Influence of Cilioretinal Arteries on Flow Density in Glaucoma Patients Measured Using Optical Coherence Tomography Angiography
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Comparability of Study Cohorts
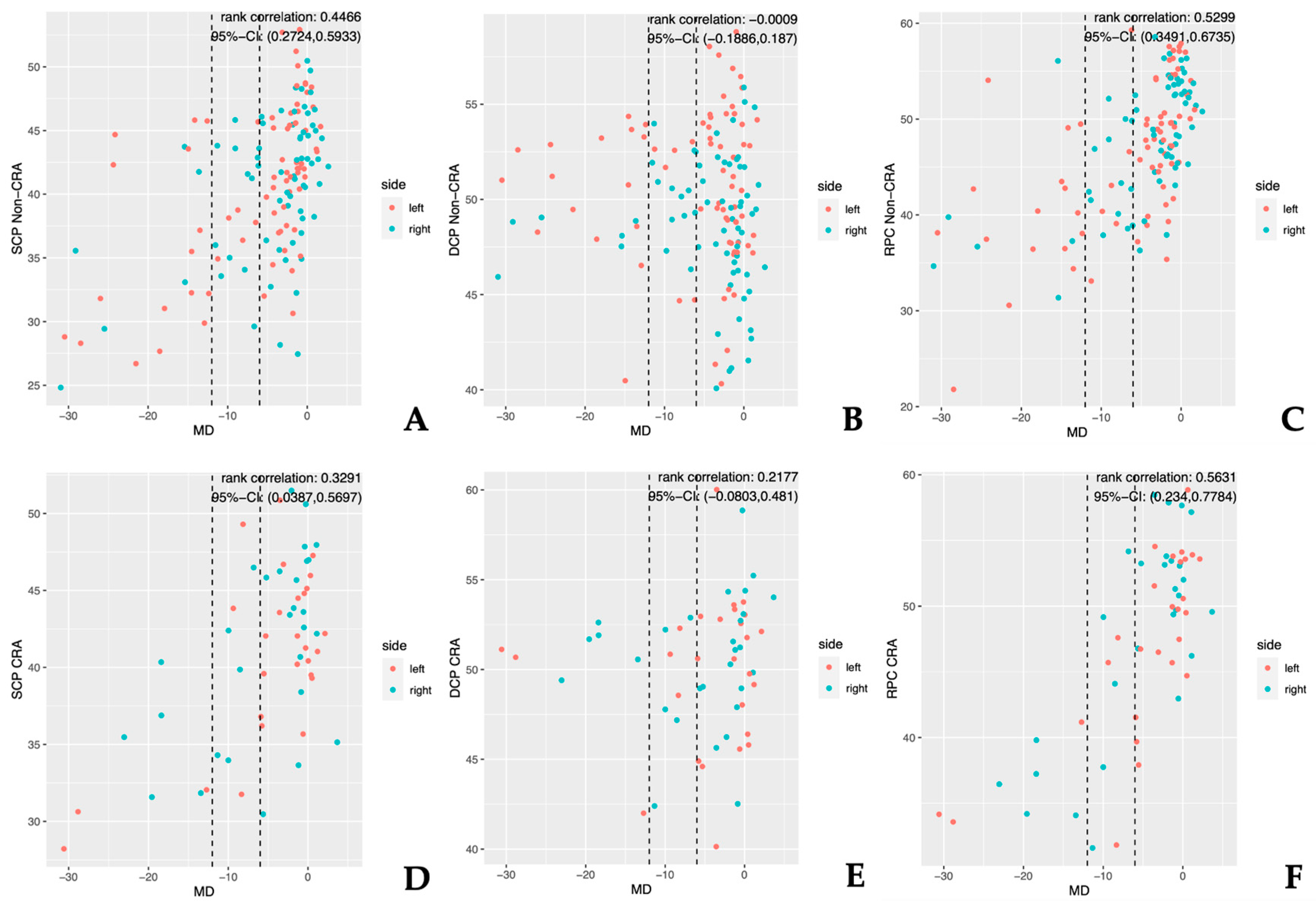
3.2. CRA Versus Non-CRA
3.3. Normal-Tension Glaucoma
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fechtner, R.D.; Weinreb, R.N. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv. Ophthalmol. 1994, 39, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, G.A. Ischemic model of optic nerve injury. Trans. Am. Ophthalmol. Soc. 2005, 103, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S. Controversies in the vascular theory of glaucomatous optic nerve degeneration. Taiwan J. Ophthalmol. 2016, 6, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Müller, H. Anatomisch-physiologische Untersuchungen über die Retina des Menschen und der Wirbeltiere. Z. Für Wissenscchaftliche Zool. 1856, 1–22. [Google Scholar]
- Hayreh, S.S. The ophthalmic artery: III. Branches. Br. J. Ophthalmol. 1962, 46, 212–247. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Jia, Y.; Morrison, J.C.; Tokayer, J.; Tan, O.; Lombardi, L.; Baumann, B.; Lu, C.D.; Choi, W.; Fujimoto, J.G.; Huang, D. Quantitative OCT angiography of optic nerve head blood flow. Biomed. Opt. Express 2012, 3, 3127–3137. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.L.; Pradhan, Z.S.; Suh, M.H.; Moghimi, S.; Mansouri, K.; Weinreb, R.N. Optical Coherence Tomography Angiography in Glaucoma. J. Glaucoma 2020, 29, 312–321. [Google Scholar] [CrossRef]
- Müller, V.C.; Storp, J.J.; Kerschke, L.; Nelis, P.; Eter, N.; Alnawaiseh, M. Diurnal variations in flow density measured using optical coherence tomography angiography and the impact of heart rate, mean arterial pressure and intraocular pressure on flow density in primary open-angle glaucoma patients. Acta Ophthalmol. 2019, 97, e844–e849. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.; Takusagawa, H.L.; Pechauer, A.D.; Edmunds, B.; Lombardi, L.; Davis, E.; Morrison, J.C.; Huang, D. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015, 133, 1045–1052. [Google Scholar] [CrossRef]
