Intranasal Fentanyl for Acute Pain Management in Children, Adults and Elderly Patients in the Prehospital Emergency Service and in the Emergency Department: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Methods and Data Extraction
2.2. Types of Studies Included
2.3. Evaluated Outcomes
2.4. Quality Assessment of Included Studies
3. Results
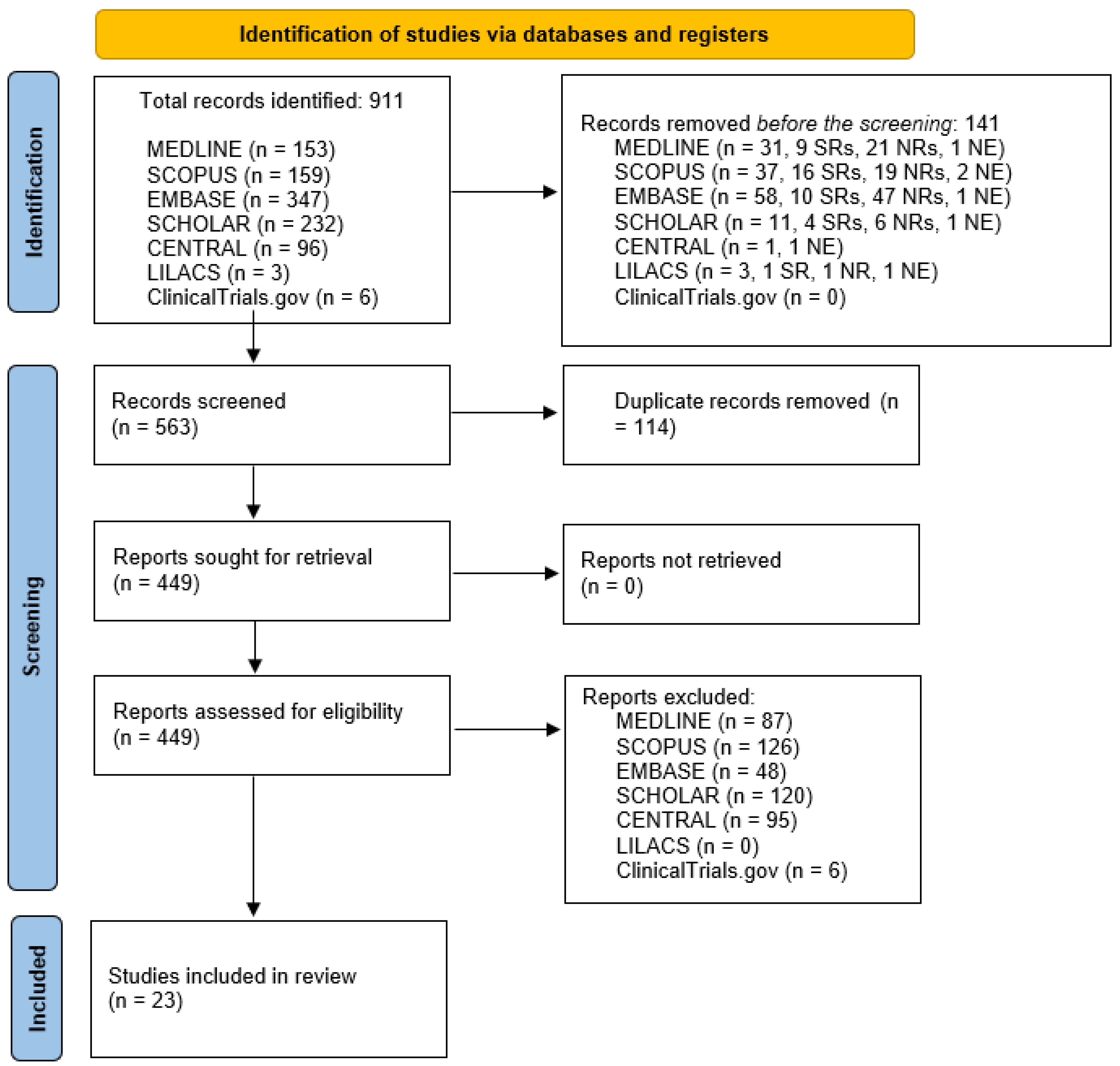
3.1. Result of the Database Research
3.2. General Characteristics of the Included Studies
| Author, Year of Publication | Type of Study * | Sample Size N (N of Women Included, (%)), N in INF Group/N in the Comparator Group (if Available) | Age £,** | Pain Scale Used | INF Dose ** | Comparator | Population, Primary Outcomes and Authors’ Conclusions |
|---|---|---|---|---|---|---|---|
| Prehospital emergency service setting | |||||||
| Murphy et al., 2017 [38] | P | 94 (44 (47)) | 11 (7–13) | FLACC or the Wong–Baker faces or the VNR according to age. | 1.5 μg/kg | INF+additional analgesia $ | In children aged 1–16 y-o, INF at a dose of 1.5 µg/kg appears to be a safe and effective analgesic in the prehospital management of acute severe pain. |
| Emergency department setting | |||||||
| Akinsola et al., 2018 [39] | P | 228 (128, (56))–180/48 | 12 ± 5 | NR | 1.5 μg/kg, two doses 5 min apart | Standard care (± oral hydrocodone, ± IV ketorolac and ± IV morphine or IV hydromorphone) | In children with pain due to vaso-occlusive crisis, INF use significantly improved time to first parenteral-opioid dose and was a safe and effective treatment for pain. |
| Anderson et al., 2022 [40] | R | 3205 (1263, (40)) | 13.7, (11.8–15.9) | NR | 2–5 μg/kg, maximum 200 μg | NC | In children, higher doses of fentanyl (2–5 μg/kg) are well tolerated without any clinically significant adverse outcomes observed over a 7-year period. |
| Borland et al., 2011 [48] | RCT | 189 (118 (63)) | 9.1 (95% CI 8.4–9.8) for INF, 8.8 (95% CI 8.1–9.5) for comparator | VAS or FPS-R | 1.5 μg/kg | 1.5 μg/kg of INF delivered with a concentration of 300 μg/mL | In children aged 3–15 y-o with pain due to suspected long bone fracture, standard concentration fentanyl and high concentration fentanyl were equivalent in reducing pain. |
| Cole et al., 2009 [41] | P | 46, (24, (52)) | 22.6 (12–36) months | FLACC | 1.5 μg/kg, a second dose of 0.5 μg/kg if persistent pain after 10 min | NC | In children aged 1–3 years with acute moderate to severe pain, INF is an effective, safe and well-tolerated mode of analgesia. |
| Crellin et al., 2010 [42] | P | 36 | 6.7, range 5–15 | VAS or Bieri faces scale-revised | 1.5 μg/kg | NC | In children aged 5–18 y-o with upper limb injuries, INF appeared to be an effective analgesic. |
