The Dark Side of Ultrasound Imaging in Parathyroid Disease
Abstract
:1. Introduction
2. Overview of Parathyroid Anatomy, Physiology, and Pathology
3. Ultrasonographic Findings of Parathyroid Glands
4. Ultrasound Mimics of Parathyroid Glands
5. Complementary Imaging Techniques
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McQueen, A.S.; Bhatia, K.S. Head and neck ultrasound: Technical advances, novel applications and the role of elastography. Clin. Radiol. 2018, 73, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Ovčariček, P.P.; Giovanella, L.; Gasset, I.C.; Hindié, E.; Huellner, M.W.; Luster, M.; Piccardo, A.; Weber, T.; Talbot, J.-N.; Verburg, F.A. The EANM practice guidelines for parathyroid imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- American Institute of Ultrasound in Medicine. AIUM Practice Guideline for the performance of thyroid and parathyroid ultrasound examination. J. Ultrasound. Med. 2003, 22, 1126–1130.
- Korwar, V.; Chang, F.Y.; Teasdale, E.; Suchett-Kaye, I.; Edwards, A.; Morgan, J. Stepwise Approach for Parathyroid Localisation in Primary Hyperparathyroidism. World J. Surg. 2020, 44, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.W.; Barraclough, B.M.; Sidhu, S.B.; Sywak, M.S.; Barraclough, B.H.; Delbridge, L.W. Two hundred consecutive parathyroid ultrasound studies by a single clinician: The impact of experience. Endocr. Pract. 2006, 12, 257–263. [Google Scholar] [CrossRef]
- Udelsman, R.; Lin, Z.; Donovan, P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann. Surg. 2011, 253, 585–591. [Google Scholar] [CrossRef]
- Giovanella, L.; Bacigalupo, L.; Treglia, G.; Piccardo, A. Will (18)F-fluorocholine PET/CT replace other methods of preoperative parathyroid imaging? Endocrine 2021, 71, 285–297. [Google Scholar] [CrossRef]
- Treglia, G.; Giovannini, E.; Di Franco, D.; Calcagni, M.L.; Rufini, V.; Picchio, M.; Giordano, A. The role of positron emission tomography using carbon-11 and fluorine-18 choline in tumors other than prostate cancer: A systematic review. Ann. Nucl. Med. 2012, 26, 451–461. [Google Scholar] [CrossRef]
- Quak, E.; Blanchard, D.; Houdu, B.; Le Roux, Y.; Ciappuccini, R.; Lireux, B.; de Raucourt, D.; Grellard, J.-M.; Licaj, I.; Bardet, S.; et al. F18-choline PET/CT guided surgery in primary hyperparathyroidism when ultrasound and MIBI SPECT/CT are negative or inconclusive: The APACH1 study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Cantisani, V.; D’Ambrosio, F.; Nielsen, M.B. Multiparametric Ultrasound of Thyroid Nodules: Where Do We Stand? Ultraschall. Med. 2017, 38, 357–359. [Google Scholar] [CrossRef]
