Defining the Age of Young Ischemic Stroke Using Data-Driven Approaches
Abstract
:1. Introduction
2. Methods
2.1. Data Source and Patient Population
2.2. Data Variables
2.3. Data Pre-Processing and Imputation
2.4. Statistical Analysis
2.5. First Approach: Phenotype Modeling
2.6. Second Approach: Machine Learning
3. Results
3.1. Patient Population and Characteristics
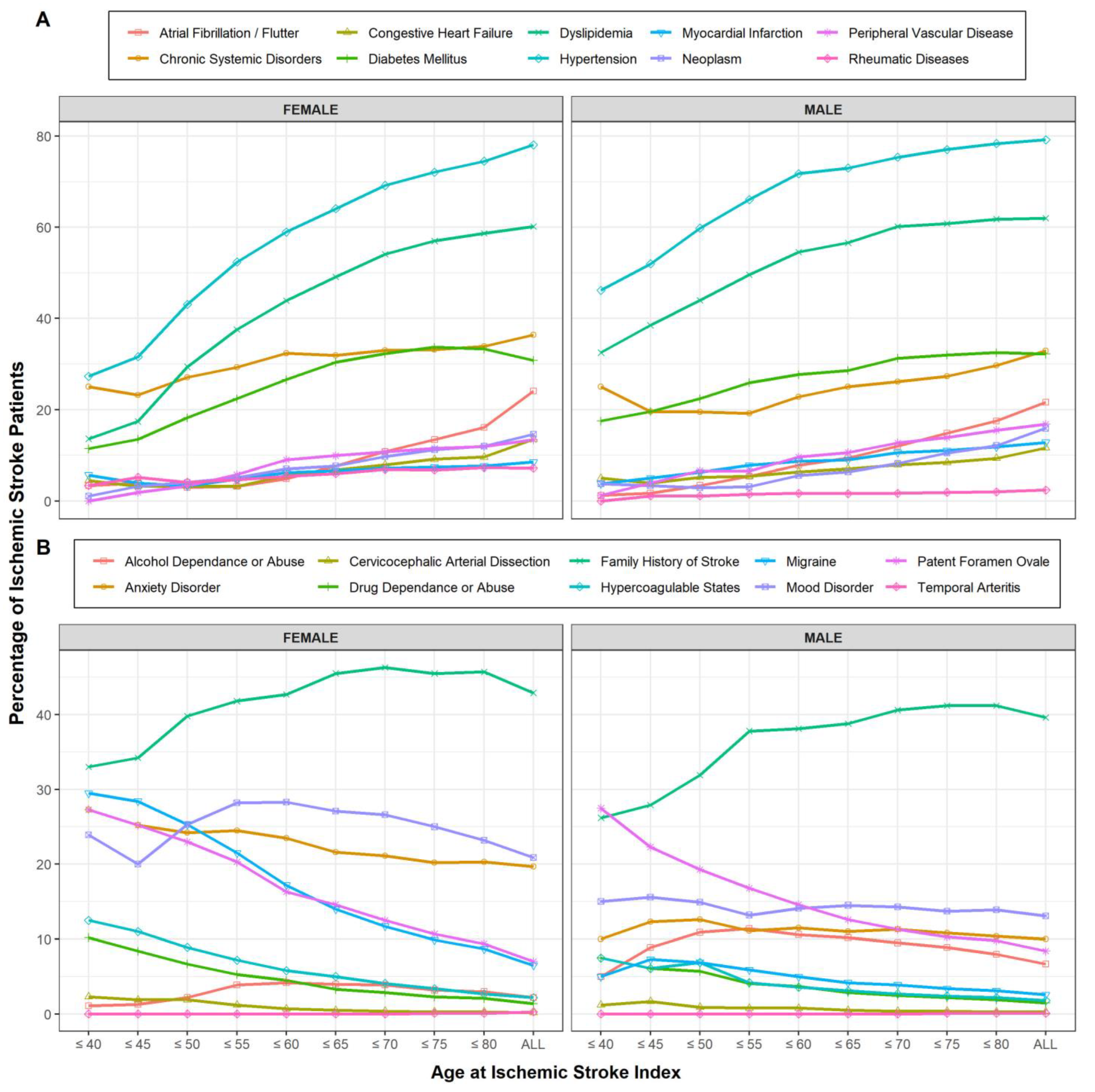
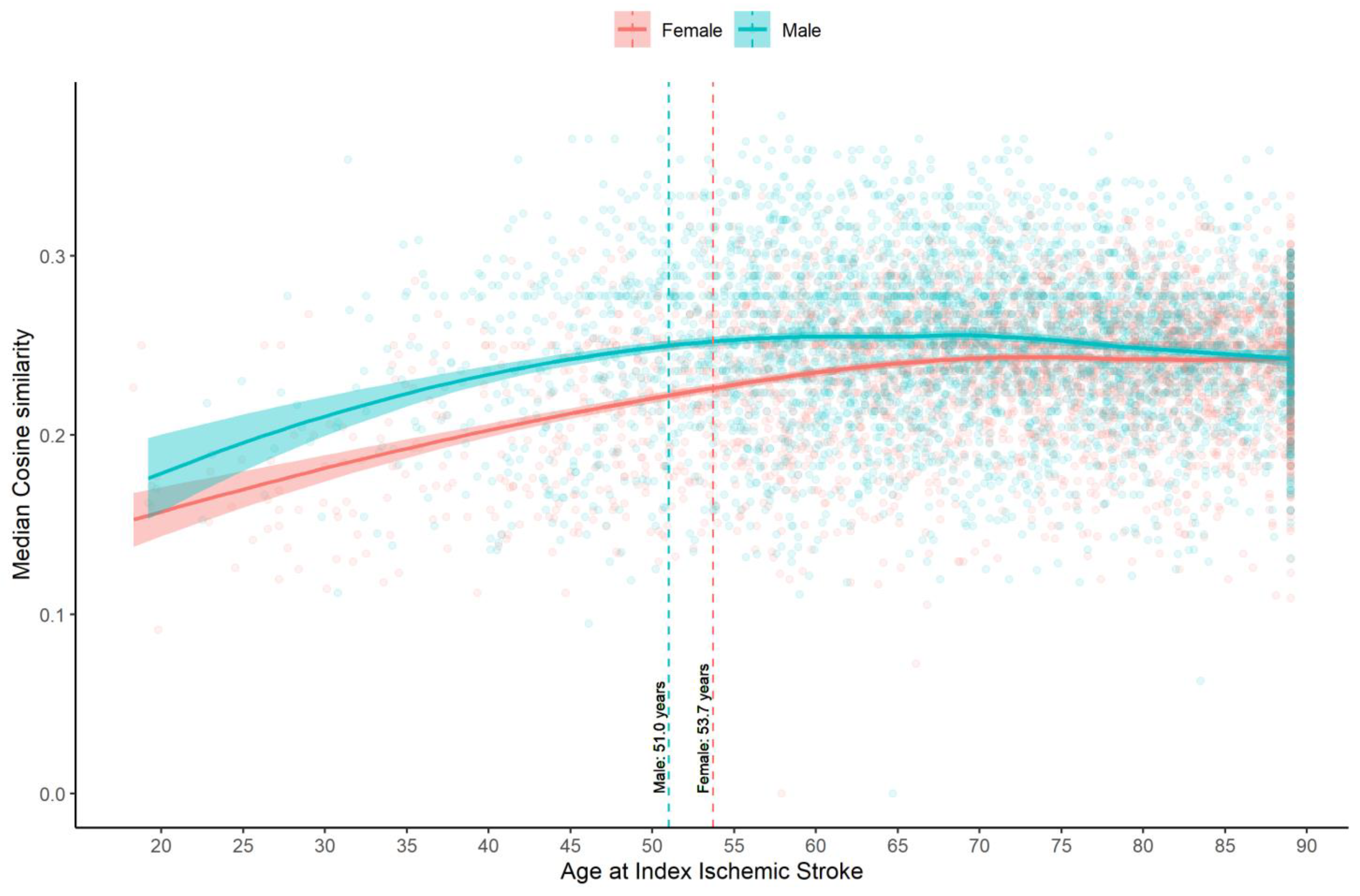
3.2. Estimating the Age Cut-Point for Young IS Using Phenotype Modeling (Approach 1)
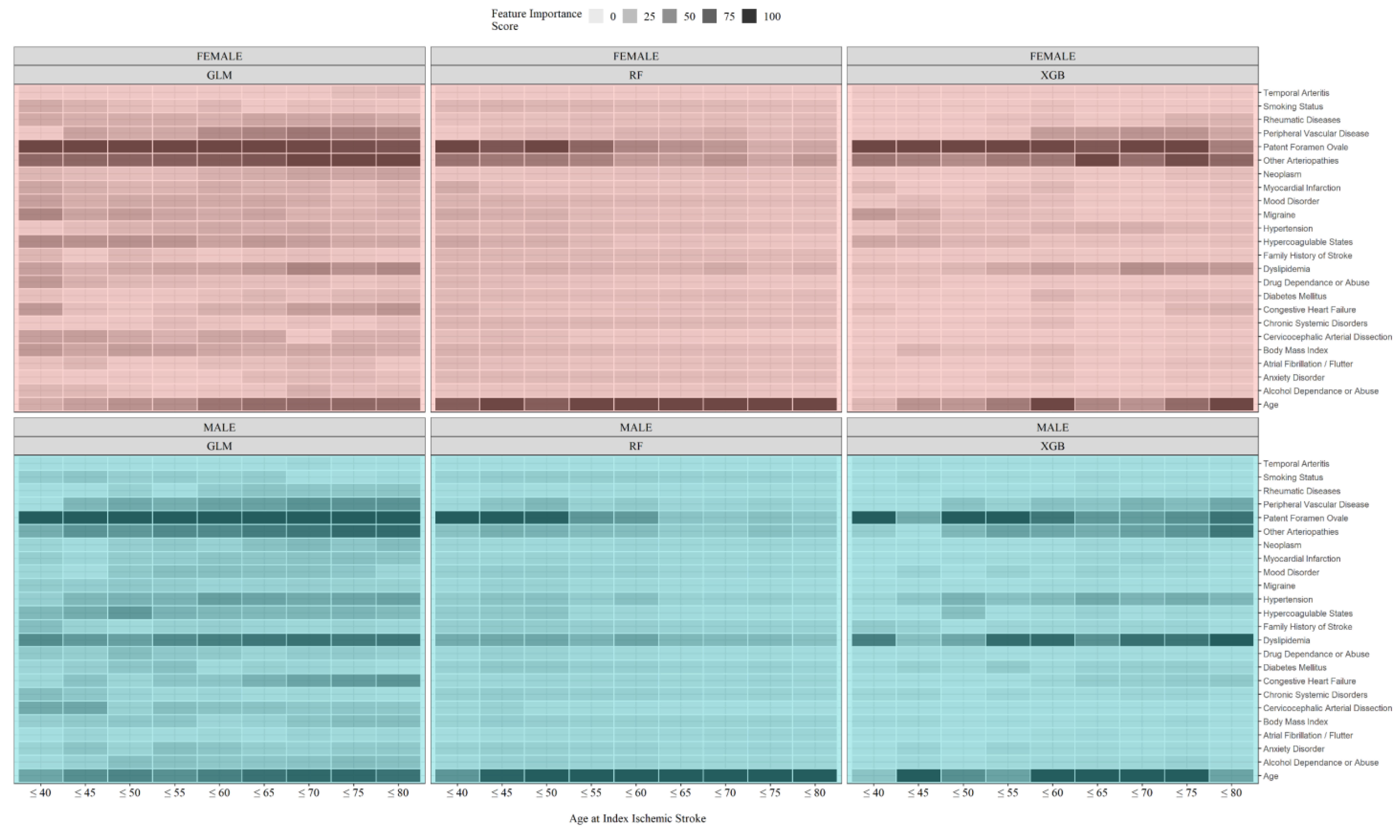
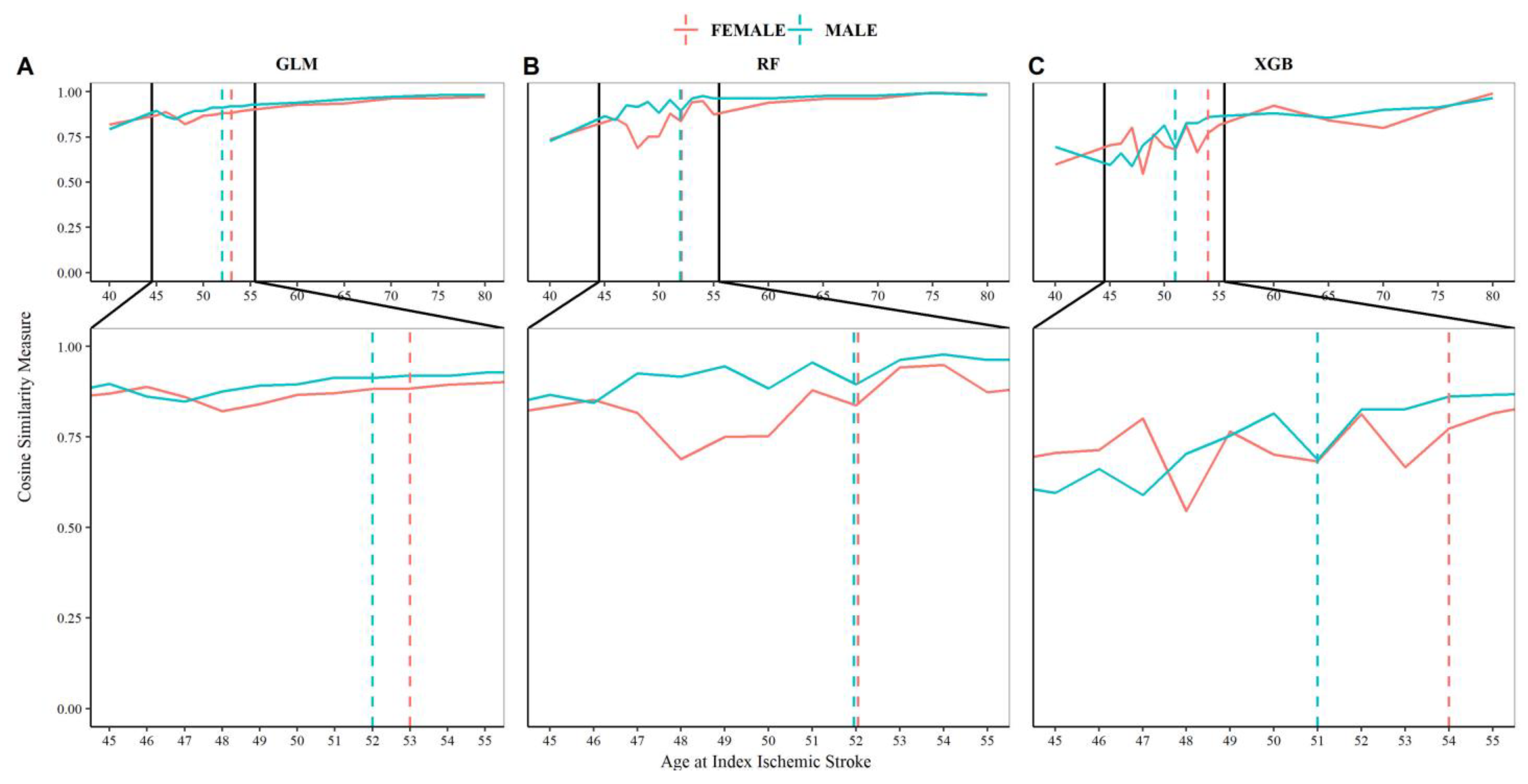
3.3. Estimating the Age Cut-Point for Young IS Using Machine Learning (Approach 2)
4. Discussion
4.1. “Young Stroke” Cutoff
4.2. Vascular Risk Factors
4.3. Rarer Stroke Etiologies and Non-Traditional Risk Factors
4.4. Gender, Genetic, and Environment Differences
4.5. Study Limitations, Strengths, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poisson, S.N.; Glidden, D.; Johnston, S.C.; Fullerton, H.J. Deaths from stroke in US young adults, 1989–2009. Neurology 2014, 83, 2110–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamurthi, R.V.; Moran, A.E.; Feigin, V.L.; Barker-Collo, S.; Norrving, B.; Mensah, G.A.; Taylor, S.; Naghavi, M.; Forouzanfar, M.H.; Nguyen, G.; et al. Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Adults Aged 20–64 Years in 1990–2013: Data from the Global Burden of Disease 2013 Study. Neuroepidemiology 2015, 45, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Putaala, J. Ischemic stroke in the young: Current perspectives on incidence, risk factors, and cardiovascular prognosis. Eur. Stroke J. 2016, 1, 28–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackland, D.T.; Roccella, E.J.; Deutsch, A.F.; Fornage, M.; George, M.G.; Howard, G.; Kissela, B.M.; Kittner, S.J.; Lichtman, J.H.; Lisabeth, L.D.; et al. Factors influencing the decline in stroke mortality a statement from the american heart association/american stroke association. Stroke 2014, 45, 315–353. [Google Scholar] [CrossRef] [Green Version]
- Hathidara, M.Y.; Saini, V.; Malik, A.M. Stroke in the Young: A Global Update. Curr. Neurol. Neurosci. Rep. 2019, 19, 91. [Google Scholar] [CrossRef]
- Ji, R.; Schwamm, L.H.; Pervez, M.A.; Singhal, A.B. Ischemic stroke and transient ischemic attack in young adults: Risk factors, diagnostic yield, neuroimaging, and thrombolysis. Arch. Neurol. 2013, 70, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Jaworek, T.; Xu, H.; Gaynor, B.J.; Cole, J.W.; Rannikmae, K.; Stanne, T.M.; Tomppo, L.; Abedi, V.; Amouyel, P.; Armstrong, N.D.; et al. Contribution of Common Genetic Variants to Risk of Early Onset Ischemic Stroke. Neurology 2022, 99, e1738–e1754. [Google Scholar] [CrossRef]
- Putaala, J.; Curtze, S.; Hiltunen, S.; Tolppanen, H.; Kaste, M.; Tatlisumak, T. Causes of death and predictors of 5-year mortality in young adults after first-ever ischemic stroke: The Helsinki young stroke registry. Stroke 2009, 40, 2698–2703. [Google Scholar] [CrossRef] [Green Version]
- Waje-Andreassen, U.; Thomassen, L.; Jusufovic, M.; Power, K.N.; Eide, G.E.; Vedeler, C.A.; Naess, H. Ischaemic stroke at a young age is a serious event–final results of a population-based long-term follow-up in Western Norway. Eur. J. Neurol. 2013, 20, 818–823. [Google Scholar] [CrossRef]
- Rutten-Jacobs, L.C.A.; Arntz, R.M.; Maaijwee, N.A.M.; Schoonderwaldt, H.C.; Dorresteijn, L.D.; Van Dijk, E.J.; De Leeuw, F.E. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA 2013, 309, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Varona, J.F.; Bermejo, F.; Guerra, J.M.; Molina, J.A. Long-term prognosis of ischemic stroke in young adults: Study of 272 cases. J. Neurol. 