- Rao, H.L.; Pradhan, Z.S.; Weinreb, R.N.; Riyazuddin, M.; Dasari, S.; Venugopal, J.P.; Puttaiah, N.K.; Rao, D.A.S.; Devi, S.; Mansouri, K.; et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS ONE 2017, 12, e0173930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Wei, E.; Wang, X.; Zhang, X.; Morrison, J.C.; Parikh, M.; Lombardi, L.H.; Gattey, D.M.; Armour, R.L.; Edmunds, B.; et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014, 121, 1322–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarmohammadi, A.; Zangwill, L.M.; Diniz-Filho, A.; Suh, M.H.; Manalastas, P.I.; Fatehee, N.; Yousefi, S.; Belghith, A.; Saunders, L.J.; Medeiros, F.A.; et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT451–OCT459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansoori, T.; Sivaswamy, J.; Gamalapati, J.S.; Balakrishna, N. Radial Peripapillary Capillary Density Measurement Using Optical Coherence Tomography Angiography in Early Glaucoma. J. Glaucoma 2017, 26, 438–443. [Google Scholar] [CrossRef]
- Geyman, L.S.; Garg, R.A.; Suwan, Y.; Trivedi, V.; Krawitz, B.D.; Mo, S.; Pinhas, A.; Tantraworasin, A.; Chui, T.Y.P.; Ritch, R.; et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: An optical coherence tomography angiography study. Br. J. Ophthalmol. 2017, 101, 1261–1268. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, S.H.; Kim, J.-A.; Kim, T.-W. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association with Glaucomatous Damage. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3004–3010. [Google Scholar] [CrossRef] [Green Version]
- Suh, M.H.; Park, J.W.; Kim, H.R. Association Between the Deep-layer Microvasculature Dropout and the Visual Field Damage in Glaucoma. J. Glaucoma 2018, 27, 543–551. [Google Scholar] [CrossRef]
- Diener, R.; Leclaire, M.D.; Eckardt, F.; Lauermann, J.L.; Alnawaiseh, M.; Eter, N.; Treder, M. Cilioretinal Arteries Influence Optic Nerve Head, Peripapillary, and Macular Vessel Densities in Healthy Eyes: An Optical Coherence Tomography Angiography Study. Retina 2021, 41, 2399–2406. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, J.; Wei, L.; Zhang, K.; He, W.; Lu, Y. Cilioretinal Arteries and Macular Vasculature in Highly Myopic Eyes: An OCT Angiography-Based Study. Ophthalmol. Retina 2020, 4, 965–972. [Google Scholar] [CrossRef]
- Hodapp, E.; Parrish, R.K.; Anderson, D.R. Clinical Decisions in Glaucoma; Mosby Incorporated: Maryland Heights, MO, USA, 1993. [Google Scholar]
- Rosner, B.; Glynn, R.J.; Lee, M.-L.T. Extension of the rank sum test for clustered data: Two-group comparisons with group membership defined at the subunit level. Biometrics 2006, 62, 1251–1259. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, M.-L.T.; He, X.; Rosner, B.; Yan, J. Wilcoxon Rank-Based Tests for Clustered Data with R Package clusrank. J. Stat. Soft. 2020, 96, 1–26. [Google Scholar] [CrossRef]