- Drudi, F.M.; Cantisani, V.; Angelini, F.; Ciccariello, M.; Messineo, D.; Ettorre, E.; Liberatore, M.; Scialpi, M. Multiparametric MRI Versus Multiparametric US in the Detection of Prostate Cancer. Anticancer Res. 2019, 39, 3101–3110. [Google Scholar] [CrossRef]
- Sidhu, P.S. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall. Med. 2015, 36, 315–317. [Google Scholar] [CrossRef]
- Cantisani, V.; Wilson, S.R. CEUS: Where are we in 2015? Eur. J. Radiol. 2015, 84, 1621–1622. [Google Scholar] [CrossRef]
- Pacini, P.; Polti, G.; Faggiano, A.; Giannetta, E.; Tarsitano, M.G.; Cantisani, V. Multiparametric ultrasound evaluation of a case of bilateral carotid body tumor. J. Ultrasound. 2021, 24, 311–315. [Google Scholar] [CrossRef]
- Yen, H.H. Progress in the Ultrasonographic Microvascular Imaging. J. Med. Ultrasound. 2018, 26, 1–2. [Google Scholar] [CrossRef]
- Parra Ramirez, P.; Santiago Hernando, A.; Barquiel Alcala, B. Potential Utility of Contrast-Enhanced Ultrasound in the Preoperative Evaluation of Primary Hyperparathyroidism. J. Ultrasound. Med. 2019, 38, 2565–2571. [Google Scholar] [CrossRef]
- Pavlovics, S.; Radzina, M.; Niciporuka, R. Contrast-Enhanced Ultrasound Qualitative and Quantitative Characteristics of Parathyroid Gland Lesions. Medicina 2021, 58, 2. [Google Scholar] [CrossRef]
- Azizi, G.; Piper, K.; Keller, J.M.; Mayo, M.L.; Puett, D.; Earp, K.M.; Malchoff, C.D. Shear wave elastography and parathyroid adenoma: A new tool for diagnosing parathyroid adenomas. Eur. J. Radiol. 2016, 85, 1586–1593. [Google Scholar] [CrossRef] [Green Version]
- Isidori, A.M.; Cantisani, V.; Giannetta, E. Multiparametric ultrasonography and ultrasound elastography in the differentiation of parathyroid lesions from ectopic thyroid lesions or lymphadenopathies. Endocrine 2017, 57, 335–343. [Google Scholar] [CrossRef]
- Ünlütürk, U.; Erdoğan, M.F.; Demir, Ö.; Çulha, C.; Gullu, S.; Başkal, N. The role of ultrasound elastography in preoperative localization of parathyroid lesions: A new assisting method to preoperative parathyroid ultrasonography. Clin. Endocrinol. 2012, 76, 492–498. [Google Scholar] [CrossRef]
- Trimboli, P.; D’Aurizio, F.; Tozzoli, R.; Giovanella, L. Measurement of thyroglobulin, calcitonin, and PTH in FNA washout fluids. Clin. Chem. Lab. Med. 2017, 55, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Politz, D.; Browarsky, I. Diagnostic aspiration of parathyroid adenomas causes severe fibrosis complicating surgery and final histologic diagnosis. Thyroid 2007, 17, 1251–1255. [Google Scholar] [CrossRef]
