Prognostic Impact of Polypharmacy following Trans-Catheter Aortic Valve Replacement
Abstract
:1. Background
2. Methods
2.1. Patient Selection
2.2. TAVR Procedure
2.3. Independent Variable and Primary Outcome
2.4. Clinical Variables
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
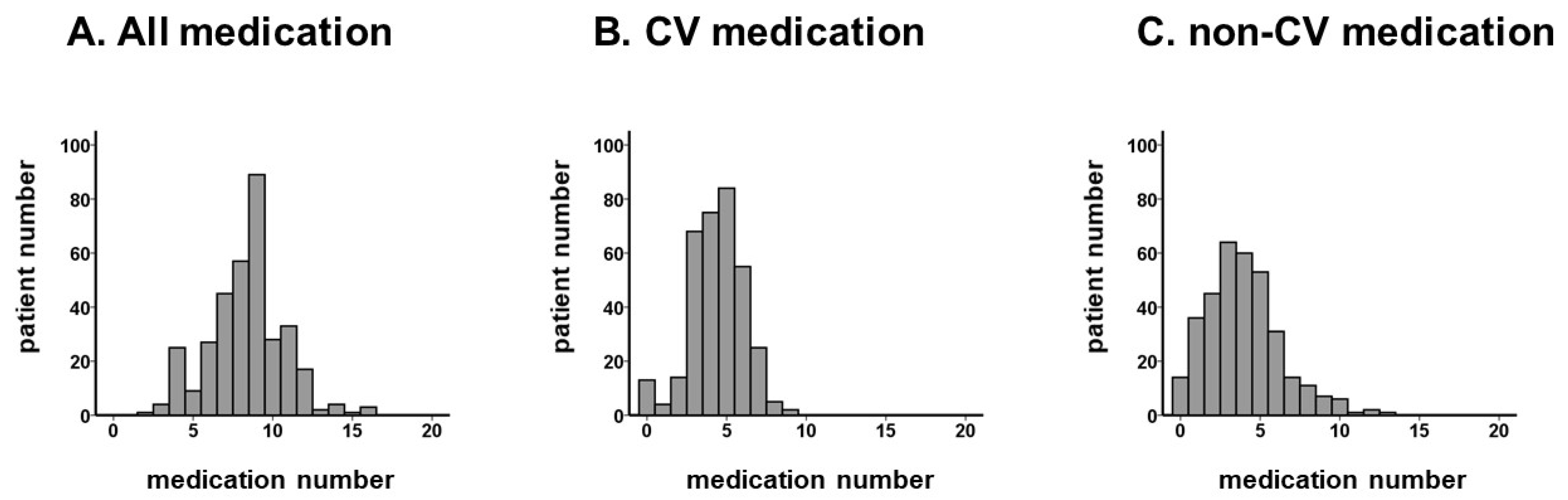
3.2. Medication Number:
3.3. Stratification of Patients’ Cohort by Polypharmacy
3.4. Impact of Polypharmacy on Clinical Outcomes
4. Discussion
4.1. Medication Number and Comorbidity
4.2. Polypharmacy and Clinical Outcome
4.3. Clinical Implication and Future Directions
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Hermiller, J.B., Jr.; Pinto, D.S.; Chetcuti, S.J.; Arshi, A.; Forrest, J.K.; Huang, J.; Yakubov, S.J. Predictors and Risk Calculator of Early Unplanned Hospital Readmission Following Contemporary Self-Expanding Transcatheter Aortic Valve Replacement from the STS/ACC TVT Registry. Cardiovasc. Revasc. Med. 2020, 21, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefevre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in Older Adults Undergoing Aortic Valve Replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenison, E.; Henderson, E.J. Multimorbidity and Frailty: Tackling Complexity in Parkinson’s Disease. J. Park. Dis. 2020, 10, S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, M.; Claggett, B.; Suzuki, K.; Hegde, S.M.; Shah, A.M.; Desai, A.S.; Lewis, E.F.; Shah, S.J.; Sweitzer, N.K.; Fang, J.C.; et al. Association of Hyper-Polypharmacy With Clinical Outcomes in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e008293. [Google Scholar] [CrossRef] [PubMed]
- Kennel, P.J.; Kneifati-Hayek, J.; Bryan, J.; Banerjee, S.; Sobol, I.; Lachs, M.S.; Safford, M.M.; Goyal, P. Prevalence and determinants of Hyperpolypharmacy in adults with heart failure: An observational study from the National Health and Nutrition Examination Survey (NHANES). BMC Cardiovasc. Disord. 2019, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Akishita, M.; Nakamura, T.; Nomura, K.; Ogawa, S.; Iijima, K.; Eto, M.; Ouchi, Y. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr. Gerontol. Int. 2012, 12, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Akishita, M.; Kameyama, Y.; Yamaguchi, K.; Yamamoto, H.; Eto, M.; Ouchi, Y. High risk of adverse drug reactions in elderly patients taking six or more drugs: Analysis of inpatient database. Geriatr. Gerontol. Int. 2012, 12, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Runganga, M.; Peel, N.M.; Hubbard, R.E. Multiple medication use in older patients in post-acute transitional care: A prospective cohort study. Clin. Interv. Aging 2014, 9, 1453–1462. [Google Scholar]
- Nishtala, P.S.; Salahudeen, M.S. Temporal Trends in Polypharmacy and Hyperpolypharmacy in Older New Zealanders over a 9-Year Period: 2005–2013. Gerontology 2015, 61, 195–202. [Google Scholar] [CrossRef]
- American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar]