- Rosner, B.; Glynn, R.J. Interval estimation for rank correlation coefficients based on the probit transformation with extension to measurement error correction of correlated ranked data. Stat. Med. 2007, 26, 633–646. [Google Scholar] [CrossRef]
- Schneider, M.; Molnar, A.; Angeli, O.; Szabo, D.; Bernath, F.; Hajdu, D.; Gombocz, E.; Mate, B.; Jiling, B.; Nagy, B.V.; et al. Prevalence of Cilioretinal Arteries: A systematic review and a prospective cross-sectional observational study. Acta Ophthalmol. 2021, 99, e310–e318. [Google Scholar] [CrossRef] [PubMed]
- Shihab, Z.M.; Beebe, W.E.; Wentlandt, T. Possible significance of cilioretinal arteries in open-angle glaucoma. Ophthalmology 1985, 92, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.; Lehmann, R.P. Cilioretinal arteries. A study based on review of stereo fundus photographs and fluorescein angiographic findings. Arch. Ophthalmol. 1976, 94, 1355–1358. [Google Scholar] [CrossRef]
- Mansouri, K. Optical coherence tomography angiography and glaucoma: Searching for the missing link. Expert Rev. Med. Devices 2016, 13, 879–880. [Google Scholar] [CrossRef] [Green Version]
- Budde, W.M.; Jonas, J.B. Influence of cilioretinal arteries on neuroretinal rim and parapapillary atrophy in glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, T. Ocular Blood Flow and Influencing Factors for Glaucoma. Asia Pac. J. Ophthalmol. 2016, 5, 38–44. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H. Retinal ganglion cell apoptotic pathway in glaucoma: Initiating and downstream mechanisms. Prog. Brain Res. 2015, 220, 37–57. [Google Scholar] [CrossRef]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef]
- De Hoz, R.; Rojas, B.; Ramírez, A.I.; Salazar, J.J.; Gallego, B.I.; Triviño, A.; Ramírez, J.M. Retinal Macroglial Responses in Health and Disease. Biomed. Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [Green Version]
- Gericke, A.; Mann, C.; Zadeh, J.K.; Musayeva, A.; Wolff, I.; Wang, M.; Pfeiffer, N.; Daiber, A.; Li, H.; Xia, N.; et al. Elevated Intraocular Pressure Causes Abnormal Reactivity of Mouse Retinal Arterioles. Oxid. Med. Cell. Longev. 2019, 2019, 9736047. [Google Scholar] [CrossRef] [Green Version]
- Alnawaiseh, M.; Lahme, L.; Eter, N.; Mardin, C. Optical coherence tomography angiography: Value for glaucoma diagnostics. Ophthalmologe 2019, 116, 602–609. [Google Scholar] [CrossRef]
- Jonas, J.B.; Fernández, M.C. Shape of the neuroretinal rim and position of the central retinal vessels in glaucoma. Br. J. Ophthalmol. 1994, 78, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Budde, W.M.; Németh, J.; Gründler, A.E.; Mistlberger, A.; Hayler, J.K. Central retinal vessel trunk exit and location of glaucomatous parapapillary atrophy in glaucoma. Ophthalmology 2001, 108, 1059–1064. [Google Scholar] [CrossRef]