- Bancos, I.; Grant, C.S.; Nadeem, S.; Stan, M.N.; Reading, C.C.; Sebo, T.J.; Algeciras-Schimnich, A.; Singh, R.J. Risks and benefits of parathyroid fine-needle aspiration with parathyroid hormone washout. Endocr. Pract. 2012, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Lentsch, E.J.; Withrow, K.P.; Ackermann, D.; Bumpous, J.M. Parathyromatosis and recurrent hyperparathyroidism. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Dhingra, S.; Mishra, S.K.; Krishnani, N. Implantation of parathyroid carcinoma along fine needle aspiration track. Langenbecks Arch. Surg. 2006, 391, 623–626. [Google Scholar] [CrossRef]
- Bollerslev, J.; Schalin-Jäntti, C.; Rejnmark, L. Management of endocrine disease: Unmet therapeutic, educational and scientific needs in parathyroid disorders. Eur. J. Endocrinol. 2019, 181, P1–P19. [Google Scholar] [CrossRef] [Green Version]
- Fancy, T.; Gallagher, D., 3rd; Hornig, J.D. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol. Clin. North Am. 2010, 43, 221–227. [Google Scholar] [CrossRef]
- Carlson, D. Parathyroid pathology: Hyperparathyroidism and parathyroid tumors. Arch. Pathol. Lab. Med. 2010, 134, 1639–1644. [Google Scholar] [CrossRef]
- Duan, K.; Hernandez, K.G.; Mete, O. Clinicopathological correlates of hyperparathyroidism. J. Clin. Pathol. 2015, 68, 771–787. [Google Scholar] [CrossRef]
- Mazeh, H.; Kouniavsky, G.; Schneider, D.F.; Makris, K.I.; Sippel, R.S.; Dackiw, A.P.; Chen, H.; Zeiger, M.A. Intrathyroidal parathyroid glands: Small, but mighty (a Napoleon phenomenon). Surgery 2012, 152, 1193–1200. [Google Scholar] [CrossRef]
- Phitayakorn, R.; McHenry, C. Incidence and location of ectopic abnormal parathyroid glands. Am. J. Surg. 2006, 191, 418–423. [Google Scholar] [CrossRef]
- Taterra, D.; Wong, L.M.; Vikse, J.; Sanna, B.; Pękala, P.; Walocha, J.; Cirocchi, R.; Tomaszewski, K.; Henry, B.M. The prevalence and anatomy of parathyroid glands: A meta-analysis with implications for parathyroid surgery. Langenbecks Arch. Surg. 2019, 404, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.K.; Kim, D.W.; Jung, S.J. Ultrasound detection of normal parathyroid glands: A preliminary study. Radiol. Med. 2017, 122, 866–870. [Google Scholar] [CrossRef]
- Erickson, L.A.; Mete, O.; Juhlin, C.C.; Perren, A.; Gill, A.J. Overview of the 2022 WHO Classification of Parathyroid Tumors. Endocr. Pathol. 2022, 33, 64–89. [Google Scholar] [CrossRef]
- Fraser, W.D. Hyperparathyroidism. Lancet 2009, 374, 145–158. [Google Scholar] [CrossRef]