| Fein et al., 2017 [49] | RCT | 49 (19 (39))–24/25 | 10.6 (5.3) for INF, 12.5 (5.1) for comparator | Modified Wong–Baker faces pain rating scale | 2 μg/kg (maximum 100 μg), single dose | SoC and IN 0.9% NaCl | In children aged 3–20 y-o with a vaso-occlusive crisis and pain score > 6 at WBFPRS, at 20 min, INF reduced vaso-occlusive crisis pain more than placebo. |
| Finn et al., 2010 [43] | R | 49 (0 (0)) | 6.2, range 1–16 | VAS | 1.5 μg/kg | NC | In children aged 1–16 y-o, this study shows INF to be both effective and safe. |
| Frey et al., 2019 [50] | RCT | 90 (29 (32))–45/45 | 12.2 (2.3) for the INF group; 11.8 (2.6) for the comparator | VAS | 2 μg/kg | 1.5 mg/kg IN ketamine | IN ketamine provides effective analgesia that is non-inferior to INF, although participants who received IN ketamine had an increase in adverse events that were minor and transient. |
| Graudins et al., 2015 [51] | RCT | 73 (27 (37))–37/36 | 9 (6–11) for INF, 7 (6–9.5) for comparator | VAS | 1.5 μg/kg | 1 mg/kg IN ketamine | In children aged 3–13 y-o with isolated limb injury and pain at least 6/10 at triage, INF and IN ketamine were associated with similar pain reduction. IN Ketamine was associated with more minor adverse events. |
| Kelly et al., 2018 [44] | R | 487 (170 (35))–376/111 | In INF group: 16.3 ± 4.8; in comparator group: 18.2 ± 3.6 | NRS | NR | Routine care, drugs and dosage NR | In children aged 1–21 y-o with acute pain due to vaso-occlusive events compared with routine care, INF demonstrated a significantly reduced time to initiation of opioid analgesic therapy when using INF. |
| Nemeth et al., 2019 [45] | P | 100 (42 (42))–19/7/5/1/1/1 | 5.5 ± 4.1 | FLACC, faces pain scale revised or NRS according to age | 2.0 μg/kg | S-ketamine, midazolam via IV, PO or PR in various combinations | In children aged 0–17 y-o with trauma for analgesia or procedural sedation, intranasal administration of fentanyl, s-ketamine and midazolam was shown to be generally rapid for achieving analgesia and/or sedation. No marked circulatory, respiratory or other SAEs were noted. |
| Quinn et al., 2021 [52] | RCT | 22 (4 (18))–11/11 | INF group: 9.58 ± 2.92; comparator group: 9.77 ± 2.51; p = 0.87 | NRS or Wong–Baker faces pain score | 1.5 μg/kg | 1 mg/kg IN ketamine | In children aged 3–17 y-o, IN ketamine was found to be inferior to IN fentanyl in relieving pain at 10 min and was found to have significantly greater rates of sedation and dizziness. No sufficient power to support the non-inferiority of IN ketamine compared with INF at 20 min after administration. |
| Reynolds et al., 2017 [53] | RCT | 82 (31 (38))–41/41 | 8 (5–11) | NRS or Wong–Baker faces pain scale | 1.5 μg/kg | 1 mg/kg IN ketamine | In children 4–17 y-o with acute pain from suspected isolated extremity fractures with pain score >3 on the Wong–Baker faces pain scale or >2 at NRS, IN ketamine was associated with more minor side effects than intranasal fentanyl. Pain relief at 20 min was similar between groups. |