- Lindenmuth, K.A.; Skuta, G.L.; Musch, D.C.; Bueche, M. Significance of cilioretinal arteries in primary open angle glaucoma. Arch. Ophthalmol. 1988, 106, 1691–1693. [Google Scholar] [CrossRef]
- Lee, S.S.; Schwartz, B. Role of the temporal cilioretinal artery in retaining central visual field in open-angle glaucoma. Ophthalmology 1992, 99, 696–699. [Google Scholar] [CrossRef]
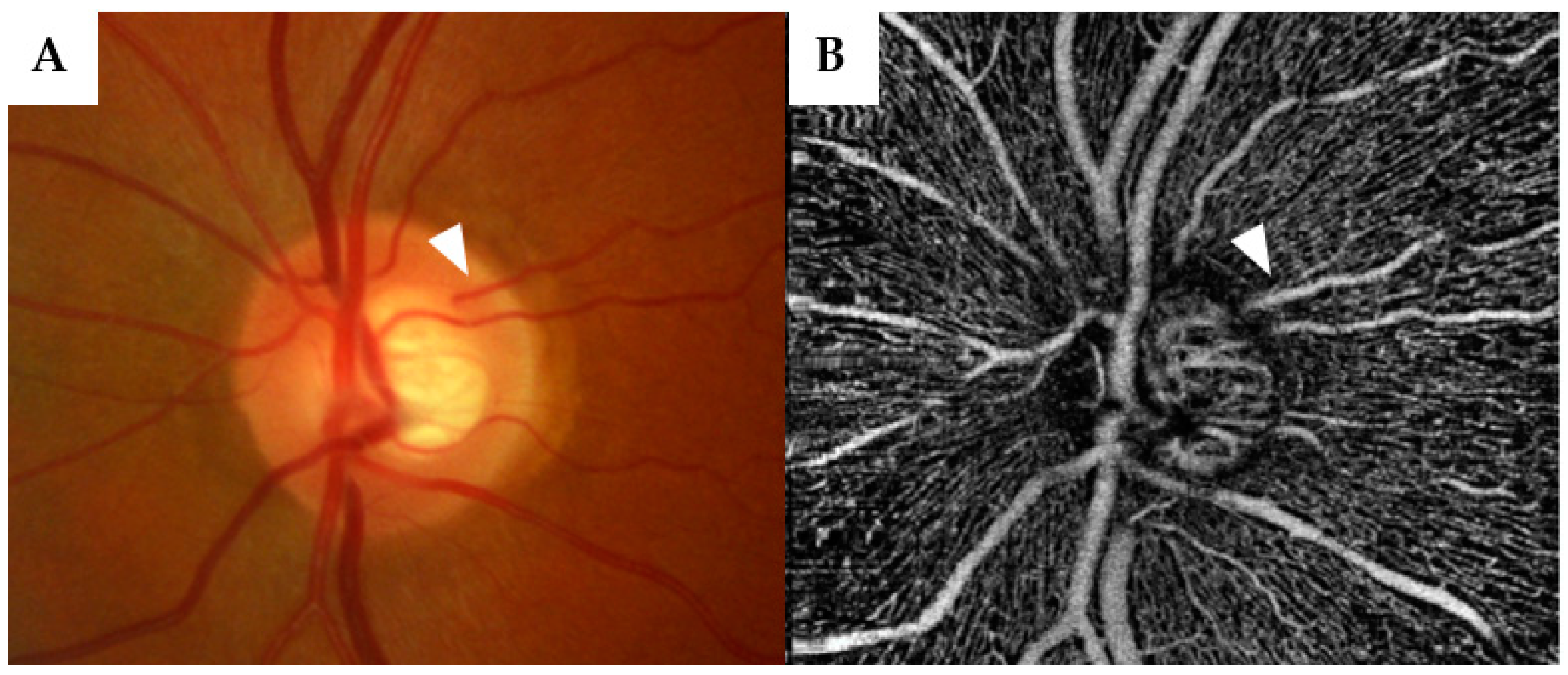
| Eyes (n) | 201 |
| Patients (n) | 134 |
| Age (years) * | 62.01 ± 15.82 |
| Gender (M:F) | 57:77 |
| Study eye (R:L) | 98:103 |
| Eyes (n) according to type of glaucoma | |
| Primary open angle glaucoma | 132 (65.7%) |
| Pseudoexfoliation glaucoma | 33 (16.4%) |
| Normal-tension glaucoma | 26 (12.9%) |
| Pigment dispersion glaucoma | 10 (5.0%) |
| Visual acuity (logMAR) ** | 0.1 (0; 0.2) |
| non-CRA | CRA | p-Value | |
|---|---|---|---|
| eyes (n) | 145 | 56 | |
| Location of CRA | |||
| Temporal (%) | 52 (92.9%) | ||
| Nasal (%) | 4 (7.1%) | ||
| Age (years) * | 66.8 (58.0; 74.1) | 62.8 (55.5; 69.5) | 0.08 |
| Gender (M:F) | 42:63 | 22:27 | 0.83 |
| Study eye (R:L) | 69:76 | 29:27 | 0.57 |
| Eyes (n) according to type of glaucoma | 0.29 | ||
| Primary open angle glaucoma | 93 (64.1%) | 39 (70.0%) | |
| Pseudoexfoliation glaucoma | 28 (19.3%) | 5 (8.9%) | |
| Normal-tension glaucoma | 17 (11.7%) | 9 (16.1%) | |
| Pigment dispersion glaucoma | 7 (4.8%) | 3 (5.4%) | |
| IOP (mmHg) * | 14 (12; 17) | 14 (12; 16.5) | 0.68 |
| MD (dB) * | −2.43 (−6.68; −0.74) | −2.06 (−8.25; −0.32) | 0.85 |
| PSD * | 2.81 (1.85; 6.74) | 2.45 (1.65; 9.40) | 0.72 |
| Visual acuity (logMAR)* | 0.10 (0.20; 0.00) | 0.10 (0.18; 0.10) | 0.91 |
| Quality index * | |||
| – macular images | 8 (7; 8) | 8 (7; 8) | 0.15 |
| – papillary images | 8 (7; 8) | 8 (7; 8) | 0.76 |
| Eyes (n) according to disease severity | 0.99 | ||
| (Hodapp-Parrish-Anderson Classification) | |||
| Group 1 (early glaucoma) | 104 (71.7%) | 40 (71.4%) | |
| Group 2 (moderate glaucoma) | 19 (13.1%) | 8 (14.3%) | |
| Group 3 (advanced glaucoma) | 22 (15.2%) | 8 (14.3%) |
| Location | Non-CRA | CRA | p-Value | |
|---|---|---|---|---|
| SCP | Whole en face | 41.73 | 42.04 | 0.81 |
| Whole en face superior hemisphere | 41.55 | 41.54 | 0.96 | |
| Whole en face inferior hemisphere | 41.34 | 42.18 | 0.71 | |
| ETDRS | 40.94 | 41.89 | 0.80 | |
