Liver Transplantation in Patients with Portal Vein Thrombosis: Revisiting Outcomes According to Surgical Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Definitions
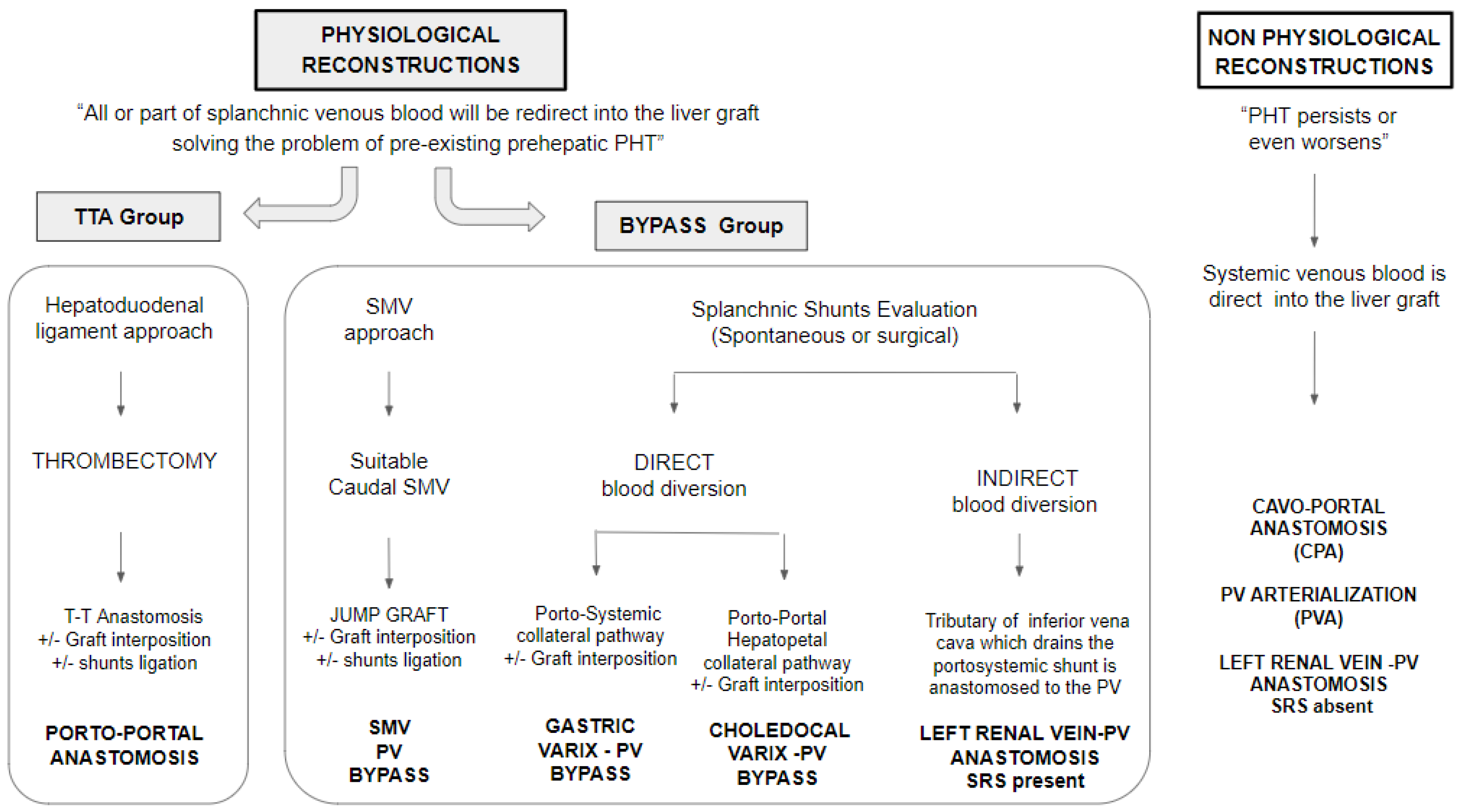
2.4. Surgical Strategies for Portal Reconstruction
2.5. Post-Operative Anticoagulation
2.6. Portal Flow Imaging Surveillance
2.7. Statistical Analysis
3. Results
3.1. Patients’ Demographic, Clinical, and Surgical Features
3.2. Post-Trasplant Outcomes
3.3. Early Mortality
3.4. Long-Term Survival
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lendoire, J.; Raffin, G.; Cejas, N.; Duek, F.; Schelotto, P.B.; Trigo, P.; Quarin, C.; Garay, V.; Imventarza, O. Liver transplantation in adult patients with portal vein thrombosis: Risk factors, management and outcome. HPB 2007, 9, 352–356. [Google Scholar] [PubMed] [Green Version]
- Mehmet, A.Y.; Bridget, G.; Darius, M.; Kaan, K. Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome. Transplantation 2000, 69, 1873–1881. [Google Scholar]
- Jamieson, N.V. Changing perspectives in portal vein thrombosis and liver transplantation. Transplantation 2000, 69, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Charco, R.; Fuster, J.; Fondevila, C.; Ferrer, J.; Mans, E.; Garcıa-Valdecasas, J.C. Portal vein thrombosis in liver transplantation. Transpl. Proc. 2005, 37, 3904–3905. [Google Scholar]
- Zanetto, A.; Rodriguez-Kastro, K.-I.; Germani, G.; Ferrarese, A.; Cillo, U.; Burra, P.; Senzolo, M. Mortality in liver transplant recipients with portal vein thrombosis—An updated meta-analysis. Transpl. Int. 2018, 31, 1318–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Dai, J.; Jia, J.; Ren, W.; Yang, M.; Li, H.; Fan, D.; Guo, X. Association between portal vein thrombosis and survival of liver transplant recipients: A systematic review and meta-analysis of observational studies. J. Gastrointestin Liver Dis. 2015, 24, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, D.; Camagni, S.; Amaduzzi, A.; Frosio, F.; Fontanella, L.; Carioli, G.; Guizzetti, M.; Zambelli, M.F.; Giovanelli, M.; Fagiuoli, S.; et al. Liver transplantation in patients with non-neoplastic portal vein thrombosis: 20 years of experience in a single center. Clin. Transpl. 2021, 36, e14501. [Google Scholar] [CrossRef] [PubMed]
