Defining Time in Acute Upper Gastrointestinal Bleeding: When Should We Start the Clock?
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Comparison between Timeframes
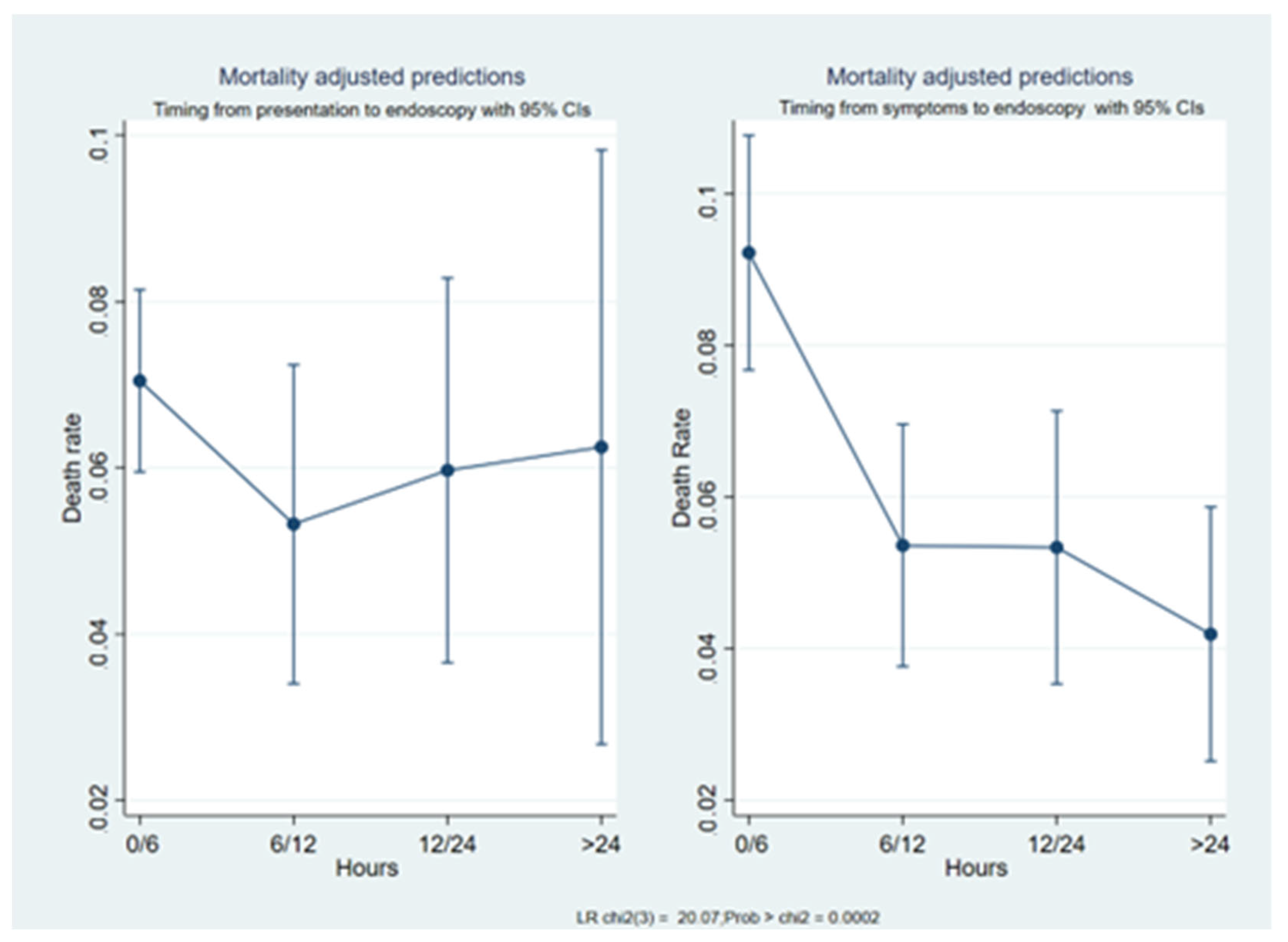
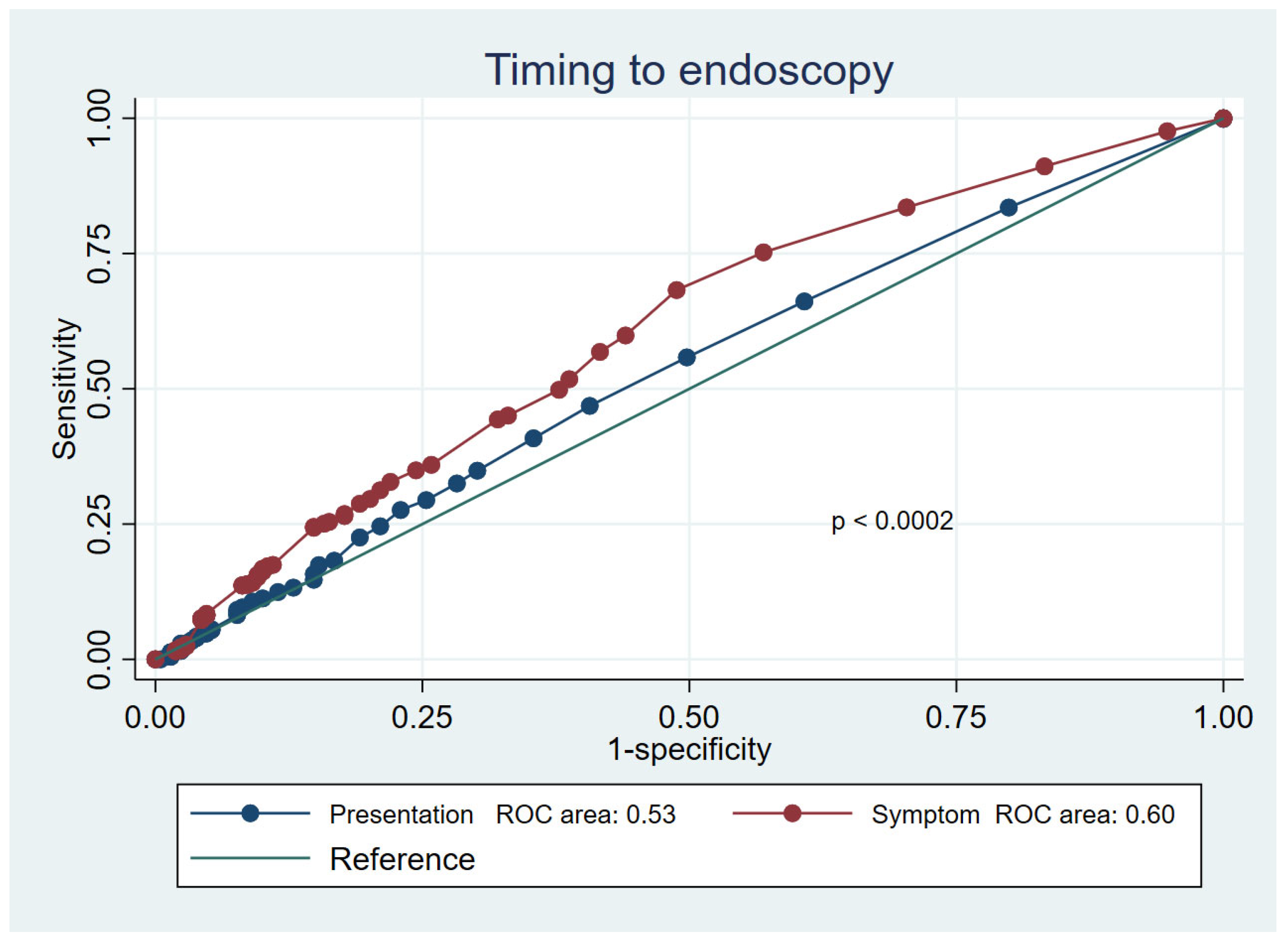
3.2. Mortality Prediction
4. Discussion
Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusano, C. Can we find a precise timing for endoscopic intervention in gastrointestinal bleeding? Dig. Endosc. 2022, 34, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.Y.W.; Yu, Y.; Tang, R.S.Y.; Chan, H.C.; Yip, H.C.; Chan, S.M.; Sung, J.J. Timing of Endoscopy for Acute Upper Gastrointestinal Bleeding. N. Engl. J. Med. 2020, 382, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.Y.W. Management of acute upper gastrointestinal bleeding: Urgent versus early endoscopy. Dig. Endosc. 2022, 34, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.F.; Chiu, P.W.Y.; Tsoi, K.K.F.; Chan, F.K.L.; Sung, J.J.Y.; Lau, J.Y.W. The effect of off-hours hospital admission on mortality and clinical outcomes for patients with upper gastrointestinal hemorrhage: A systematic review and meta-analysis of 20 cohorts. United Eur. Gastroenterol. J. 2018, 6, 367–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.L.; Cohen, A.J.; Nayor, J.; Claggett, B.L.; Saltzman, J.R. Timing of upper endoscopy influences outcomes in patients with acute nonvariceal upper GI bleeding. Gastrointest. Endosc. 2017, 85, 945–952.e1. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Dumonceau, J.-M.; Kuipers, E.J.; Lanas, A.; Sanders, D.S.; Kurien, M.; Rotondano, G.; Hucl, T.; Dinis-Ribeiro, M.; Marmo, R.; et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, a1–a46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, L.; Barkun, A.N.; Saltzman, J.R.; Martel, M.; Leontiadis, G.I. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am. J. Gastroenterol. 2021, 116, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Marmo, R.; Rotondano, G.; Koch, M.; Del Piano, M.; Bianco, M.; Zambelli, A.; Di Matteo, G.; Cipolletta, L. Timing of endoscopy and mortality from NonVariceal Upper Gastrointestinal Bleeding (NVUGIB): The sooner is not always the better. Dig. Liver Dis. 2011, 43, S118. [Google Scholar] [CrossRef]