2004, 251, 1507–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béjot, Y.; Daubail, B.; Jacquin, A.; Durier, J.; Osseby, G.V.; Rouaud, O.; Giroud, M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: The dijon stroke registry. J. Neurol. Neurosurg. Psychiatry 2014, 85, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Cabral, N.L.; Freire, A.T.; Conforto, A.B.; Dos Santos, N.; Reis, F.I.; Nagel, V.; Guesser, V.V.; Safanelli, J.; Longo, A.L. Increase of stroke incidence in young adults in a middle-income country a 10-year population-based study. Stroke 2017, 48, 2925–2930. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Anyaehie, M.; Demiraj, F.; Bavishi, S.; Shahjouei, S.; Li, J.; Abedi, V.; Zand, R. Comparison of Long-Term Outcomes and Associated Factors between Younger and Older Rural Ischemic Stroke Patients. J. Clin. Med. 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, R.J.; Bastarache, L.; Denny, J.C. R PheWAS: Data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics 2014, 30, 2375–2376. [Google Scholar] [CrossRef] [Green Version]
- van Alebeek, M.E.; Arntz, R.M.; Ekker, M.S.; Synhaeve, N.E.; AMM Maaijwee, N.; Schoonderwaldt, H.; van der Vlugt, M.J.; van Dijk, E.J.; Rutten-Jacobs, L.C.; de Leeuw, F.-E. Risk factors and mechanisms of stroke in young adults: The FUTURE study. J. Cereb. Blood Flow Metab. 2018, 38, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Sacco, R.L.; Wolf, P.A.; Gorelick, P.B. Risk factors and their management for stroke prevention: Outlook for 1999 and beyond. Neurology 1999, 53, S15–S24. [Google Scholar]
- Kokotailo, R.A.; Hill, M.D. Coding of Stroke and Stroke Risk Factors Using International Classification of Diseases, Revisions 9 and 10. Stroke 2005, 36, 1776–1781. [Google Scholar] [CrossRef] [Green Version]
- Arboix, A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J. Clin. Cases 2015, 3, 418. [Google Scholar] [CrossRef]
- Surtees, P.G.; Wainwright, N.W.J.; Luben, R.N.; Wareham, N.J.; Bingham, S.A.; Khaw, K.T. Psychological distress, major depressive disorder, and risk of stroke. Neurology 2008, 70, 788–794. [Google Scholar] [CrossRef]
- Lambiase, M.J.; Kubzansky, L.D.; Thurston, R.C. Prospective study of anxiety and incident stroke. Stroke 2014, 45, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Abedi, V.; Li, J.; Shivakumar, M.K.; Avula, V.; Chaudhary, D.P.; Shellenberger, M.J.; Khara, H.S.; Zhang, Y.; Lee, M.T.M.; Wolk, D.M.; et al. Increasing the Density of Laboratory Measures for Machine Learning Applications. J. Clin. Med. 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, X.S.; Chaudhary, D.; Avula, V.; Mudiganti, S.; Husby, H.; Shahjouei, S.; Afshar, A.; Stewart, W.F.; Yeasin, M.; et al. Imputation of missing values for electronic health record laboratory data. NPJ Digit. Med. 2021, 4, 147. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. 2013. Available online: http://www.r-project.org/ (accessed on 30 October 2022).
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; R Core Team; et al. Caret: Classification and Regression Training. R Package Version Vol. 6.0 81. 2015. Available online: https://CRAN.R-project.org/package=caret (accessed on 30 October 2022).
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V.; Tang, Y.; Cho, H.; Chen, K.; Mitchell, R.; Cano, I.; Zhou, T.; et al. Extreme Gradient Boosting. R Package Version 1.1.1.1. 2019. Available online: https://cran.r-project.org/web/packages/xgboost/index.html (accessed on 30 October 2022).
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- YoungStroke—Raising Awareness about Treatment and Management of Stroke in Young Adults. Available online: https://youngstroke.org/ (accessed on 30 October 2022).
