Can Alterations in Cerebrovascular CO2 Reactivity Be Identified Using Transfer Function Analysis without the Requirement for Carbon Dioxide Inhalation?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Participants
2.3. Study Design
2.4. Measurements
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
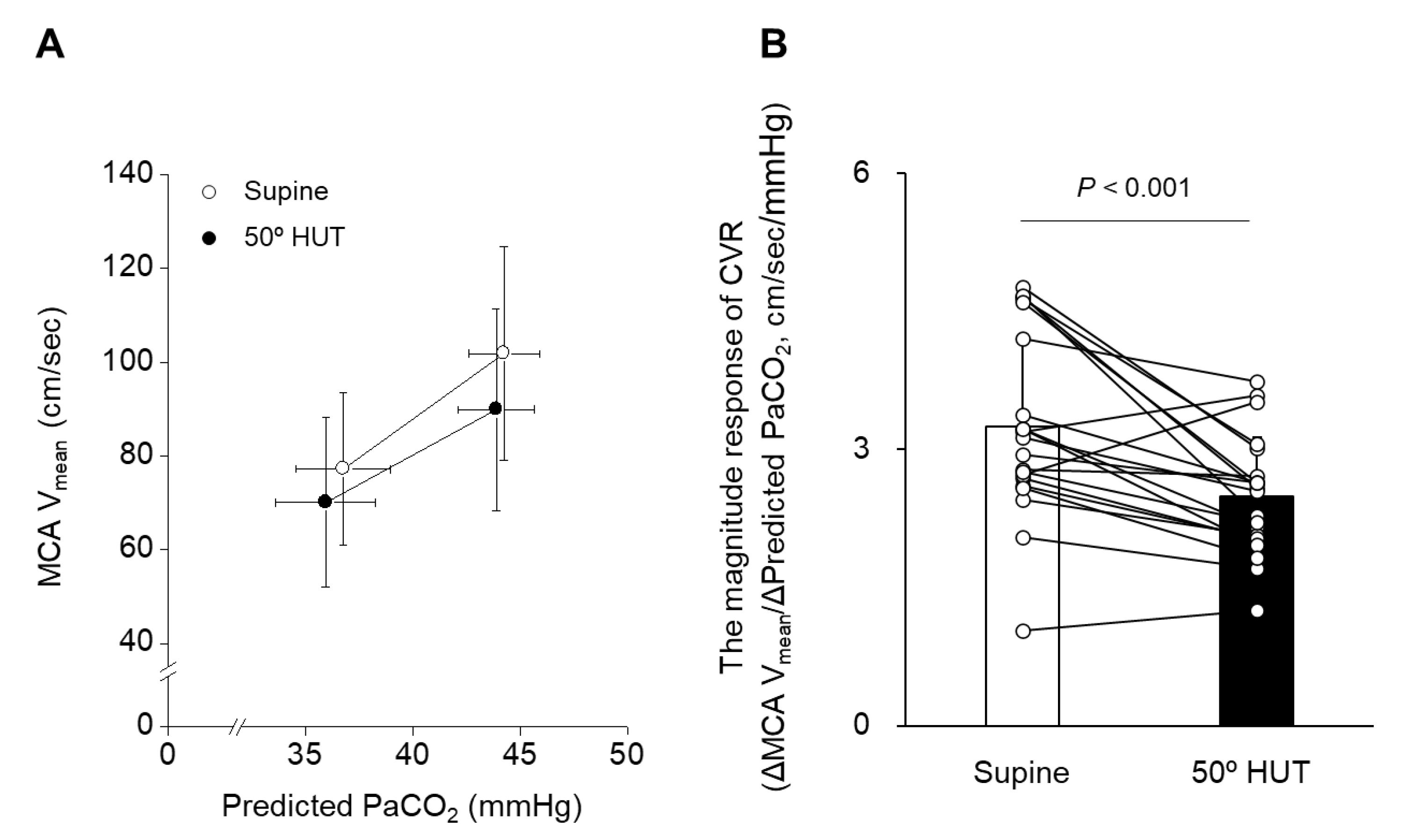
3.1. Hemodynamic Response to Orthostatic Stress (50° HUT)
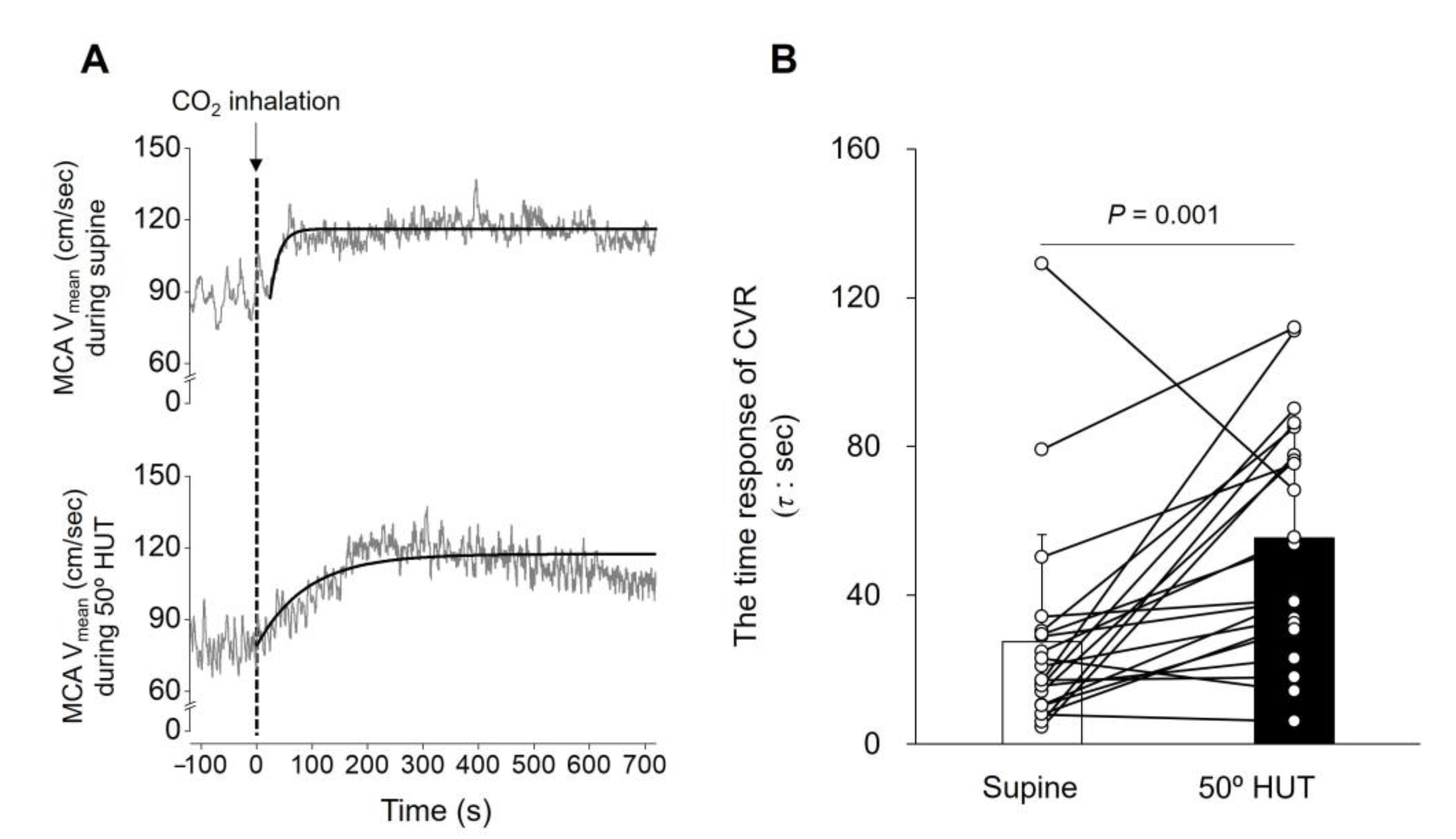
3.2. Time Domain Analysis Determined CVR
3.3. TFA-Determined CVR without CO2 Inhalation
4. Discussion
Perspectives and Significance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogoh, S. Interaction between the respiratory system and cerebral blood flow regulation. J. Appl. Physiol. 2019, 127, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Hayashi, N.; Inagaki, M.; Ainslie, P.N.; Miyamoto, T. Interaction between the ventilatory and cerebrovascular responses to hypo- and hypercapnia at rest and during exercise. J. Physiol. 2008, 586, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Shibata, S.; Ito, G.; Miyamoto, T. Dynamic characteristics of cerebrovascular reactivity or ventilatory response to change in carbon dioxide. Exp. Physiol. 2020, 105, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Chesler, M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Mandell, D.M.; Han, J.S.; Poublanc, J.; Crawley, A.P.; Stainsby, J.A.; Fisher, J.A.; Mikulis, D.J. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in Patients with arterial steno-occlusive disease: Comparison with arterial spin labeling MRI. Stroke 2008, 39, 2021–2028. [Google Scholar] [CrossRef] [Green Version]
- Richiardi, J.; Monsch, A.U.; Haas, T.; Barkhof, F.; Van de Ville, D.; Radu, E.W.; Kressig, R.W.; Haller, S. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2015, 36, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Glodzik, L.; Randall, C.; Rusinek, H.; de Leon, M.J. Cerebrovascular reactivity to carbon dioxide in Alzheimer’s disease. J. Alzheimers. Dis. 2013, 35, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Markus, H.; Cullinane, M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001, 124, 457–467. [Google Scholar] [CrossRef]