- Hales, C.M.; Servais, J.; Martin, C.B.; Kohen, D. Prescription Drug Use Among Adults Aged 40–79 in the United States and Canada. NCHS Data Brief 2019, 1–8. [Google Scholar]
- Green, P.; Woglom, A.E.; Genereux, P.; Daneault, B.; Paradis, J.M.; Schnell, S.; Hawkey, M.; Maurer, M.S.; Kirtane, A.J.; Kodali, S.; et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: A single-center experience. JACC Cardiovasc. Interv. 2012, 5, 974–981. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswami, A.; Steinman, M.A.; Goyal, P.; Zullo, A.R.; Anderson, T.S.; Birtcher, K.K.; Goodlin, S.J.; Maurer, M.S.; Alexander, K.P.; Rich, M.W.; et al. Deprescribing in Older Adults With Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 73, 2584–2595. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Kjeldsen, K.P.; Delpon, E.; Semb, A.G.; Cerbai, E.; Dobrev, D.; Savarese, G.; Sulzgruber, P.; Rosano, G.; Borghi, C.; et al. Facing the challenge of polypharmacy when prescribing for older people with cardiovascular disease. A review by the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Mizukami, K.; Tomita, N.; Arai, H.; Ohrui, T.; Eto, M.; Takeya, Y.; Isaka, Y.; Rakugi, H.; Sudo, N.; et al. Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese: Report of the Japan Geriatrics Society Working Group on “Guidelines for medical treatment and its safety in the elderly”. Geriatr. Gerontol. Int. 2016, 16, 983–1001. [Google Scholar] [CrossRef] [PubMed]



| Total (N = 345) | PP (N = 88) | Non-PP (N = 257) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 85 (83, 89) | 85 (81, 88) | 85 (83, 88) | 0.27 |
| Men | 99 (29%) | 27 (31%) | 72 (28%) | 0.36 |
| Body surface area, m2 | 1.38 (1.28, 1.50) | 1.37 (1.25, 1.51) | 1.39 (1.29, 1.50) | 0.43 |
| STS score | 4.6 (3.9, 6.1) | 4.7 (4.0, 6.3) | 4.6 (3.9, 6.0) | 0.34 |
| Vital signs | ||||
| Systolic blood pressure, mmHg | 117 (106, 128) | 117 (102, 125) | 117 (106, 128) | 0.32 |
| Pulse rate, bpm | 70 (63, 78) | 68 (63, 77) | 70 (63, 78) | 0.83 |
| Comorbidity | ||||
| Atrial fibrillation | 44 (13%) | 11 (13%) | 33 (13%) | 0.55 |
| Hypertension | 252 (73%) | 64 (73%) | 188 (73%) | 0.52 |
| Diabetes mellitus | 61 (18%) | 18 (20%) | 43 (17%) | 0.26 |
| Dyslipidemia | 165 (48%) | 46 (52%) | 119 (46%) | 0.20 |
| Coronary artery disease | 88 (26%) | 32 (36%) | 56 (22%) | 0.006 * |
| History of stroke | 45 (13%) | 13 (15%) | 32 (12%) | 0.35 |
| History of heart failure | 138 (40%) | 40 (45%) | 98 (38%) | 0.14 |
| Chronic obstructive pulmonary disease | 21 (6%) | 6 (7%) | 15 (6%) | 0.46 |
| Peripheral artery disease | 77 (22%) | 25 (28%) | 52 (20%) | 0.076 |
| Frailty | ||||
| Mini-mental state examination, points | 26 (23, 28) | 26 (23, 28) | 26 (24, 29) | 0.38 |
| CSHA score, points | 4 (3, 4) | 4 (3, 4) | 4 (3, 5) | 0.52 |
| Echocardiography | ||||
| Aortic valve peak velocity, m/s | 2.1 (1.7, 2.4) | 2.1 (1.8, 2.4) | 2.0 (1.7, 2.4) | 0.62 |
| LVDd, mm | 45 (41, 49) | 46 (42, 49) | 45 (41, 49) | 0.45 |