- Smajlović, D. Strokes in young adults: Epidemiology and prevention. Vasc. Health Risk Manag. 2015, 11, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Kissela, B.M.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Adeoye, O.; Flaherty, M.L.; Khatri, P.; Ferioli, S.; De Los Rios La Rosa, F.; et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology 2012, 79, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Putaala, J.; Yesilot, N.; Waje-Andreassen, U.; Pitkäniemi, J.; Vassilopoulou, S.; Nardi, K.; Odier, C.; Hofgart, G.; Engelter, S.; Burow, A.; et al. Demographic and geographic vascular risk factor differences in european young adults with ischemic stroke: The 15 cities young stroke study. Stroke 2012, 43, 2624–2630. [Google Scholar] [CrossRef] [Green Version]
- Siriratnam, P.; Godfrey, A.; O’Connor, E.; Pearce, D.; Hu, C.; Low, A.; Hair, C.; Oqueli, E.; Sharma, A.; Kraemer, T.; et al. Prevalence and risk factors of ischaemic stroke in the young: A regional Australian perspective. Intern. Med. J. 2020, 50, 698–704. [Google Scholar] [CrossRef]
- Treadwell, S.D.; Robinson, T.G. Cocaine use and stroke. Postgrad. Med. J. 2007, 83, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Bonita, R.; Stewart, A.; Nissinen, A.; Salonen, J.T. Hypertension, cigarette smoking, and the decline in stroke incidence in eastern Finland. Stroke 1991, 22, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towfighi, A.; Saver, J.L. Stroke declines from third to fourth leading cause of death in the United States: Historical perspective and challenges ahead. Stroke. 2011, 42, 2351–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, R.G.; Jorge, R.E. Post-stroke depression: A review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Howard, G.; Kleindorfer, D.O.; Cushman, M.; Long, D.L.; Jasne, A.; Judd, S.E.; Higginbotham, J.C.; Howard, V.J. Contributors to the Excess Stroke Mortality in Rural Areas in the United States. Stroke 2017, 48, 1773–1778. [Google Scholar] [CrossRef]
- Stamler, J.; Stamler, R.; Neaton, J.D.; Wentworth, D.; Daviglus, M.L.; Garside, D.; Dyer, A.R.; Liu, K.; Greenland, P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy. Findings for 5 large cohorts of young adult and middle-aged men and women. J. Am. Med. Assoc. 1999, 282, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Joubert, J.; Prentice, L.F.; Moulin, T.; Liaw, S.-T.S.T.; Joubert, L.B.; Preux, P.-M.P.M.; Ware, D.; De Bustos, E.M.; Mclean, A. Stroke in rural areas and small communities. Stroke 2008, 39, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- Lisabeth, L.; Bushnell, C. Menopause and stroke: An epidemiologic review. Lancet Neurol 2012, 11, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Nichols, H.B.; Trentham-Dietz, A.; Hampton, J.M.; Titus-Ernstoff, L.; Egan, K.M.; Willett, W.C.; Newcomb, P.A. From Menarche to Menopause: Trends among US Women Born from 1912 to 1969. Am. J. Epidemiol. 2006, 164, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Lisabeth, L.D.; Beiser, A.S.; Brown, D.L.; Murabito, J.M.; Kelly-Hayes, M.; Wolf, P.A. Age at natural menopause and risk of ischemic stroke the framingham heart study. Stroke 2009, 40, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedi, V.; Lambert, C.; Chaudhary, D.; Rieder, E.; Avula, V.; Hwang, W.; Li, J.; Zand, R. Defining the Age of Young Ischemic Stroke Using Data-Driven Approaches. J. Clin. Med. 2023, 12, 2600. https://doi.org/10.3390/jcm12072600
Abedi V, Lambert C, Chaudhary D, Rieder E, Avula V, Hwang W, Li J, Zand R. Defining the Age of Young Ischemic Stroke Using Data-Driven Approaches. Journal of Clinical Medicine. 2023; 12(7):2600. https://doi.org/10.3390/jcm12072600
Chicago/Turabian StyleAbedi, Vida, Clare Lambert, Durgesh Chaudhary, Emily Rieder, Venkatesh Avula, Wenke Hwang, Jiang Li, and Ramin Zand. 2023. "Defining the Age of Young Ischemic Stroke Using Data-Driven Approaches" Journal of Clinical Medicine 12, no. 7: 2600. https://doi.org/10.3390/jcm12072600
APA StyleAbedi, V., Lambert, C., Chaudhary, D., Rieder, E., Avula, V., Hwang, W., Li, J., & Zand, R. (2023). Defining the Age of Young Ischemic Stroke Using Data-Driven Approaches. Journal of Clinical Medicine, 12(7), 2600. https://doi.org/10.3390/jcm12072600