- Laine, L.; Spiegel, B.; Rostom, A.; Moayyedi, P.; Kuipers, E.J.; Bardou, M.; Sung, J.J.Y.; Barkun, A.N. Methodology for Randomized Trials of Patients With Nonvariceal Upper Gastrointestinal Bleeding: Recommendations From an International Consensus Conference. Am. J. Gastroenterol. 2010, 105, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.W.; Lu, Y.W.; Teller, T.; Sekhon, H.K.; Wu, B.U. A modified Glasgow Blatchford Score improves risk stratification in upper gastrointestinal bleed: A prospective comparison of scoring systems. Aliment. Pharmacol. Ther. 2012, 36, 782–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Daabiss, M. American Society of Anaesthesiologists physical status classification. Indian J. Anaesth. 2011, 55, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Rockall, A.T.; Logan, R.F.; Devlin, H.B.; Northfield, T.C. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996, 38, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, J.R.; Tabak, Y.P.; Hyett, B.H.; Sun, X.; Travis, A.C.; Johannes, R.S. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest. Endosc. 2011, 74, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Blatchford, O.; Murray, W.R.; Blatchford, M. A risk score to predict need for treatment for uppergastrointestinal haemorrhage. Lancet 2000, 356, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Marmo, R.; Koch, M.; Cipolletta, L.; Capurso, L.; Grossi, E.; Cestari, R.; Bianco, A.M.; Pandolfo, N.; Dezi, A.; Casetti, T.; et al. Predicting mortality in non-variceal upper gastrointestinal bleeders: Validation of the Italian PNED Score and Prospective Comparison with the Rockall Score. Am. J. Gastroenterol. 2010, 105, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Marmo, R.; Soncini, M.; de Franchis, R. Patient’s performance status should dictate transfusion strategy in nonvariceal acute upper gastrointestinal bleeding (NV- AUGIB): A prospective multicenter cohort study: Transfusion strategy in NV- AUGIB. Dig. Liver Dis. 2020, 52, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Laursen, S.B.; Leontiadis, G.I.; Stanley, A.J.; Møller, M.H.; Hansen, J.M.; de Muckadell, O.B.S. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: A nationwide cohort study. Gastrointest. Endosc. 2017, 85, 936–944.e3. [Google Scholar] [CrossRef] [PubMed]