| Ruffin et al., 2022 [54] | RCT | 34 (17 (50)), 17/17 | INF group: 3.1 years; comparator group: 1.8 years, p = 0.06 | Faces, FLACC or VAS according to age | 1.5 μg/kg | PO administered acetaminophen+hydrocodone, 0.15 mg/kg hydrocodone | In 6 months–18 y-o children with painful infectious mouth conditions, INF seems to be a safe and effective alternative to acetaminophen with hydrocodone in reducing pain. |
| Saunders et al., 2010 [46] | P | 81 (32 (39)) | 8 ± 3.7 | Wong–Baker faces scale or VAS according to age | 2 μg/kg | NC | In children aged 3–18 y-o with moderate to severe pain on the Wong–Baker faces scale or VAS, a single dose of INF provides effective analgesia for paediatric ED patients with painful orthopaedic trauma within 10 min of administration. |
| Schaefer et al., 2015 [47] | R | 54 (36 (66))–7/47 | INF group: 7.7 ± 4.7 comparator group: 13.4 ± 3.8, p = 0.018 | NRS or faces pain score | 1.1 to 1.5 μg/kg | IV opioids administration | INF administration reduces the time from physician encounter to opioid administration in paediatric patients. |
| Younge et al., 1999 [30] | RCT | 47 (30 (63))–24/23 | INF group: mean 6.6 (SD NR) comparator: 7.1 mean (SD NR), p = 0.053 | NR | 1 μg/kg | 0.2 mg/kg IM morphine | In children aged 3–10 y-o, INF provides effective pain relief for children requiring opioid analgesia in the ED. It appears as effective, with better tolerance to administration, as IMM |
| Author, Year of Publication | Type of Study * | Sample Size N (N of Women Included, (%)), N in INF Group/N in the Comparator Group (if Available) | Age in Years ** | Pain Scale Used | INF Dose | Comparator | Population, Primary Outcomes and Authors’ Conclusions |
|---|---|---|---|---|---|---|---|
| Prehospital emergency service setting | |||||||
| Tanguay et al., 2020 [55] | R (subgroup analysis for patients aged 18–70 y-o) | 729–402/327 | 59 ± 19.9 | NRS | 1.5 μg/kg, maximum dose of 100 μg, 50 μg in patients with general comorbidities £: | 1.5 μg/kg SC fentanyl | In patients aged 18–70 y-o, both INF and SCF are feasible, effective and safe for managing acute severe pain in the prehospital setting. We also found that a greater proportion of older patients in the INF group experienced pain relief, even though they received a lower dose of fentanyl. |
| Emergency department setting | |||||||
| Assad et al., 2023 [56] | R | 95–31/64 | 31.1 (10.4) for INF, 31.8 (9.2) for comparator, p = 0.5 | NR | 50 μg or 100 μg | IV morphine, 0.1 mg/kg | INF provided similar pain reduction compared to IV morphine in the treatment of adults with VOC presenting to the ED; however, there was a trend in readmission within 48 h. No significant difference in adverse events between the groups. |
| Belkouch et al., 2015 [57] | P | 23 (11 (47.8)) | 51.3 | VAS | 1.5 µg/kg | NC | In patients admitted for renal colic, INF provides quick pain relief and its use is safe. |