- Haight, T.J.; Bryan, R.N.; Erus, G.; Davatzikos, C.; Jacobs, D.R.; D’Esposito, M.; Lewis, C.E.; Launer, L.J. Vascular risk factors, cerebrovascular reactivity, and the default-mode brain network. Neuroimage 2015, 115, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Junejo, R.T.; Braz, I.D.; Lucas, S.J.E.; van Lieshout, J.J.; Lip, G.Y.H.; Fisher, J.P. Impaired Cerebrovascular Reactivity in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 73, 1230–1232. [Google Scholar] [CrossRef]
- Smoliński, Ł.; Członkowska, A. Cerebral vasomotor reactivity in neurodegenerative diseases. Neurol. Neurochir. Pol. 2016, 50, 455–462. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Duffin, J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1473–R1495. [Google Scholar] [CrossRef] [Green Version]
- Peebles, K.; Celi, L.; McGrattan, K.; Murrell, C.; Thomas, K.; Ainslie, P.N. Human cerebrovascular and ventilatory CO2 reactivity to end-tidal, arterial and internal jugular vein PCO2. J. Physiol. 2007, 584, 347–357. [Google Scholar] [CrossRef]
- Sato, K.; Sadamoto, T.; Hirasawa, A.; Oue, A.; Subudhi, A.W.; Miyazawa, T.; Ogoh, S. Differential blood flow responses to CO(2) in human internal and external carotid and vertebral arteries. J. Physiol. 2012, 590, 3277–3290. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, S.; Strandgaard, S.; Uemura, K.; Ito, K.; Kutsuzawa, T. Cerebrovascular CO2 reactivity in normotensive and hypertensive man. Stroke 1976, 7, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Ogoh, S.; Ainslie, P.N.; Miyamoto, T. Onset responses of ventilation and cerebral blood flow to hypercapnia in humans: Rest and exercise. J. Appl. Physiol. 2009, 106, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Poulin, M.J.; Liang, P.J.; Robbins, P.A. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J. Appl. Physiol. 1996, 81, 1084–1095. [Google Scholar] [CrossRef]
- Poublanc, J.; Crawley, A.P.; Sobczyk, O.; Montandon, G.; Sam, K.; Mandell, D.M.; Dufort, P.; Venkatraghavan, L.; Duffin, J.; Mikulis, D.J.; et al. Measuring cerebrovascular reactivity: The dynamic response to a step hypercapnic stimulus. J. Cereb. Blood Flow Metab. 2015, 35, 1746–1756. [Google Scholar] [CrossRef]
- McKetton, L.; Sobczyk, O.; Duffin, J.; Poublanc, J.; Sam, K.; Crawley, A.P.; Venkatraghavan, L.; Fisher, J.A.; Mikulis, D.J. The aging brain and cerebrovascular reactivity. Neuroimage 2018, 181, 132–141. [Google Scholar] [CrossRef]