| n = 3166 | |
|---|---|
| Age, mean [± SD] | 68.2 [±15.7] |
| Male gender, n (%) | 2137 (67.5) |
| Hemodynamic instability, yes, n (%) | 226 (7.1) |
| Outpatients, n (%) | 2672 (84.4) |
| Bleeding source, n (%) | |
| Non-variceal | 2632 (83.1) |
| Variceal | 534 (16.9) |
| ASA score, n (%) | |
| I | 842 (26.6) |
| II | 1100 (34.7) |
| III | 1029 (32.5) |
| IV | 195 (6.2) |
| “Presentation-to-Endoscopy” timeframe, number of patients (%) | |
| 0–6 h | 2072 (65.4) |
| 6–12 h | 520 (16.4) |
| 12–24 h | 399 (12.6) |
| ≥24 h | 175 (5.5) |
| “Symptoms-to-Endoscopy” timeframe, number of patients (%) | |
| 0–6 h | 1304 (41.2) |
| 6–12 h | 745 (23.5) |
| 12–24 h | 578 (18.3) |
| ≥24 h | 539 (17) |
| Rockall score, mean (95% C.I.) | 4 (3–5) |
| Glasgow-Blatchford score, mean (95% C.I.) | 7 (5–9) |
| ABC score, mean (95% C.I.) | 5 (3–6) |
| AIMS65 score, mean (95% C.I.) | 1 (1–2) |
| PNED score, mean (95% C.I.) | 3 (1–6) |
| Endoscopy performed | |
| On call, number (%) | 904 (28.5) |
| Weekend, number (%) | 748 (23.6) |
| Clinical outcomes | |
| Transfusion, yes (%) | 1885 (59.5) |
| Re-bleeding, yes (%) | 192 (6.1) |
| Need for interventional radiology, yes (%) | 30 (0.9) |
| Need for surgery, yes (%) | 102 (3.2) |
| Mortality, yes (%) | 209 (6.6) |
| Length of stay, days [±SD] | 9.5 [±9] |
| Symptoms-to-Endoscopy Group | Presentation-to-Endoscopy Group | ||||
|---|---|---|---|---|---|
| 0–6 h | 6–12 h | 12–24 h | ≥24 h | Total | |
| 0–6 h | 1229 | 22 | 28 | 25 | 1304 |
| 6–12 h | 405 | 307 | 26 | 7 | 745 |
| 12–24 h | 195 | 145 | 225 | 13 | 578 |
| ≥24 h | 243 | 46 | 120 | 130 | 539 |
| Total | 2072 | 520 | 399 | 175 | 3166 |
| Timeframe | Odds Ratio | z | p > |z| | 95% Confidence Interval |
|---|---|---|---|---|
| 0–6 h | Reference | |||
| 6–12 h | 0.75 | −1.35 | 0.177 | 0.50–1.14 |
| 12–24 h | 0.84 | −0.74 | 0.457 | 0.54–1.32 |
| ≥24 h | 0.88 | −0.38 | 0.705 | 0.47–1.67 |
| _cons | 0.08 | −30.05 | 0.000 | 0.06–0.09 |