| Nasr Isfahani et al., 2022 [58] | RCT | 125 (9 (8)) –44/40 in IN ketamine/41 in IN placebo | INF group: 30.51 ± 10.77; for placebo group: 32.25 ± 13.23; IN ketamine group: 31.26 ± 12.07 (p > 0.05) | VAS | 1 µg/kg | 1 mg/kg IN ketamine and IN placebo | In patients with isolated limb trauma aged 15–65 y-o with moderate to severe pain (at least 45 mm at VAS), the efficacy of INF and IN ketamine was similar in reduction of pain 40 min after the administration. IN ketamine has a reduced time of onset. The rate of minor adverse events after IN l ketamine was higher than INF without serious adverse events registered. |
| Nazemian et al., 2020 [59] | RCT | 220 (96 (43)) –110/110 | NR | NRS | 2 µg/kg + 60 mg IM ketorolac | 1 µg/kg IV fentanyl + 60 mg IM ketorolac | In patients with renal colic pain, INF in combination with ketorolac is a fast-acting, non-invasive, convenient and effective method to manage pain in these patients. |
| Author, Year of Publication | Type of Study * | Sample Size N (N of Women Included, (%)), N in INF Group/N in Comparator Group (if Available) | Age in Years ** | Pain Scale Used | INF Dose | Comparator | Population, Primary Outcomes and Authors’ Conclusions |
|---|---|---|---|---|---|---|---|
| Prehospital emergency service setting | |||||||
| Tanguay et al., 2020 [55] | R (subgroup analysis for patients aged >70 y-o) | 380–202/195 | NR | NRS | 50 μg | 50 μg SC fentanyl | In patients aged >70 y-o with severe pain, both INF and SCF are feasible, effective and safe for managing acute severe pain in the prehospital setting. We also found that a greater proportion of older patients in the INF group experienced pain relief, even though they received a lower dose of fentanyl. |
3.3. Efficacy of Intranasal Fentanyl
3.3.1. Efficacy in Children, PHES Setting
3.3.2. Efficacy in Children, ED Setting
3.3.3. Efficacy in Adults, PHES Setting
3.3.4. Efficacy in Adults, ED Setting
3.3.5. Efficacy in the Elderly, PHES Setting
3.3.6. Efficacy in the Elderly, ED Setting
3.4. Safety of Intranasal Fentanyl
3.4.1. Safety in Children, PHES Setting
3.4.2. Safety in Children, ED Setting
3.4.3. Safety in Adults, PHES Setting
3.4.4. Safety in Adults, ED Setting
3.4.5. Safety in Elderly, PHES Setting
3.4.6. Safety in the Elderly; ED Setting
3.5. Risk of Bias for the Outcome of Efficacy in Reducing Pain in the Included RCTs
4. Discussion
4.1. Implications for Clinical Practice
4.2. Limitations of the Present Systematic Review
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Table A1, Table A2 and Table A3: Efficacy of INF in Children, Adults and Elderly Patients, Respectively
| Author, Year of Publication | Cause of Pain * | Pain at Baseline ** | Time Point Evaluated, Pain after INF | Time Point Evaluated, Pain after Comparator | p Value | Other Efficacy Outcomes Evaluated after INF | Other Efficacy Outcomes Evaluated after Comparator | p Value |