- Duffin, J.; Sobczyk, O.; Crawley, A.P.; Poublanc, J.; Mikulis, D.J.; Fisher, J.A. The dynamics of cerebrovascular reactivity shown with transfer function analysis. Neuroimage 2015, 114, 207–216. [Google Scholar] [CrossRef]
- Chacon, M.; Araya, C.; Panerai, R.B. Non-linear multivariate modeling of cerebral hemodynamics with autoregressive Support Vector Machines. Med. Eng. Phys. 2011, 33, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Devitt, D.L.; Hughson, R.L. Two-breath CO(2) test detects altered dynamic cerebrovascular autoregulation and CO(2) responsiveness with changes in arterial P(CO(2)). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R627–R632. [Google Scholar] [CrossRef] [PubMed]
- Regan, R.E.; Fisher, J.A.; Duffin, J. Factors affecting the determination of cerebrovascular reactivity. Brain Behav. 2014, 4, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, A.E.; Allen, J.E.; Dahdaleh, N.S.; Drebot, I.I.; Coryell, M.W.; Wunsch, A.M.; Lynch, C.M.; Faraci, F.M.; Howard, M.A., III; Welsh, M.J.; et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 2009, 139, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Elsaid, N.; Saied, A.; Kandil, H.; Soliman, A.; Taher, F.; Hadi, M.; Giridharan, G.; Jennings, R.; Casanova, M.; Keynton, R.; et al. Impact of stress and hypertension on the cerebrovasculature. Front. Biosci. 2021, 26, 1643–1652. [Google Scholar] [CrossRef]
- Karim, H.T.; Tudorascu, D.L.; Butters, M.A.; Walker, S.; Aizenstein, H.J.; Andreescu, C. In the grip of worry: Cerebral blood flow changes during worry induction and reappraisal in late-life generalized anxiety disorder. Transl. Psychiatry 2017, 7, e1204. [Google Scholar] [CrossRef]
- Van den Bergh, O.; Zaman, J.; Bresseleers, J.; Verhamme, P.; Van Diest, I. Anxiety, pCO2 and cerebral blood flow. Int. J. Psychophysiol. 2013, 89, 72–77. [Google Scholar] [CrossRef]
- Ogoh, S.; Suzuki, K.; Washio, T.; Tamiya, K.; Saito, S.; Bailey, T.G.; Shibata, S.; Ito, G.; Miyamoto, T. Does respiratory drive modify the cerebral vascular response to changes in end-tidal carbon dioxide? Exp. Physiol. 2019, 104, 1363–1370. [Google Scholar] [CrossRef]
- Duffin, J. Measuring the respiratory chemoreflexes in humans. Respir. Physiol. Neurobiol. 2011, 177, 71–79. [Google Scholar] [CrossRef]