- Pepe, J.; Cipriani, C.; Pilotto, R.; De Lucia, F.; Castro, C.; Lenge, L.; Russo, S.; Guarnieri, V.; Scillitani, A.; Carnevale, V.; et al. Sporadic and hereditary primary hyperparathyroidism. J. Endocrinol. Invest. 2011, 34, 40–44. [Google Scholar]
- Dugonjić, S.; Šišić, M.; Radulović, M.; Ajdinović, B. Positive (99m)Tc-MIBI and the subtraction parathyroid scan are related to intact parathyroid hormone but not to total plasma calcium in primary hyperparathyroidism. Hell J. Nucl. Med. 2017, 20, 46–50. [Google Scholar]
- El Lakis, M.; Nockel, P.; Gaitanidis, A. Probability of Positive Genetic Testing Results in Patients with Family History of Primary Hyperparathyroidism. J. Am. Coll. Surg. 2018, 226, 933–938. [Google Scholar] [CrossRef]
- Jamal, S.A.; Miller, P.D. Secondary and tertiary hyperparathyroidism. J. Clin. Densitom. 2013, 16, 64–68. [Google Scholar] [CrossRef]
- Nasrallah, M.P.; Fraker, D.L.; LiVolsi, V.A. Parathyroid carcinoma in the setting of tertiaryhyperparathyroidism after renal transplant. Endocr. Pathol. 2014, 25, 433–435. [Google Scholar] [CrossRef]
- Moe, S.M. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur. J. Clin. Invest. 2006, 36, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Huppert, B.J.; Reading, C.C. Parathyroid sonography: Imaging and intervention. J. Clin. Ultrasound. 2007, 35, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Dietrich, C.F.; David, E.; Mastroeni, G.; Spagnolo, O.V.; Sidhu, P.; Letizia, C.; Messineo, D.; D’Ambrosio, F.; Radzina, M.; et al. Ultrasound and ultrasound-related techniques in endocrine diseases. Minerva Endocrinol. 2018, 43, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Guilmette, J.; Sadow, P.M. Parathyroid Pathology. Surg. Pathol. Clin. 2019, 12, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Acar, T.; Ozbek, S.S.; Ertan, Y.; Kavukcu, G.; Tuncyurek, M.; Icoz, R.G.; Akyildiz, M.M.; Makay, O.; Acar, S. Variable sonographic spectrum of parathyroid adenoma with a novel ultrasound finding: Dual concentric echo sign. Med. Ultrason. 2015, 17, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Moloudi, F.; Ghasemi-rad, M. The role of colour Doppler ultrasonography in the preoperative localization of parathyroid adenomas. Endocr. J. 2012, 59, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Vitetta, G.M.; Ravera, A.; Mensa, G.; Fuso, L.; Neri, P.; Carriero, A.; Cirillo, S. Actual role of color-doppler high-resolution neck ultrasonography in primary hyperparathyroidism: A clinical review and an observational study with a comparison of (99m)Tc-sestamibi parathyroid scintigraphy. J. Ultrasound. 2019, 22, 291–308. [Google Scholar] [CrossRef]