- Bhangui, P.; Lim, C.; Levesque, E.; Salloum, C.; Lahat, E.; Feray, C.; Azoulay, D. Novel classification of non-malignant portal vein thrombosis: A guide to surgical decision-making during liver transplantation. J. Hepat. 2019, 71, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications—A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Feng, S.; Goodrich, N.; Bragg-Gresham, J.; Dykstra, D.; Punch, J.; DebRoy, M.; Greenstein, S.; Merion, R. Characteristics Associated with Liver Graft Failure: The Concept of a Donor Risk Index. Am. J. Transpl. 2006, 6, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhu, J.; Choi, G.-S.; Kwon, C.H.D.; Kim, J.M.; Joh, J.-W. Portal vein thrombosis during liver transplantation: The risk of extra-anatomical portal vein reconstruction. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.W.; Law, M.S.N.; Lim, J.C.L.; Ng, C.H.; Tan, D.J.H.; Tay, P.W.L.; Syn, N.; Tham, H.Y.; Huang, D.Q.; Siddiqui, M.S.; et al. Meta-analysis and systematic review: Prevalence, graft failure, mortality, and post-operative thrombosis in liver transplant recipients with pre-operative portal vein thrombosis. Clin. Transpl. 2022, 36, e14520. [Google Scholar] [CrossRef] [PubMed]
- Tzakis, A.G.; Kirkegaard, P.; Pinna, A.D.; Jovine, E.; Misiakos, E.P.; Maziotti, A.; Dodson, F.; Khan, F.; Nery, J.; Rasmussen, A.; et al. Liver transplantation with cavoportal hemitransposition in the presence of diffuse portal vein thrombosis. Transplantation 1998, 65, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, G.; Weppler, D.; Nishida, S.; Moon, J.; Levi, D.; Kato, T.; Tzakis, A.G. Ten-Year Experience in Porto-Caval Hemitransposition for Liver Transplantation in the Presence of Portal Vein Thrombosis. Am. J. Transplant. 2007, 7, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Nishida, S.; Levi, D.M.; Selvaggi, G.; Tekin, A.; Fan, J.; Ruiz, P.; Tzakis, A.G. When and why portal vein thrombosis matters in liver transplantation: A critical audit of 174 cases. Ann. Surg. 2014, 259, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Zanello, M.; Grazi, G.L.; Ercolani, G.; Cescon, M.; Del Gaudio, M.; Cucchetti, A.; Pinna, A.D. Portal vein thrombosis and liver transplantation: Evolution during 10 years of experience at the University of Bologna. Ann. Surg. 2011, 253, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Llado, L.; Azoulay, D.; Quintini, C.; Rayar, M.; Salloum, C.; D´amico, G.; Ramos, E.; Fabregat, J.; Compagnon, P.; Eshkenazy, R.; et al. Renoportal Anastomosis during Liver Transplantation in Patients with Portal Vein Thrombosis: First long-term Results from a Multicenter Study. Ann. Surg. 2021, 23, S692. [Google Scholar] [CrossRef]