|---|---|---|---|---|---|---|---|---|
| Prehospital emergency service setting | ||||||||
| Murphy et al., 2016 [38] | A | 10 (8–10) in INF alone group; 9 (8–10) in the comparator group | 10 min: 5 (2–7) | 5 min: 5 (2.5–7) | NR | NR | NR | NR |
| Emergency department setting | ||||||||
| Akinsola et al., 2018 [39] | M | NR | First time reassessment NR, exact pain value NR Time of disposition NR, exact pain value NR | First time reassessment NR, exact pain value NR Time of disposition NR, pain value NR | <0.05 for first time reassessment <0.01 for time of disposition | Time to first parenteral opioid (min SD): 29 ± 15 LOS: 215 ± 86 min Admission rate: 86 (48%) Rate of patient/parent satisfaction after INF: exact value NR | Time to first parenteral opioid (min SD): 77 ± 44 LOS: 197 ± 67 Admission rate: 34 ± 71 | Time to first parenteral opioid: <0.0001 LOS:0.028 Admission rate: 0.004 |
| Anderson et al., 2022 [40] | A | NR | NR | NR | NR | NR | NR | NR |
| Borland et al., 2011 [48] | T | For INF: 80.0 (60.0–95.5), for comparator: 77.5 (60.0–100) | 10 min: 49.5 (26.5–68.5) 20 min: 27.5 (18.5–56.5) 30 min: 10.0–46.0) | 10 min: 43.0 (15.2–66.0) 20 min: 35.0 (9.0–57.0) 30 min: 21.5 (4.75–51.0) | Baseline pain: 0.881 10 min: 0.176 20 min: 0.758 30 min: 0.662 | Additional analgesia: 42 (43%) | Additional analgesia: 25 (27%) | 0.028 |
| Cole et al., 2009 [41] | T | 8 (5–10) | 10 min: 2 (0–4) 30 min: 0 (0–2) | NC | Second dose of INF: 2 (4), rescue analgesia: 1 (2.2) | |||
| Crellin et al., 2010 [42] | T | 7 (5–10) | 5 min: 5 (4–8) 30 min: 2 (1–40) | NC | <0.001 for each time point | Second dose: 1, IV morphine: 1 | NC | - |
| Fein et al., 2016 [49] | M | 10 (8–10) for INF, 9 (8–10) for comparator | 10 min: 8 (6–8) 20 min: 6.5 (4–8) 30 min: 8 (5–8) | 10 min: 8 (7–9) 20 min: 8 (6–9) 30 min: 8 (6–8) | Baseline pain: p > 0.05 10 min: >0.05 20 min: 0.048 30 min: >0.05 | NR | NR | - |
| Finn et al., 2010 [43] | A | Media 91/100, range 85–100 | 5 min: 52/100 (range 40–75) 30 min: 16/100 (range 8–42) | NC | - | Further opiate analgesia: 1 | NC | - |
| Frey et al., 2018 [50] | T | 72 (18.6) for INF, 74.7 (15.3) for comparator | ££ 15 min: −25.3 (−30.3–−23.3) 30 min: −31.9 (−37.2–−26.6) 60 min: −29.0 (−35.1–−22.8) | ££ 15 min: −24 (−29.3–−19.4) 30 min: −30.6 (−35.8–−25.4) 60 min: −27.7 (−33.8–−21.6) | 15 min: no difference 30 min: no difference 60 min: no difference | Needing rescue analgesia: 9 (21) | Needing rescue analgesia: 11 (25) | RR 0.89 (95% CI 0.5–1.6) |
| Graudins et al., 2015 [51] | T | INF group: 80 (70–100), IN ketamine group: 80 (69–96) p > 0.05 | ££ 15 min: 30 (15–40) 30 min: 40 (20–45) 60 min: 50 (20–60) | ££ 15 min: 30 (16–42) 30 min: 45 (20–60) 60 min: 50 (30–61) | £££ 15 min: 0 (−20–20) 30 min: 5 (−10–20) 60 min: 0 (−13–13) | Needing rescue analgesia: 12 (32) | Needing rescue analgesia: 5 (36) | Difference in IN ketamine vs. INF: −18 (−37–3) |