- Hayakawa, N.; Nakamoto, Y.; Kurihara, K.; Yasoda, A.; Kanamoto, N.; Miura, M.; Inagaki, N.; Togashi, K. A comparison between 11C-methionine PET/CT and MIBI SPECT/CT for localization of parathyroid adenomas/hyperplasia. Nucl. Med. Commun. 2015, 36, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Ruda, J.M.; Hollenbeak, C.S.; Stack, B.C., Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol. Head Neck Surg. 2005, 132, 359–372. [Google Scholar] [CrossRef]
- Xue, J.; Liu, Y.; Ji, T.; Zhao, A.; Liang, Y.; Deng, H.; Wang, Q.; Zhang, Y.; Yang, L.; Yang, A. Comparison between technetium-99m methoxyisobutylisonitrile scintigraphy and ultrasound in the diagnosis of parathyroid adenoma and parathyroid hyperplasia. Nucl. Med. Commun. 2018, 39, 1129–1137. [Google Scholar] [CrossRef]
- Strambu, V.; Bratucu, M.; Garofil, D.; Paic, V.; Zurzu, M.; Tigora, A.; Popa, F.; Radu, P. The Value of Imaging of the Parathyroid Glands in Secondary Hyperparathyroidism. Chirurgia 2019, 114, 541–549. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, W.; Zhou, J.; Zhou, W. Role of ultrasound in the differentiation of parathyroid carcinoma and benign parathyroid lesions. Clin. Radiol. 2020, 75, 179–184. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Talat, N.; Patel, P.; Mulholland, N.J.; Schulte, K.-M. Ultrasound features of malignancy in the preoperative diagnosis of parathyroid cancer: A retrospective analysis of parathyroid tumours larger than 15 mm. Eur. Radiol. 2011, 21, 1865–1873. [Google Scholar] [CrossRef]
- Platz Batista da Silva, N.; Jung, E.M.; Jung, F. VueBox(R) perfusion analysis of contrast-enhanced ultrasound (CEUS) examinations in patients with primary hyperparathyroidism for preoperative detection of parathyroid gland adenoma. Clin. Hemorheol. Microcirc. 2018, 70, 423–431. [Google Scholar] [CrossRef]
- Ratniece, M.; Tauvena, E.; Pavlovics, S.; Niciporuka, R.; Liepa, M.; Prieditis, P.; Ozolins, A.; Gardovskis, J.; Radzina, M.; Narbuts, Z. Large Parathyroid Tumor 8 Years after Thyroid Surgery: A Case Report. Case Rep. Oncol. 2022, 15, 528–534. [Google Scholar] [CrossRef]
- Heller, M.T.; Yip, L.; Tublin, M.E. Sonography of intrathyroid parathyroid adenomas: Are there distinctive features that allow for preoperative identification? Eur. J. Radiol. 2013, 82, e22–e27. [Google Scholar] [CrossRef]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in Parathyroid Imaging. Magn. Reson. Imaging Clin. N Am. 2018, 26, 151–166. [Google Scholar] [CrossRef]