| Fovea | 18.24 | 19.73 | 0.26 | |
| Para Fovea | 43.84 | 44.47 | 0.94 | |
| Para Fovea superior hemisphere | 43.66 | 44.07 | 0.82 | |
| Para Fovea inferior hemisphere | 43.79 | 44.93 | 0.70 | |
| Para Fovea temporal | 42.29 | 42.56 | 0.78 | |
| Para Fovea superior | 44.7 | 44.95 | 0.99 | |
| Para Fovea nasal | 42.80 | 44.36 | 0.27 | |
| Para Fovea inferior | 45.66 | 45.73 | 0.63 | |
| DCP | Whole en face | 49.6 | 50.77 | 0.39 |
| Whole en face superior hemisphere | 49.25 | 51.36 | 0.07 | |
| Whole en face inferior hemisphere | 48.87 | 50.52 | 0.11 | |
| ETDRS | 48.58 | 50.74 | 0.05 | |
| Fovea | 33.11 | 33.31 | 0.33 | |
| Para Fovea | 51.68 | 52.91 | 0.42 | |
| Para Fovea superior hemisphere | 50.91 | 53.63 | 0.03 | |
| Para Fovea inferior hemisphere | 50.88 | 52.78 | 0.10 | |
| Para Fovea temporal | 50.49 | 52.06 | 0.23 | |
| Para Fovea superior | 51.11 | 53.81 | 0.03 | |
| Para Fovea nasal | 50.96 | 53.88 | 0.03 | |
| Para Fovea inferior | 50.77 | 53.8 | 0.05 | |
| RPC | Whole en face | 48.34 | 49.54 | 0.79 |
| Whole en face capillaries | 42.19 | 43.77 | 0.89 | |
| Inside disc all | 54.93 | 55.05 | 0.96 | |
| Inside disc capillaries | 47.20 | 47.04 | 0.93 | |
| Peripapillary all | 50.85 | 51.26 | 0.60 | |
| Peripapillary capillaries | 44.85 | 45.68 | 0.94 | |
| Superior hemisphere all | 51.74 | 52.17 | 0.91 | |
| Inferior hemisphere all | 49.91 | 51.9 | 0.72 | |
| Superior hemisphere capillaries | 45.65 | 46.86 | 0.84 | |
| Inferior hemisphere capillaries | 43.79 | 46.25 | 0.92 | |
| Nasal superior | 42.92 | 44.07 | 0.77 | |
| Nasal inferior | 40.29 | 41.59 | 0.59 | |
| Inferior nasal | 42.53 | 43.60 | 0.93 | |
| Inferior temporal | 50.61 | 54.59 | 0.80 | |
| Temporal inferior | 47.63 | 48.76 | 0.59 | |
| Temporal superior | 51.6 | 53.08 | 0.37 | |
| Superior temporal | 48.00 | 46.52 | 0.26 | |
| Superior nasal | 41.52 | 41.93 | 0.63 |
| Group 1 MD: ≥ −6 dB (Early) | Group 2 MD: −6–≥ −12 dB (Moderate) | Group 3 MD: < −12 dB (Advanced) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Non-CRA | CRA | p-Value | Non-CRA | CRA | p-Value | Non-CRA | CRA | p-Value | |
| SCP | Whole en face | 42.46 | 43.01 | 0.57 | 38.75 | 41.13 | 0.74 | 32.68 | 31.95 | 0.76 |
| Whole en face superior hemisphere | 42.16 | 42.64 | 0.60 | 41.2 | 42.33 | 0.76 | 34.15 | 34.38 | 0.67 | |
| Whole en face inferior hemisphere | 42.34 | 43.53 | 0.42 | 40.92 | 44.11 | 0.38 | 30.32 | 31.16 | 0.84 | |
| ETDRS | 41.82 | 42.54 | 0.49 | 39.26 | 42.44 | 0.38 | 32.36 | 31.67 | 0.92 | |
| Fovea | 19.38 | 20.8 | 0.39 | 14.49 | 18.27 | 0.47 | 16.00 | 15.9 | 0.87 | |