- Claassen, J.A.; Meel-van den Abeelen, A.S.; Simpson, D.M.; Panerai, R.B.; on Behalf of the international Cerebral Autoregulation Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow Metab. 2016, 36, 665–680. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Saito, S.; Washio, T.; Bailey, D.M.; Ogoh, S. Acute Gravitational Stress Selectively Impairs Dynamic Cerebrovascular Reactivity in the Anterior Circulation Independent of Changes to the Central Respiratory Chemoreflex. Front. Physiol. 2022, 12, 749255. [Google Scholar] [CrossRef]
- Burma, J.S.; Macaulay, A.; Copeland, P.; Khatra, O.; Bouliane, K.J.; Smirl, J.D. Comparison of cerebrovascular reactivity recovery following high-intensity interval training and moderate-intensity continuous training. Physiol. Rep. 2020, 8, e14467. [Google Scholar] [CrossRef]
- Ogoh, S.; Volianitis, S.; Nissen, P.; Wray, D.W.; Secher, N.H.; Raven, P.B. Carotid baroreflex responsiveness to head-up tilt-induced central hypovolaemia: Effect of aerobic fitness. J. Physiol. 2003, 551, 601–608. [Google Scholar] [CrossRef]
- Ogoh, S.; Nakahara, H.; Okazaki, K.; Bailey, D.M.; Miyamoto, T. Cerebral hypoperfusion modifies the respiratory chemoreflex during orthostatic stress. Clin. Sci. 2013, 125, 37–44. [Google Scholar] [CrossRef]
- Jacobi, M.S.; Patil, C.P.; Saunders, K.B. Transient, steady-state and rebreathing responses to carbon dioxide in man, at rest and during light exercise. J. Physiol. 1989, 411, 85–96. [Google Scholar] [CrossRef]
- Miyamoto, T.; Bailey, D.M.; Nakahara, H.; Ueda, S.; Inagaki, M.; Ogoh, S. Manipulation of central blood volume and implications for respiratory control function. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1669–H1678. [Google Scholar] [CrossRef]
- Poulin, M.J.; Liang, P.J.; Robbins, P.A. Fast and slow components of cerebral blood flow response to step decreases in end-tidal PCO2 in humans. J. Appl. Physiol. 1998, 85, 388–397. [Google Scholar] [CrossRef]
- Mitsis, G.D.; Poulin, M.J.; Robbins, P.A.; Marmarelis, V.Z. Nonlinear modeling of the dynamic effects of arterial pressure and CO2 variations on cerebral blood flow in healthy humans. IEEE Trans. Biomed. Eng. 2004, 51, 1932–1943. [Google Scholar] [CrossRef]