- Trimboli, P.; Castellana, M.; Virili, C.; Havre, R.F.; Bini, F.; Marinozzi, F.; D’Ambrosio, F.; Giorgino, F.; Giovanella, L.; Prosch, H.; et al. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: A systematic review and meta-analysis using histological standard of reference. Radiol. Med. 2020, 125, 406–415. [Google Scholar] [CrossRef]
- Barbaros, U.; Erbil, Y.; Salmashoğlu, A. The characteristics of concomitant thyroid nodules cause false-positive ultrasonography results in primary hyperparathyroidism. Am. J. Otolaryngol. 2009, 30, 239–243. [Google Scholar] [CrossRef]
- Scerrino, G.; Attard, M.; Piccolo, C.L. The coexistence of primary hyperparathyroidism and thyroid nodules: Should the preoperative work-up of the parathyroid and the thyroid diseases be specifically adjusted? G. Chir. 2016, 37, 123–129. [Google Scholar] [CrossRef]
- Treglia, G.; Trimboli, P.; Huellner, M. Imaging in primary hyperparathyroidism: Focus on the evidence-based diagnostic performance of different methods. Minerva Endocrinol. 2018, 43, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Radzina, M.; Ratniece, M.; Putrins, D.S.; Saule, L.; Cantisani, V. Performance of Contrast-Enhanced Ultrasound in Thyroid Nodules: Review of Current State and Future Perspectives. Cancers 2021, 13, 5469. [Google Scholar] [CrossRef] [PubMed]
- Rago, T.; Scutari, M.; Santini, F. Real-time elastosonography: Useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J. Clin. Endocrinol. Metab. 2010, 95, 5274–5280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batur, A.; Atmaca, M.; Yavuz, A.; Ozgokce, M.; Bora, A.; Bulut, M.D.; Arslan, H.; Toktas, O.; Alpaslan, M. Ultrasound Elastography for Distinction Between Parathyroid Adenomas and Thyroid Nodules. J. Ultrasound. Med. 2016, 35, 1277–1282. [Google Scholar] [CrossRef]
- Chandramohan, A.; Therese, M.; Abhraham, D. Can ARFI elastography be used to differentiate parathyroid from thyroid lesions? J. Endocrinol. Invest. 2018, 41, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Ghervan, C.; Silaghi, A.; Nemes, C. Parathyroid incidentaloma detected during thyroid sonography—Prevalence and significance beyond images. Med. Ultrason. 2012, 14, 187–191. [Google Scholar]
- Rohan, K.; Ramesh, A.; Sureshkumar, S. Evaluation of B-Mode and Color Doppler Ultrasound in the Diagnosis of Malignant Cervical Lymphadenopathy. Cureus 2020, 12, e9819. [Google Scholar] [CrossRef]
- Liu, H.; Liao, Q.; Wang, Y.; Hu, Y.; Zhu, Q.; Wang, L.; Liu, Q.; Li, J.; Jiang, Y. A new tool for diagnosing parathyroid lesions: Angio plus ultrasound imaging. J. Thorac. Dis. 2019, 11, 4829–4834. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Liu, J. Value of Qualitative and Quantitative Contrast-Enhanced Ultrasound Analysis in Preoperative Diagnosis of Cervical Lymph Node Metastasis from Papillary Thyroid Carcinoma. J. Ultrasound. Med. 2020, 39, 73–81. [Google Scholar] [CrossRef]