| Overall (165) | TTA (123) | Bypass (42) | p-Value | |
|---|---|---|---|---|
| Centre; n (%) | 0.90 | |||
| 76 (46.1) | 57 (46.3) | 19 (45.2) | |
| 89 (53.9) | 66 (53.7) | 23 (54.8) | |
| RECIPIENT | ||||
| Male gender; n (%) | 119 (72.1) | 91 (74.0) | 28 (66.7) | 0.36 |
| Age (ys) at transplantation; median (IQR) | 56 (49–61) | 56 (49–61) | 54.6 (47.8–61) | 0.37 |
| BMI; median (IQR) | 25.1 (23–28.3) | 25.4 (23–28.7) | 24.7 (227–27.5) | 0.17 |
| Waiting time (mo) in list; median (IQR) | 7 (2.6–19.6) | 6.8 (2.6–17.1) | 9.9 (2.8–25.6) | 0.15 |
| Liver disease; n (%) | 0.035 | |||
| 52 (31.5) | 42 (34.1) | 10 (23.8) | |
| 24 (14.5) | 20 (16.3) | 4 (9.5) | |
| 62 (37.6) | 47 (38.2) | 15 (35.7) | |
| 27 (16.4) | 14 (11.4) | 13 (31) | |
| Pre-LT positioning of TIPPS; n (%) | 13 (7.9) | 11 (8.9) | 2 (4.8) | 0.52 |
| Pre-LT MELD; median (IQR) | 18 (14–25) | 18 (14–25) | 17 (14–23 | 0.58 |
| Pre-LT ascites; n (%) | 95 (57.6) | 68 (55.3) | 27 (64.3) | 0.31 |
| Pre-LT oesophageal varices; n (%) | 147(90.2) | 107 (88.4) | 40 (95.2) | 0.25 |
| Hospitalization at moment of LT; n (%) | 57 (34.8) | 45 (36.6) | 12 (29.3) | 0.39 |
| DONOR | ||||
| Age (ys); median (IQR) | 63.0 (50.0–74.7) | 61.0 (49.0–73.0) | 71.2 (51.6–75.0) | 0.088 |
| BMI; median (IQR) | 25.3 (23.4–27.7) | 25.0 (23.4–27.7) | 25.4 (23.5–27.8) | 0.60 |
| Male gender; n (%) | 89 (53.9) | 71 (57.7) | 18 (42.9) | 0.095 |
| Cause of death; n (%) | 0.25 | |||
| 113 (68.5) | 82 (66.7) | 31 (73.8 | |
| 15 (9.1) | 10 (8.1) | 5 (11.9) | |
| 27 (16.4) | 24 (19.5) | 3 (7.1) | |
| 10 (6.1) | 7 (5.7) | 3 (7.1) | |
| HBcAb positivity; n (%) | 30 (18.2) | 25 (20.3) | 5 (11.9) | 0.22 |
| HCV positivity; n (%) | 6 (3.6) | 5 (4.1) | 1 (2.4) | 1 |
| Donor risk index; median (IQR) | 1.7 (1.4–1.9) | 1.5 (1.4–1.9) | 1.8 (1.5–1.9) | 0.014 |
| INTRAOPERATORY VARIABLES | ||||
| LT length in min; median (IQR) | 425.0 (370–500) | 420 (360–490) | 447.5 (390–540) | 0.091 |
| CIT in min; median (IQR) | 400 (335–465) | 395 (331–465) | 400 (350–480) | 0.55 |
| Yerdel grade; n (%) | <0.001 | |||
| 73 (44.2) | 67 (54.5) | 6 (14.3) | |
| 71 (43) | 50 (40.7) | 21 (50) | |
| 21 (12.7) | 6 (4.9) | 15 (35.7) | |
| Right split graft; n (%) | 5 (3) | 4 (3.3) | 1 (2.4) | 1 |
| Caval anastomosis; n (%) | 0.36 | |||
| 97 (58.8) | 69 (56.1) | 28 (66.7) | |