| Para Fovea | 45.07 | 45.73 | 0.77 | 42.08 | 44.09 | 0.67 | 35.11 | 33.79 | 0.91 | |
| Para Fovea superior hemisphere | 44.11 | 44.84 | 0.37 | 43.1 | 43.3 | 0.49 | 36.66 | 36.14 | 0.76 | |
| Para Fovea inferior hemisphere | 44.92 | 46.02 | 0.46 | 43.18 | 48.35 | 0.28 | 31.55 | 33.23 | 0.68 | |
| Para Fovea temporal | 43.38 | 43.74 | 0.90 | 39.15 | 43.92 | 0.38 | 31.86 | 33.7 | 0.90 | |
| Para Fovea superior | 44.96 | 45.88 | 0.61 | 45.06 | 44.18 | 0.91 | 37.66 | 36.15 | 0.92 | |
| Para Fovea nasal | 43.39 | 45.86 | 0.16 | 43.71 | 44.83 | 0.45 | 36.89 | 36.30 | 0.76 | |
| Para Fovea inferior | 46.33 | 47.63 | 0.94 | 44.71 | 50.83 | 0.28 | 32.32 | 35.08 | 0.29 | |
| DCP | Whole en face | 49.55 | 50.85 | 0.31 | 50.58 | 49.71 | 0.81 | 49.26 | 50.9 | 0.54 |
| Whole en face superior hemisphere | 48.25 | 51.64 | 0.13 | 50.99 | 50.83 | 0.68 | 49.25 | 51.34 | 0.17 | |
| Whole en face inferior hemisphere | 48.81 | 50.46 | 0.19 | 49.16 | 50.87 | 0.82 | 48.71 | 50.52 | 0.20 | |
| ETDRS | 48.34 | 50.84 | 0.10 | 49.82 | 49.66 | 0.82 | 48.58 | 50.69 | 0.18 | |
| Fovea | 34.22 | 35.97 | 0.18 | 31.96 | 29.35 | 0.64 | 30.22 | 30.43 | 0.94 | |
| Para Fovea | 51.36 | 52.49 | 0.33 | 53.34 | 51.44 | 0.55 | 51.63 | 53.35 | 0.48 | |
| Para Fovea superior hemisphere | 50.25 | 53.6 | 0.07 | 53.17 | 52.17 | 0.82 | 51.45 | 53.74 | 0.09 | |
| Para Fovea inferior hemisphere | 50.55 | 52.32 | 0.13 | 52.24 | 53.99 | 0.66 | 50.85 | 53.18 | 0.46 | |
| Para Fovea temporal | 50.38 | 52.5 | 0.23 | 52.08 | 53.41 | 0.87 | 50.43 | 51.50 | 0.79 | |
| Para Fovea superior | 50.84 | 53.18 | 0.07 | 53.01 | 51.35 | 0.82 | 51.71 | 54.43 | 0.10 | |
| Para Fovea nasal | 50.42 | 53.67 | 0.08 | 52.51 | 54.44 | 0.62 | 52.07 | 53.44 | 0.39 | |
| Para Fovea inferior | 50.57 | 52.98 | 0.14 | 51.71 | 53.86 | 0.58 | 52.47 | 54.32 | 0.06 | |
| RPC | Whole en face | 49.81 | 51.43 | 0.43 | 42.71 | 44.9 | 0.80 | 39.10 | 35.32 | 0.24 |
| Whole en face capillaries | 43.94 | 45.33 | 0.66 | 38.22 | 40.49 | 0.45 | 29.34 | 28.74 | 0.39 | |
| Inside disc all | 56.9 | 57.04 | 0.69 | 53.8 | 56.03 | 0.76 | 49.87 | 49.94 | 0.99 | |
| Inside disc capillaries | 49.02 | 49.05 | 0.99 | 46.41 | 47.06 | 0.66 | 42.31 | 44.73 | 0.67 | |
| Peripapillary all | 52.25 | 54.14 | 0.23 | 43.9 | 46.51 | 0.75 | 37.45 | 34.37 | 0.36 | |
| Peripapillary capillaries | 46.81 | 48.57 | 0.41 | 39.62 | 42.29 | 0.72 | 28.38 | 27.69 | 0.28 | |
| Superior hemisphere all | 52.96 | 54.32 | 0.51 | 45.41 | 46.1 | 0.66 | 38.48 | 37.57 | 0.52 | |
| Inferior hemisphere all | 52.92 | 53.44 | 0.20 | 45.61 | 52.55 | 0.49 | 36.02 | 32.51 | 0.28 | |