- Cotoi, L.; Borcan, F.; Sporea, I.; Amzar, D.; Schiller, O.; Schiller, A.; Dehelean, C.A.; Pop, G.N.; Stoian, D. Shear Wave Elastography in Diagnosing Secondary Hyperparathyroidism. Diagnostics 2019, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Mete, O.; Asa, S.L.; Gill, A.J.; Kimura, N.; de Krijger, R.R.; Tischler, A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2022, 33, 90–114. [Google Scholar] [CrossRef]
- Rubenthaler, J.; Lutz, J.; Reiser, M. Title Page—Paraganglioma of the Head and Neck: Follow-Up of Interventional Procedures with CEUS. Ultraschall. Med. 2015, 36, 541–543. [Google Scholar]
- Puliani, G.; Sesti, F.; Feola, T.; Di Leo, N.; Polti, G.; Verrico, M.; Modica, R.; Colao, A.; Lenzi, A.; Isidori, A.M.; et al. Natural History and Management of Familial Paraganglioma Syndrome Type 1: Long-Term Data from a Large Family. J. Clin. Med. 2020, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Valentino, M.; Quiligotti, C.; Carone, L. Branchial cleft cyst. J. Ultrasound. 2013, 16, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, A.T.; King, A.D.; Metreweli, C. Second branchial cleft cysts: Variability of sonographic appearances in adult cases. AJNR Am. J. Neuroradiol. 2000, 21, 315–319. [Google Scholar]
- Sadick, M.; Overhoff, D.; Baessler, B.; von Spangenberg, N.; Krebs, L.; Wohlgemuth, W.A. Peripheral Vascular Anomalies—Essentials in Periinterventional Imaging. Rofo 2020, 192, 150–162. [Google Scholar] [CrossRef]
- Majewska, N.K.; Stajgis, P.; Wykrętowicz, M. Peripheral vascular malformations—Modern imaging. Pol. J. Radiol. 2018, 83, e253–e259. [Google Scholar] [CrossRef]
- Ovčariček, P.P.; Giovanella, L.; Hindie, E.; Huellner, M.W.; Talbot, J.-N.; Verburg, F.A. An essential practice summary of the new EANM guidelines for parathyroid imaging. Q J. Nucl. Med. Mol. Imaging 2022, 66, 93–103. [Google Scholar]
- Alharbi, A.A.; Alshehri, F.M.; Albatly, A.A. [(18)F]Fluorocholine Uptake of Parathyroid Adenoma Is Correlated with Parathyroid Hormone Level. Mol. Imaging Biol. 2018, 20, 857–867. [Google Scholar] [CrossRef]
- Piccardo, A.; Trimboli, P.; Rutigliani, M. Additional value of integrated (18)F-choline PET/4D contrast-enhanced CT in the localization of hyperfunctioning parathyroid glands and correlation with molecular profile. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 766–775. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Miner Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