| Left ventricular ejection fraction, % | 64 (57, 72) | 64 (59, 72) | 64 (57, 71) | 0.45 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 10.4 (9.7, 11.1) | 10.4 (9.6, 11.1) | 10.4 (9.7, 11.3) | 0.59 |
| Serum albumin, g/dL | 3.4 (3.0, 3.6) | 3.3 (2.9, 3.5) | 3.4 (3.1, 3.7) | 0.013 * |
| Serum sodium, mEq/L | 139 (138, 141) | 140 (138, 141) | 139 (137, 141) | 0.39 |
| Serum potassium, mEq/L | 4.3 (4.0, 4.6) | 4.3 (4.0, 4.4) | 4.3 (4.0, 4.6) | 0.32 |
| eGFR, mL/min/1.73 m2 | 49.4 (35.7, 62.4) | 44 (32, 57) | 52 (38, 66) | 0.006 * |
| Total cholesterol, mg/dL | 157 (135, 173) | 148 (131, 165) | 158 (137, 174) | 0.026 * |
| Low density lipoprotein cholesterol, mg/dL | 88 (2, 105) | 83 (66, 95) | 89 (73, 109) | 0.024 * |
| Plasma B-type natriuretic peptide, pg/mL | 107 (57, 224) | 118 (68, 294) | 94 (55, 196) | 0.041 * |
| C-reactive protein, mg/dL | 0.7 (0.3, 2.0) | 1.2 (0.4, 2.8) | 0.6 (0.3, 1.9) | 0.36 |
| Death (N = 21) | Readmission (N = 88) | |
|---|---|---|
| Cardiovascular | ||
| Heart failure | 2 | 18 |
| Stroke | 1 | 9 |
| Sudden death | 1 | 0 |
| Arrhythmia | 0 | 5 |
| Non cardiovascular | ||
| Unknown or others | 7 | 5 |
| Infection | 7 | 22 |
| Fixture | 0 | 10 |
| Renal failure | 3 | 3 |
| Malignancy | 0 | 11 |
| Gastrointestinal bleeding | 0 | 5 |
| Unadjusted Analyses | Adjusted Analyses | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Mortality | ||||
| Medication number per one medicine | 1.51 (1.24–1.79) | <0.001 * | 1.58 (1.24–1.88) | <0.001 * |
| PP versus non-PP | 19.5 (5.61–65.6) | <0.001 * | 21.4 (6.06–74.8) | <0.001 * |
| HF readmission | ||||
| Medication number per one medicine | 1.31 (1.06–1.58) | 0.005 * | 1.29 (1.03–1.58) | 0.018 * |
| PP versus non-PP | 5.21 (2.01–13.9) | 0.001 * | 4.52 (1.63–12.9) | 0.004 * |
| All readmission | ||||
| Medication number per one medicine | 1.24 (1.13–1.45) | <0.001 * | 1.29 (1.15–1.34) | <0.001 * |
| PP versus non-PP | 3.76 (2.56–5.89) | <0.001 * | 3.39 (2.12–5.39) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Narang, N.; Ushijima, R.; Sobajima, M.; Fukuda, N.; Ueno, H.; Kinugawa, K. Prognostic Impact of Polypharmacy following Trans-Catheter Aortic Valve Replacement. J. Clin. Med. 2023, 12, 2598. https://doi.org/10.3390/jcm12072598
Imamura T, Narang N, Ushijima R, Sobajima M, Fukuda N, Ueno H, Kinugawa K. Prognostic Impact of Polypharmacy following Trans-Catheter Aortic Valve Replacement. Journal of Clinical Medicine. 2023; 12(7):2598. https://doi.org/10.3390/jcm12072598
Chicago/Turabian StyleImamura, Teruhiko, Nikhil Narang, Ryuichi Ushijima, Mitsuo Sobajima, Nobuyuki Fukuda, Hiroshi Ueno, and Koichiro Kinugawa. 2023. "Prognostic Impact of Polypharmacy following Trans-Catheter Aortic Valve Replacement" Journal of Clinical Medicine 12, no. 7: 2598. https://doi.org/10.3390/jcm12072598
APA StyleImamura, T., Narang, N., Ushijima, R., Sobajima, M., Fukuda, N., Ueno, H., & Kinugawa, K. (2023). Prognostic Impact of Polypharmacy following Trans-Catheter Aortic Valve Replacement. Journal of Clinical Medicine, 12(7), 2598. https://doi.org/10.3390/jcm12072598