| Superior hemisphere capillaries | 47.72 | 48.65 | 0.69 | 38.45 | 38.52 | 0.66 | 29.69 | 29.66 | 0.99 | |
| Inferior hemisphere capillaries | 47.19 | 47.45 | 0.35 | 38.27 | 46.57 | 0.49 | 27.13 | 24.83 | 0.27 | |
| Nasal superior | 44.40 | 45.65 | 0.25 | 40.95 | 37.08 | 0.66 | 27.42 | 25.89 | 0.83 | |
| Nasal inferior | 42.21 | 43.68 | 0.26 | 37.87 | 46.98 | 0.62 | 30.34 | 28.80 | 0.79 | |
| Inferior nasal | 45.37 | 45.68 | 0.55 | 36.26 | 41.17 | 0.49 | 21.47 | 18.13 | 0.63 | |
| Inferior temporal | 52.63 | 55.21 | 0.14 | 36.47 | 55.63 | 0.62 | 25.24 | 18.79 | 0.25 | |
| Temporal inferior | 48.92 | 51.92 | 0.14 | 48.71 | 46.74 | 0.98 | 35.81 | 32.76 | 0.38 | |
| Temporal superior | 52.11 | 53.76 | 0.40 | 53.02 | 55.96 | 0.25 | 40.51 | 42.68 | 0.60 | |
| Superior temporal | 50.76 | 50.79 | 0.77 | 39.35 | 29.49 | 0.31 | 25.27 | 25.96 | 0.67 | |
| Superior nasal | 44.04 | 44.33 | 0.91 | 35.87 | 27.67 | 0.37 | 25.93 | 23.37 | 0.47 | |
| CRA | Non-CRA | ||||||
|---|---|---|---|---|---|---|---|
| Location | Estimate | Lower 95%-CI Bound | Upper 95%-CI Bound | Estimate | Lower 95%-CI Bound | Upper 95%-CI Bound | |
| SCP | Whole en face | 0.33 | 0.04 | 0.57 | 0.45 | 0.27 | 0.59 |
| Whole en face superior hemisphere | 0.40 | 0.01 | 0.69 | 0.47 | 0.24 | 0.65 | |
| Whole en face inferior hemisphere | 0.30 | −0.16 | 0.65 | 0.56 | 0.36 | 0.72 | |
| ETDRS | 0.30 | −0.16 | 0.65 | 0.54 | 0.33 | 0.69 | |
| Fovea | 0.16 | −0.16 | 0.45 | 0.26 | 0.08 | 0.42 | |
| Para Fovea | 0.34 | 0.02 | 0.60 | 0.43 | 0.26 | 0.58 | |
| Para Fovea superior hemisphere | 0.26 | −0.35 | 0.72 | 0.46 | 0.21 | 0.65 | |
| Para Fovea inferior hemisphere | 0.34 | −0.07 | 0.66 | 0.52 | 0.31 | 0.69 | |
| Para Fovea temporal | 0.35 | −0.20 | 0.74 | 0.52 | 0.30 | 0.68 | |
| Para Fovea superior | 0.22 | −0.21 | 0.59 | 0.43 | −0.93 | 0.99 | |
| Para Fovea nasal | 0.25 | −0.16 | 0.58 | 0.43 | 0.26 | 0.55 | |
| Para Fovea inferior | 0.32 | 0.01 | 0.57 | 0.54 | 0.33 | 0.69 | |
| DCP | Whole en face | 0.22 | −0.08 | 0.48 | 0.00 | −0.19 | 0.19 |
| Whole en face superior hemisphere | 0.25 | −0.32 | 0.69 | −0.11 | −0.48 | 0.3 | |
| Whole en face inferior hemisphere | 0.36 | −0.14 | 0.72 | 0.05 | −0.37 | 0.46 | |
| ETDRS | 0.29 | −0.35 | 0.75 | 0.00 | −0.45 | 0.44 | |
| Fovea | 0.23 | −0.18 | 0.57 | 0.25 | 0.07 | 0.41 | |
| Para Fovea | 0.16 | −0.18 | 0.47 | −0.03 | −0.23 | 0.16 | |
| Para Fovea superior hemisphere | 0.25 | −0.66 | 0.87 | −0.14 | −0.50 | 0.26 | |
| Para Fovea inferior hemisphere | 0.26 | −0.35 | 0.71 | −0.02 | −0.40 | 0.36 | |
