Song, I.C.; Moon, W.K. The effects of clinically used MRI contrast agents on the biological properties of human mesenchymal stem cells. NMR Biomed. 2010, 23, 514–522. [Google Scholar] [CrossRef] [PubMed]







| Gene | Primer Sequence | Size (bp) |
|---|---|---|
| CD34 | Forward: 5′-AGCCATGTGCTCACACATCA-3′ | 257 |
| Reverse: 5′-CAAACACTCGGGCCTAACCT-3′ | ||
| CD45 | Forward: 5′-CCAAGAGTGGCTCAGAAGGG-3′ | 450 |
| Reverse: 5′-CTGGGCTCATGGGACCATTT-3′ | ||
| CD73 | Forward: 5′-TGCATCGATATGGCCAGTCC-3′ | 208 |
| Reverse: 5′-AATCCATCCCCACCGTTGAC-3′ | ||
| CD90 | Forward: 5′-GACCCAGGACGGAGCTATTG-3′ | 177 |
| Reverse: 5′-TCATGCTGGATGGGCAAGTT-3′ |
| Gene | Primer Sequence | Size (bp) |
|---|---|---|
| B2m | Forward: 5′-CGTGCTTGCCATTCAGAAA-3′ | 244 |
| Reverse: 5′-ATATACATCGGTCTCGGTGG-3′ | ||
| Bax | Forward: 5′-CTGCAGAGGATGATTGCTGA-3′ | 174 |
| Reverse: 5′-GATCAGCTCGGGCACTTTAG-3′ | ||
| Bcl-2 | Forward: 5′-ATCGCTCTGTGGATGACTGAGTAC-3′ | 134 |
| Reverse: 5′-AGAGACAGCCAGGAGAAATCAAAC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalli, R.; Mehrabani, D.; Zare, S.; Saeedi Moghadam, M.; Jamhiri, I.; Manafi, N.; Mehrabani, G.; Ghabanchi, J.; Razeghian Jahromi, I.; Rasouli-Nia, A.; et al. Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI. J. Clin. Med. 2023, 12, 2395. https://doi.org/10.3390/jcm12062395
Jalli R, Mehrabani D, Zare S, Saeedi Moghadam M, Jamhiri I, Manafi N, Mehrabani G, Ghabanchi J, Razeghian Jahromi I, Rasouli-Nia A, et al. Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI. Journal of Clinical Medicine. 2023; 12(6):2395. https://doi.org/10.3390/jcm12062395
Chicago/Turabian StyleJalli, Reza, Davood Mehrabani, Shahrokh Zare, Mahdi Saeedi Moghadam, Iman Jamhiri, Navid Manafi, Golshid Mehrabani, Janan Ghabanchi, Iman Razeghian Jahromi, Aghdass Rasouli-Nia, and et al. 2023. "Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI" Journal of Clinical Medicine 12, no. 6: 2395. https://doi.org/10.3390/jcm12062395