- Mitsis, G.D.; Zhang, R.; Levine, B.D.; Marmarelis, V.Z. Cerebral hemodynamics during orthostatic stress assessed by nonlinear modeling. J. Appl. Physiol. 2006, 101, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Sobczyk, O.; Battisti-Charbonney, A.; Fierstra, J.; Mandell, D.M.; Poublanc, J.; Crawley, A.P.; Mikulis, D.J.; Duffin, J.; Fisher, J.A. A conceptual model for CO(2)-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage 2014, 92, 56–68. [Google Scholar] [CrossRef]
- Lythgoe, D.J.; Williams, S.C.; Cullinane, M.; Markus, H.S. Mapping of cerebrovascular reactivity using BOLD magnetic resonance imaging. Magn. Reson. Imaging 1999, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Toft, P.B.; Leth, H.; Lou, H.C.; Pryds, O.; Peitersen, B.; Henriksen, O. Local vascular CO2 reactivity in the infant brain assessed by functional MRI. Pediatr. Radiol. 1995, 25, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Simpson, D.M.; Deverson, S.T.; Mahony, P.; Hayes, P.; Evans, D.H. Multivariate dynamic analysis of cerebral blood flow regulation in humans. IEEE Trans. Biomed. Eng. 2000, 47, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazraji, B.K.; Shoemaker, L.N.; Gati, J.S.; Szekeres, T.; Shoemaker, J.K. Reactivity of larger intracranial arteries using 7 T MRI in young adults. J. Cereb. Blood Flow Metab. 2019, 39, 1204–1214. [Google Scholar] [CrossRef]
- Miller, K.B.; Howery, A.J.; Rivera-Rivera, L.A.; Johnson, S.C.; Rowley, H.A.; Wieben, O.; Barnes, J.N. Age-Related Reductions in Cerebrovascular Reactivity Using 4D Flow MRI. Front. Aging Neurosci. 2019, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Giller, C.A.; Bowman, G.; Dyer, H.; Mootz, L.; Krippner, W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 1993, 32, 737–741. [Google Scholar] [CrossRef]
- Serrador, J.M.; Picot, P.A.; Rutt, B.K.; Shoemaker, J.K.; Bondar, R.L. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000, 31, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.J.; Crabtree, D.C.; Palta, M.; Skatrud, J.B. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J. Appl. Physiol. 1995, 79, 205–213. [Google Scholar] [CrossRef]
- Xie, A.; Skatrud, J.B.; Crabtree, D.C.; Puleo, D.S.; Goodman, B.M.; Morgan, B.J. Neurocirculatory consequences of intermittent asphyxia in humans. J. Appl. Physiol. 2000, 89, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Altose, M.D.; McCauley, W.C.; Kelsen, S.G.; Cherniack, N.S. Effects of hypercapnia and inspiratory flow-resistive loading on respiratory activity in chronic airways obstruction. J. Clin. Investig. 1977, 59, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Flenley, D.C.; Franklin, D.H.; Millar, J.S. The hypoxic drive to breathing in chronic bronchitis and emphysema. Clin. Sci. 1970, 38, 503–518. [Google Scholar] [CrossRef] [Green Version]