| Para Fovea temporal | 0.32 | −0.35 | 0.78 | 0.02 | −0.51 | 0.53 | |
| Para Fovea superior | 0.10 | −0.98 | 0.99 | −0.20 | −0.59 | 0.26 | |
| Para Fovea nasal | 0.28 | −0.15 | 0.62 | −0.06 | −0.36 | 0.26 | |
| Para Fovea inferior | 0.13 | −0.25 | 0.47 | −0.05 | −0.37 | 0.29 | |
| RPC | Whole en face | 0.56 | 0.23 | 0.78 | 0.53 | 0.35 | 0.67 |
| Whole en face capillaries | 0.57 | −1.00 | 1.00 | 0.57 | 0.36 | 0.73 | |
| Inside disc all | 0.38 | −0.46 | 0.86 | 0.31 | 0.03 | 0.55 | |
| Inside disc capillaries | 0.27 | −0.82 | 0.94 | 0.27 | −0.05 | 0.54 | |
| Peripapillary all | 0.61 | 0.20 | 0.84 | 0.53 | 0.35 | 0.67 | |
| Peripapillary capillaries | 0.59 | 0.43 | 0.72 | 0.59 | 0.37 | 0.75 | |
| Superior hemisphere all | 0.52 | −0.75 | 0.97 | 0.53 | 0.30 | 0.70 | |
| Inferior hemisphere all | 0.46 | −0.03 | 0.77 | 0.55 | 0.33 | 0.72 | |
| Superior hemisphere capillaries | 0.53 | −0.06 | 0.85 | 0.54 | 0.31 | 0.71 | |
| Inferior hemisphere capillaries | 0.53 | −0.28 | 0.90 | 0.56 | 0.33 | 0.72 | |
| Nasal superior | 0.49 | −0.03 | 0.80 | 0.55 | 0.32 | 0.72 | |
| Nasal inferior | 0.28 | −0.12 | 0.61 | 0.42 | 0.25 | 0.54 | |
| Inferior nasal | 0.48 | −0.07 | 0.81 | 0.48 | 0.25 | 0.66 | |
| Inferior temporal | 0.62 | 0.07 | 0.88 | 0.56 | 0.30 | 0.74 | |
| Temporal inferior | 0.41 | −0.26 | 0.82 | 0.46 | −0.99 | 1.00 | |
| Temporal superior | 0.07 | −1.00 | 1.00 | 0.40 | 0.12 | 0.62 | |
| Superior temporal | 0.65 | −0.66 | 0.98 | 0.46 | 0.16 | 0.69 | |
| Superior nasal | 0.55 | 0.14 | 0.81 | 0.46 | 0.12 | 0.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, J.A.; Storp, J.J.; Diener, R.; Danzer, M.F.; Esser, E.L.; Eter, N.; Brücher, V.C. Influence of Cilioretinal Arteries on Flow Density in Glaucoma Patients Measured Using Optical Coherence Tomography Angiography. J. Clin. Med. 2023, 12, 2458. https://doi.org/10.3390/jcm12072458
Zimmermann JA, Storp JJ, Diener R, Danzer MF, Esser EL, Eter N, Brücher VC. Influence of Cilioretinal Arteries on Flow Density in Glaucoma Patients Measured Using Optical Coherence Tomography Angiography. Journal of Clinical Medicine. 2023; 12(7):2458. https://doi.org/10.3390/jcm12072458
Chicago/Turabian StyleZimmermann, Julian Alexander, Jens Julian Storp, Raphael Diener, Moritz Fabian Danzer, Eliane Luisa Esser, Nicole Eter, and Viktoria Constanze Brücher. 2023. "Influence of Cilioretinal Arteries on Flow Density in Glaucoma Patients Measured Using Optical Coherence Tomography Angiography" Journal of Clinical Medicine 12, no. 7: 2458. https://doi.org/10.3390/jcm12072458