| Kelly et al., 2017 [44] | M | 9 (8–10) in INF group, 8 (7–9) in comparator group | First time point at 59 (36) min: 8 (7–9), first decrease in pain score: 0 (0–2), second time point at 70 (47) min: 7 (6–9), second decrease in pain score: 1 (0–2) | First time point at 59 (36) min: 7 (6–8), first decrease in pain score: 1 (0–2), second time point at 90 (51) min: 7 (5–8), second decrease in pain score: 1 (0–3) | <0.001 at first and second time point, first decrease in pain score: 0.838, second decrease in pain score: 0.369 | Time to first opioid administration: 29 + 16 min, patients treated in <30 min: 252 (67%) | Time to first opioid administration: 78 (57), patients treated in <30 min: 6 (5%) | <0.001 for time to first opioid <0.001 for patients treated in <30 min |
| Nemeth et al., 2019 [45] | T | 6 (2–10) | 2 (0–6) | Ranging from 1 to 6, including all patients: 2 (0–6) | >0.05 | IN repetition: 1 (5%), need for IV supplementation: 1 (5%) | IN repetition total: 3 (9%), IV supplementation: 2 (6%) | >0.05 for all comparisons |
| Quinn et al., 2021 [52] | A | For INF group: 8 (6–10), for comparator group: 8 (5–10) | 30 min: median 1 (IQR NR), 60 min: median 2 (IQR NR) | 30 min: 2 (IQR NR) 60 min: 2 (IQR NR) | At baseline: 0.87 30 min: 0.18 60 min: 0.5 | Rescue medication: 0 (0) | Rescue medication: 0 (0) | - |
| Reynolds et al., 2017 [53] | T | For INF group: 69 ± 26, for comparator group: 73 ± 26 | 20 min: reduction of pain equal to 35 ± 29, 60 min: reduction of pain equal to 44 ± 28 | 20 min: reduction of pain equal to 44 ± 36, 60 min: reduction of pain equal to 42 ± 32 | At baseline: mean difference 4 (95% CI: −7–15), 20 min: mean difference 9 (95% CI: −5–23), 60 min: mean difference −2 (95% CI: −16–13) | Clinically significant reduction at 20 min: 35 (80%) | Clinically significant reduction of pain at 20 min: 30 (77%) | Clinically significant reduction of pain at 20 min: 0.77 |
| Ruffin et al., 2022 [54] | M | NR | 15 min: 1.7 (95% CI: 0.7–2.6), 30 min: 0.6 (95% CI 0–1.2) | 15 min: 2.9 (95% CI 1.7–4.0), 30 min: 1.6 (95% CI 0.7–2.5) | 15 min: 0.088 30 min: 0.059 | Parental perception in: dehydration at 60 min, from 1 to 4: 1.7 (1.4–1.9). Change in dehydration −1.1 (−1.4 to −0.9). Pain at 60 min 2.2 (1.5–3.0). Overall satisfaction, from 1 to 7: 6.4 (5.9–6.8). Admission to hospital: 0 (0%). | Parental perception in: dehydration at 60 min, from 1 to 4: 1.5 (1.2–1.8). Change in dehydration −1.4 (−1.7 to −1.0). Pain at 60 min 2.4 (1.4–3.5). Overall satisfaction, from 1 to 7: 6.5 (6.0–6.9). Admission to hospital: 2 (12%). | Parental perception in: dehydration at 60 min, from 1 to 4:0.367. Change in dehydration 0.265. Pain at 60 min 0.770. Overall satisfaction, from 1 to 7: 0.707. Admission to hospital: 0.485. |