- Scano, G.; Spinelli, A.; Duranti, R.; Gorini, M.; Gigliotti, F.; Goti, P.; Milic-Emili, J. Carbon dioxide responsiveness in COPD patients with and without chronic hypercapnia. Eur. Respir. J. 1995, 8, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Van de Ven, M.J.; Colier, W.N.; Van der Sluijs, M.C.; Kersten, B.T.; Oeseburg, B.; Folgering, H. Ventilatory and cerebrovascular responses in normocapnic and hypercapnic COPD patients. Eur. Respir. J. 2001, 18, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, L.; Casucci, G.; Haider, T.; Brandstatter, E.; Pocecco, E.; Ehrenbourg, I.; Burtscher, M. Autonomic and cerebrovascular abnormalities in mild COPD are worsened by chronic smoking. Eur. Respir. J. 2008, 32, 1458–1465. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, S.E.; Pialoux, V.; Leigh, R.; Poulin, M.J. Decreased cerebrovascular response to CO2 in post-menopausal females with COPD: Role of oxidative stress. Eur. Respir. J. 2012, 40, 1354–1361. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.; Gelinas, J.C.M.; Ainslie, P.N.; Smirl, J.D.; Agar, G.; Melzer, B.; Rolf, J.D.; Eves, N.D. Cerebrovascular function in patients with chronic obstructive pulmonary disease: The impact of exercise training. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H380–H391. [Google Scholar] [CrossRef]

| Supine (n = 21) | 50° HUT (n = 21) | Linear Mixed-Effects Model | |||||
|---|---|---|---|---|---|---|---|
| Variables | p-Values | ||||||
| Without CO2 Inhalation | With CO2 Inhalation | Without CO2 Inhalation | With CO2 Inhalation | Condition | Time | Interaction | |
| HR, beats/min | 64.4 ± 10.7 | 70.1 ± 12.6 | 80.5 ± 16.1 | 83.2 ± 14.7 | <0.001 | <0.001 | 0.176 |
| MAP, mmHg | 93.0 ± 8.9 | 97.2 ± 9.8 | 92.9 ± 9.2 | 98.6 ± 8.4 | 0.567 | <0.001 | 0.476 |
| MCA Vmean, cm/s | 77.2 ± 16.3 | 101.9 ± 22.9 | 70.1 ± 18.1 | 89.9 ± 21.5 | <0.001 | <0.001 | 0.083 |
| RR, breath/min | 16.4 ± 3.7 | 17.9 ± 5.3 | 15.9 ± 3.4 | 17.9 ± 4.8 | 0.622 | 0.001 | 0.690 |
| , L/min | 8.8 ± 1.4 | 18.6 ± 3.7 | 8.8 ± 1.4 | 18.5 ± 4.7 | 0.946 | <0.001 | 0.985 |