| 65 (39.4) | 52 (42.3) | 13 (31.0) | |
| 3 (1.8) | 2 (1.6) | 1 (2.4) | |
| Use of vascular graft for portal anastomosis; n (%) | 32 (19.4) | 3 (2.4) | 29 (69.0) | <0.001 |
| Biliary anastomosis; n (%) | 0.083 | |||
| 133 (80.6) | 102 (82.9) | 31 (73.8) | |
| 28 (17.0) | 20 (16.3) | 8 (19.0) | |
| 4 (2.4) | 1 (0.8) | 3 (7.1) | |
| Overall (165) | Alive at 90 Days (144) | Deceased at 90 Days (21) | p-Value | |
|---|---|---|---|---|
| Portal vein reconstruction; n (%) | 0.050 | |||
| 123 (74.5) | 111 (77.1) | 12 (57.1) | |
| 42 (25.5) | 33 (22.9) | 9 (42.9) | |
| Specific of portal vein reconstruction; n (%) | 0.026 | |||
| 123 (74.5) | 111 (77.1) | 12 (57.1) | |
| 11 (6.7) | 7 (4.9) | 4 (19) | |
| 21 (12.7) | 19 (13.2) | 2 (9.5) | |
| 10 (6.1) | 7 (4.9) | 3 (14.3) | |
| Use of vascular graft for portal anastomosis; n (%) | 32 (19.4) | 24 (16.7) | 8 (38.1) | 0.02 |
| Biliary anastomosis; n (%) | 0.005 | |||
| 133 (80.6) | 119 (82.6) | 14 (66.7) | |
| 28 (17.0) | 24 (16.7) | 4 (19.0) | |
| 4 (2.4) | 1 (0.7) | 3 (14.3) | |
| Amount of intraoperative red blood cells transfusions in ml; median (IQR) | 1000 (520–1750) | 1000 (500–1680) | 2240 (1300–3055) | 0.003 |
| Centre; n (%) | 0.043 | |||
| 76 (46.1) | 62 (43.1) | 14 (66.7) | |
| 89 (53.9) | 82 (56.9) | 7 (33.3) |
| Overall | Stratified Centre 1 (14 Events) | Stratified Centre 2 (7 Events) | |
|---|---|---|---|
| Portal reconstruction | |||
| 1 (ref) | 1 (ref) | 1 (ref) |
| 2.52 (0.96–6.58) | 3.64 (1.14–11.64) | 1.12 (0.20–6.43) |
| p-value = 0.059 | p-value = 0.029 | p-value = 0.897 | |
| Yerdel grade | |||
| 1 (ref) | 1 (ref) | 1 (ref) |
| 0.91 (0.34–2.44) | 0.82 (0.26–2.62) | 1.14 (0.20–6.51) |
| p-value = 0.849 | p-value = 0.735 | p-value = 0.887 | |
| Centre | |||
| 1 (ref) | ||
| 0.39 (0.15–1.01) | NA | NA |
| p-value = 0.051 |
| Recipient Age | Meld | Yerdel Grade | Graft Type | PV Anastomosis | RE-LT | Days LT-Death | CAUSE OF DEATH | |
|---|---|---|---|---|---|---|---|---|
| BYPASS | 46 | 14 | 3 | Split (I + IV–VIII) | Gastric varix | 0 | 0 | Intraoperative cardiac arrest due to massive hemorrage |
| 44 | 44 | 3 | whole | SMV (with VG) | 0 | 2 | PNF | |
| 56 | 13 | 2 | whole | Gastric varix | 0 | 5 | Septic shock (intestinal ischemia) | |
| 59 | 15 | 2 | whole | SMV (with VG) | 0 | 5 | Septic shock | |
| 50 | 8 | 3 | whole | RPA (with VG) | 0 | 28 | Intracranial hemorrage | |