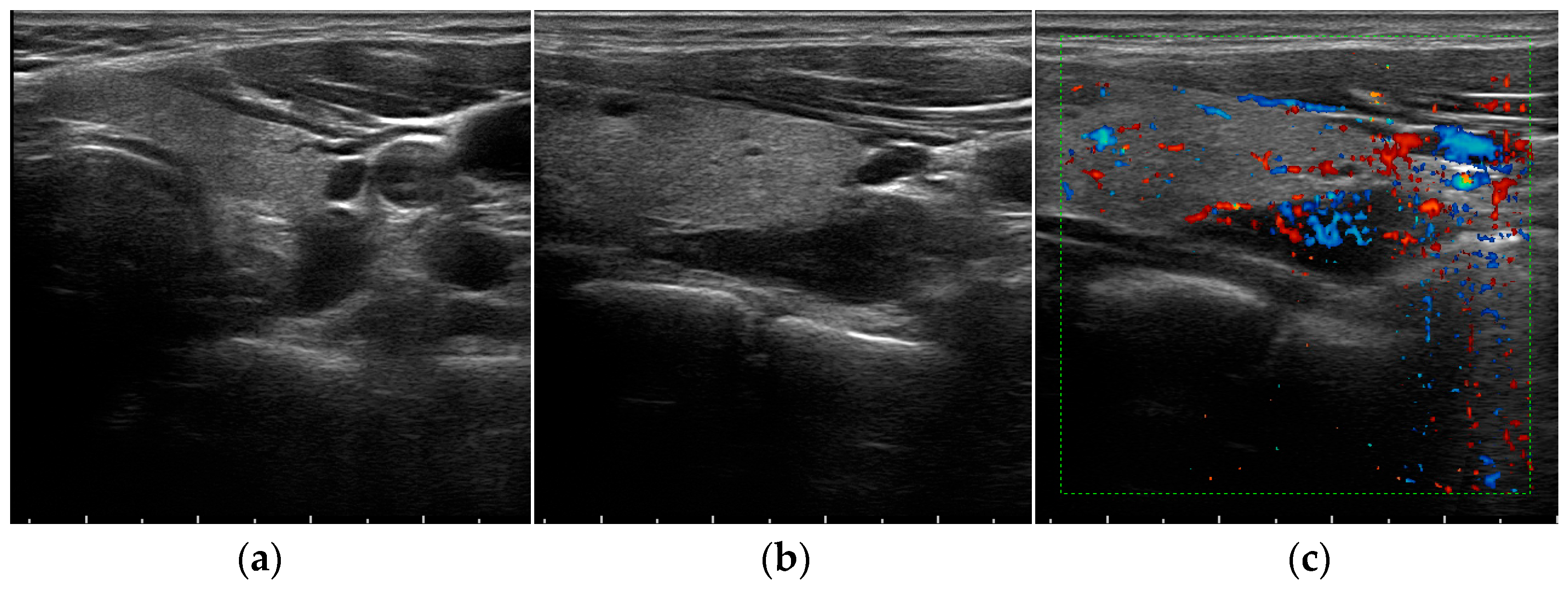

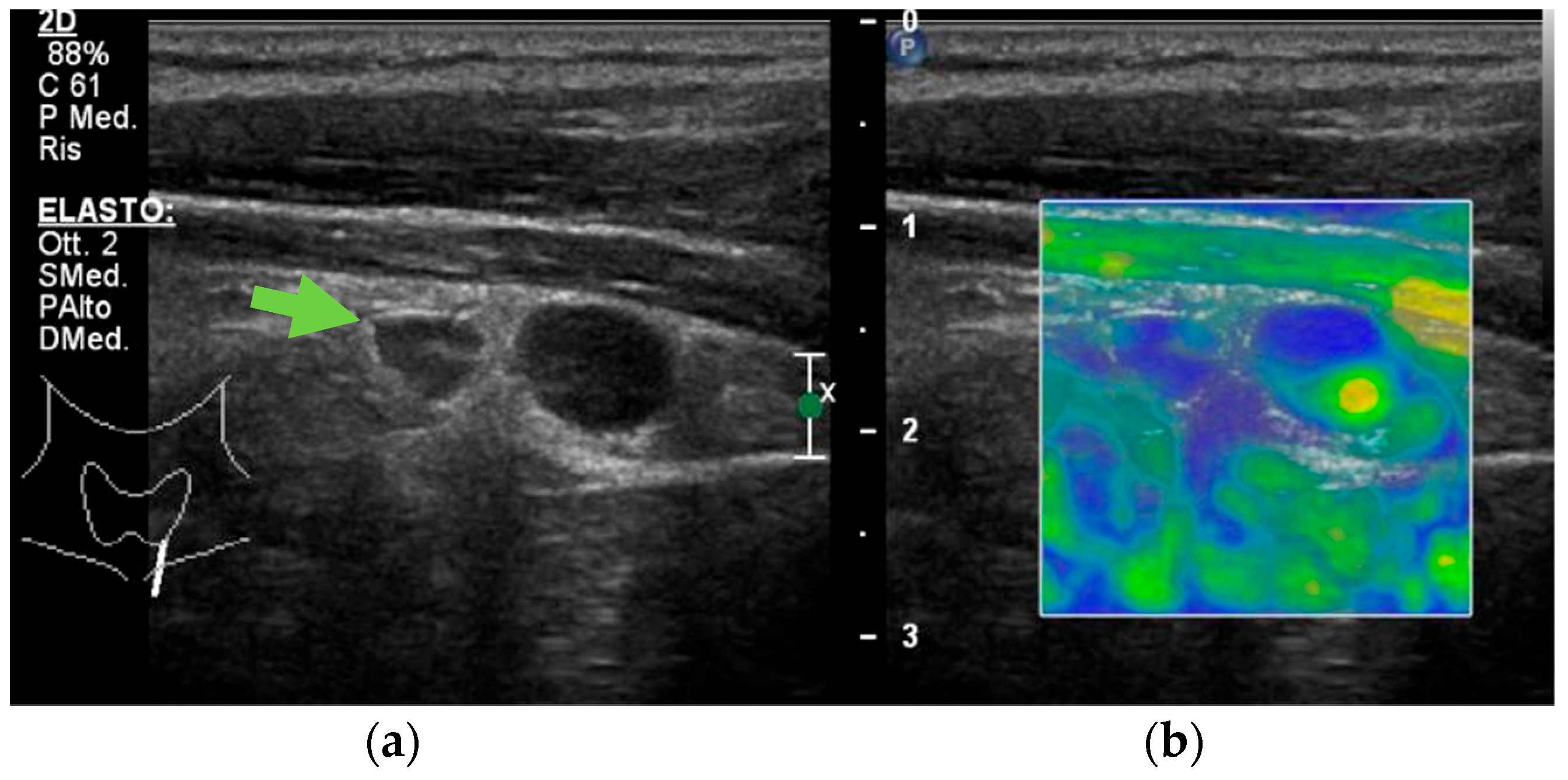



| B-Mode | Color Doppler US | CEUS | USE | |
|---|---|---|---|---|
| Parathyroid hyperplasia | More than one symmetrically or asymmetrically enlarged, hypoechoic, oval shaped, lobulated gland. Significantly smaller than adenoma. Cystic inclusions may be seen. | Feeding polar vessels entering the pole and then extending around the periphery. | Fast intense homogeneous enhancement. Fast homogeneous wash-out. | Stiffer than proper parathyroid glands. |
| Parathyroid adenoma | Enlarged, circumscribed, hypoechoic, oval shaped lesion, delineated by hyperechoic halo. Cystic inclusions may be seen. | Feeding polar vessels entering the pole and then extending around the periphery. | Early peripheral hyperenhancement. Central wash-out in the later phases. | Stiffer than hyperplastic parathyroid glands. |
| Parathyroid carcinoma | Length > 3 cm, depth/width ratio > 1. Lobulated, heterogeneous, hypoechoic lesion. Irregular borders. Thick capsule. Intranodular calcifications. Cystic inclusions may be seen. | Intralesional disordered vascularity. | Early heterogeneous enhancement. Early homogeneous wash-out. | Stiffer than proper, hyperplastic, and adenomatous parathyroid glands. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centello, R.; Sesti, F.; Feola, T.; Sada, V.; Pandozzi, C.; Di Serafino, M.; Pacini, P.; Cantisani, V.; Giannetta, E.; Tarsitano, M.G. The Dark Side of Ultrasound Imaging in Parathyroid Disease. J. Clin. Med. 2023, 12, 2487. https://doi.org/10.3390/jcm12072487
Centello R, Sesti F, Feola T, Sada V, Pandozzi C, Di Serafino M, Pacini P, Cantisani V, Giannetta E, Tarsitano MG. The Dark Side of Ultrasound Imaging in Parathyroid Disease. Journal of Clinical Medicine. 2023; 12(7):2487. https://doi.org/10.3390/jcm12072487
Chicago/Turabian StyleCentello, Roberta, Franz Sesti, Tiziana Feola, Valentina Sada, Carla Pandozzi, Marco Di Serafino, Patrizia Pacini, Vito Cantisani, Elisa Giannetta, and Maria Grazia Tarsitano. 2023. "The Dark Side of Ultrasound Imaging in Parathyroid Disease" Journal of Clinical Medicine 12, no. 7: 2487. https://doi.org/10.3390/jcm12072487
APA StyleCentello, R., Sesti, F., Feola, T., Sada, V., Pandozzi, C., Di Serafino, M., Pacini, P., Cantisani, V., Giannetta, E., & Tarsitano, M. G. (2023). The Dark Side of Ultrasound Imaging in Parathyroid Disease. Journal of Clinical Medicine, 12(7), 2487. https://doi.org/10.3390/jcm12072487