| Vt, mL | 576.4 ± 122.9 | 1126.4 ± 335.7 | 583.0 ± 111.7 | 1110.8 ± 300.6 | 0.900 | <0.001 | 0.754 |
| PETCO2, mmHg | 38.9 ± 2.5 | 47.4 ± 1.9 | 38.0 ± 2.7 | 47.0 ± 2.0 | 0.009 | <0.001 | 0.317 |
| Predicted PaCO2, mmHg | 36.8 ± 2.2 | 44.2 ± 1.7 | 36.0 ± 2.3 | 43.9 ± 1.8 | 0.007 | <0.001 | 0.262 |
| Variables | Supine (n = 21) | 50° HUT (n = 21) | p-Values |
|---|---|---|---|
| t0, s | 8.5 ± 5.6 | 10.5 ± 4.6 | 0.132 |
| y0, cm/s | 77.2 ± 16.3 | 70.1 ± 18.1 | <0.001 |
| G | 26.1 ± 10.6 | 23.0 ± 7.8 | 0.102 |
| τ, s | 27.5 ± 28.9 | 55.4 ± 31.5 | 0.001 |
| Variables | Supine (n = 21) | 50° HUT (n = 21) | p-Values | |
|---|---|---|---|---|
| Phase, radian | VLF | −0.38 ± 0.59 | 0.31 ± 0.78 | 0.003 |
| LF | −0.32 ± 0.61 | 0.04 ± 0.61 | 0.064 | |
| HF | −0.12 ± 0.44 | −0.07 ± 0.49 | 0.705 | |
| Gain, db | VLF | 4.58 ± 1.57 | 3.75 ± 2.20 | 0.099 |
| LF | 4.17 ± 1.85 | 3.69 ± 2.15 | 0.126 | |
| HF | 6.46 ± 4.84 | 6.90 ± 4.63 | 0.664 | |
| Coherence | VLF | 0.47 ± 0.22 | 0.50 ± 0.18 | 0.594 |
| LF | 0.37 ± 0.22 | 0.41 ± 0.17 | 0.601 | |
| HF | 0.31 ± 0.19 | 0.35 ± 0.10 | 0.396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogoh, S.; Watanabe, H.; Saito, S.; Fisher, J.P.; Iwamoto, E. Can Alterations in Cerebrovascular CO2 Reactivity Be Identified Using Transfer Function Analysis without the Requirement for Carbon Dioxide Inhalation? J. Clin. Med. 2023, 12, 2441. https://doi.org/10.3390/jcm12062441
Ogoh S, Watanabe H, Saito S, Fisher JP, Iwamoto E. Can Alterations in Cerebrovascular CO2 Reactivity Be Identified Using Transfer Function Analysis without the Requirement for Carbon Dioxide Inhalation? Journal of Clinical Medicine. 2023; 12(6):2441. https://doi.org/10.3390/jcm12062441
Chicago/Turabian StyleOgoh, Shigehiko, Hironori Watanabe, Shotaro Saito, James P. Fisher, and Erika Iwamoto. 2023. "Can Alterations in Cerebrovascular CO2 Reactivity Be Identified Using Transfer Function Analysis without the Requirement for Carbon Dioxide Inhalation?" Journal of Clinical Medicine 12, no. 6: 2441. https://doi.org/10.3390/jcm12062441
APA StyleOgoh, S., Watanabe, H., Saito, S., Fisher, J. P., & Iwamoto, E. (2023). Can Alterations in Cerebrovascular CO2 Reactivity Be Identified Using Transfer Function Analysis without the Requirement for Carbon Dioxide Inhalation? Journal of Clinical Medicine, 12(6), 2441. https://doi.org/10.3390/jcm12062441