| Timeframe | Odds Ratio | z | p > |z| | 95% Confidence Interval |
|---|---|---|---|---|
| 0–6 h | Reference | |||
| 6–12 h | 0.55 | −3.15 | 0.002 | 0.37–0.80 |
| 12–24 h | 0.57 | −2.65 | 0.008 | 0.38–0.87 |
| ≥24 h | 0.45 | −3.39 | 0.001 | 0.29–0.72 |
| _cons | 0.10 | −23.91 | 0.000 | 0.08–0.12 |
| Timeframe | Odds Ratio | z | p > |z| | 95% Confidence Interval |
|---|---|---|---|---|
| Symptoms-to-endoscopy | ||||
| 0–6 h | Reference | |||
| 6–12 h | 0.62 | −2.08 | 0.038 | 0.40–0.97 |
| 12–24 h | 0.60 | −1.97 | 0.049 | 0.36–1.00 |
| ≥24 h | 0.46 | −2.80 | 0.005 | 0.27–0.79 |
| Presentation-to-endoscopy | ||||
| 0–6 h | Reference | |||
| 6–12 h | 0.94 | −0.25 | 0.802 | 0.56–1.56 |
| 12–24 h | 1.06 | 0.19 | 0.848 | 0.61–1.84 |
| ≥24 h | 1.20 | 0.49 | 0.623 | 0.58–2.49 |
| Bleeding severity | 1.76 | 2.50 | 0.013 | 1.13–2.74 |
| ASA score | ||||
| ASA I | Reference | |||
| ASA II | 4.34 | 3.81 | 0.000 | 2.04–9.26 |
| ASA III | 8.60 | 5.76 | 0.000 | 4.13–17.89 |
| ASA IV | 47.98 | 9.97 | 0.000 | 22.42–102.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marmo, R.; Soncini, M.; Bucci, C.; Marmo, C.; Riccioni, M.E.; on behalf of the GISED Study Group. Defining Time in Acute Upper Gastrointestinal Bleeding: When Should We Start the Clock? J. Clin. Med. 2023, 12, 2542. https://doi.org/10.3390/jcm12072542
Marmo R, Soncini M, Bucci C, Marmo C, Riccioni ME, on behalf of the GISED Study Group. Defining Time in Acute Upper Gastrointestinal Bleeding: When Should We Start the Clock? Journal of Clinical Medicine. 2023; 12(7):2542. https://doi.org/10.3390/jcm12072542
Chicago/Turabian StyleMarmo, Riccardo, Marco Soncini, Cristina Bucci, Clelia Marmo, Maria Elena Riccioni, and on behalf of the GISED Study Group. 2023. "Defining Time in Acute Upper Gastrointestinal Bleeding: When Should We Start the Clock?" Journal of Clinical Medicine 12, no. 7: 2542. https://doi.org/10.3390/jcm12072542
APA StyleMarmo, R., Soncini, M., Bucci, C., Marmo, C., Riccioni, M. E., & on behalf of the GISED Study Group. (2023). Defining Time in Acute Upper Gastrointestinal Bleeding: When Should We Start the Clock? Journal of Clinical Medicine, 12(7), 2542. https://doi.org/10.3390/jcm12072542