| 53 | 33 | 3 | whole | RPA (with VG) | 0 | 62 | Cerebral Cryptoccosis | |
| 66 | 14 | 3 | whole | RPA | 0 | 74 | MOF | |
| 59 | 18 | 4 | whole | SMV (with VG) | 0 | 6 | Intracranial hemorrage | |
| 51 | 17 | 4 | whole | SMV | 1 (PNF) | 33 | Intracranial hemorrage | |
| TTA | 59 | 15 | 2 | whole | TTA | 0 | 0 | Intraoperative cardiac arrest |
| 48 | 15 | 2 | whole | TTA | 1 (PNF) | 2 | Intraoperative cardiac arrest (at the end of surgery) | |
| 56 | 14 | 2 | whole | TTA | 0 | 3 | Heart failure and pulmonary hypertension | |
| 60 | 15 | 2 | whole | TTA | 0 | 21 | Intracranial hemorrage | |
| 53 | 11 | 3 | whole | TTA | 0 | 23 | Acute AMR | |
| 47 | na | 2 | whole | TTA | 0 | 27 | MOF | |
| 60 | 25 | 3 | whole | TTA | 0 | 37 | Septic shock | |
| 47 | 21 | 2 | whole | TTA | 0 | 1 | PNF | |
| 56 | 12 | 3 | whole | TTA | 1 (HAT) | 73 | Hemorragic Pancreatitis | |
| 65 | 25 | 2 | whole | TTA | 0 | 16 | Septic shock | |
| 44 | 9 | 4 | whole | TTA | 0 | 38 | Septic shock | |
| 48 | 42 | 3 | whole | TTA | 0 | 39 | Hemorragic shock (splenic aneurism rupture) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinelli, D.; Cescon, M.; Ravaioli, M.; Neri, F.; Amaduzzi, A.; Serenari, M.; Carioli, G.; Siniscalchi, A.; Colledan, M. Liver Transplantation in Patients with Portal Vein Thrombosis: Revisiting Outcomes According to Surgical Techniques. J. Clin. Med. 2023, 12, 2457. https://doi.org/10.3390/jcm12072457
Pinelli D, Cescon M, Ravaioli M, Neri F, Amaduzzi A, Serenari M, Carioli G, Siniscalchi A, Colledan M. Liver Transplantation in Patients with Portal Vein Thrombosis: Revisiting Outcomes According to Surgical Techniques. Journal of Clinical Medicine. 2023; 12(7):2457. https://doi.org/10.3390/jcm12072457
Chicago/Turabian StylePinelli, Domenico, Matteo Cescon, Matteo Ravaioli, Flavia Neri, Annalisa Amaduzzi, Matteo Serenari, Greta Carioli, Antonio Siniscalchi, and Michele Colledan. 2023. "Liver Transplantation in Patients with Portal Vein Thrombosis: Revisiting Outcomes According to Surgical Techniques" Journal of Clinical Medicine 12, no. 7: 2457. https://doi.org/10.3390/jcm12072457
APA StylePinelli, D., Cescon, M., Ravaioli, M., Neri, F., Amaduzzi, A., Serenari, M., Carioli, G., Siniscalchi, A., & Colledan, M. (2023). Liver Transplantation in Patients with Portal Vein Thrombosis: Revisiting Outcomes According to Surgical Techniques. Journal of Clinical Medicine, 12(7), 2457. https://doi.org/10.3390/jcm12072457