| Saunders et al., 2010 [46] | T | For Wong–Baker group: 5 (4–6), for VAS group: 70 mm (95% CI: 63–77) | For Wong–Baker group: 10 min: 3 (2–5) 20 min: 2 (1–4) 30 min: 2 (1–3), for VAS group: 10 min: 21 mm (95 %CI: 14–28) 20 min: 25 mm (95% CI: 15–34) 30 min: 27mm (95% CI: 16–37) | NC | - | Rescue analgesia: 7 (9%), provider satisfaction score: 79 mm (95% CI: 74–83 mm), parent’s mean satisfaction score: 74 mm (95% CI: 69–79), patient’s mean satisfaction score: 62 mm (95% CI: 53–70) | NC | - |
| Schaefer et al., 2015 [47] | A | 7.19 ± 2.49 | NR | NR | - | Time to opioid administration: 20.43 ± 11.54 min | Time to opioid administration: 42.04 ± 31.55 min | Time to opioids administration: 0.038 |
| Younge et al., 1999 [30] | T | INF group: NR, comparator group: NR | 10 min: median 1 (IQR NR) 20 min: median 1 (IQR NR) 30 min: median 1 (IQR NR) | 10 min: median 2 (IQR NR) 20 min: 2 (IQR NR) 30 min: 1 (IQR NR) | At baseline: 0.46 10 min: 0.014 20 min: 0.64 30 min: >0.05 | Tolerance score: exact value NR | Tolerance score: exact value NR | Tolerance score: <0.001 for better INF |
| Author, Year of Publication | Cause of Pain * | Pain at Baseline ^ | Time Point Evaluated, Pain after INF | Time Point Evaluated, Pain after Comparator | p Value | Other Efficacy Outcome Evaluated after INF ^ | Other Efficacy Outcomes Evaluated after Comparator^ | p Value |
|---|---|---|---|---|---|---|---|---|
| Prehospital emergency service setting | ||||||||
| Tanguay et al., 2020 [55] | A | 8.9 ± 1.1 | Time point NR, pain reduction at least 1.5 points: exact value NR, pain reduction at least 3 points: exact value NR | Time point NR, pain reduction at least 1.5 points: exact value NR, pain reduction at least 3 points: exact value NR | (Log rank test: proportion of patients with reduction in pain at least 1.5 points, p = 0.050; proportion of patients with a reduction in pain at least 3 points: p = 0.003) for INF higher than subcutaneous fentanyl | INF administration mean time = 9 min 36 s ± 3 min 32 s | Subcutaneous fentanyl, mean time = 9 min 30 s ± 3 min 46 s | >0.05 |
| Emergency department | ||||||||
| Assad et al., 2022 [56] | M | NR | Time point NR, pain reduction of 17.25% | Time point NR, pain reduction of 17.15% | 0.1% (−9.3–9.5%) | Time to first rescue medication: 22.4 (16.4) min Median IV opiates: 8 (6–14) MME Readmission rate: 2 (6.5%) Discharged home from ED: 5 (16%) | Time to first rescue medication: 27.3 (15.6) min Median IV opiate dose: 6 (5.7–9.3) MME Readmission rate: 13 (20.9%) Discharged home from ED: 41 (66%) | Time to first rescue medication: 0.22 Median IV opiate dose: 0.03 Readmission rate: 0.06 Discharged home from ED: 0.02 |
| Belkouch et al., 2015 [57] | M | 82.2 (59–100) mm | 5 min: 48 mm (36–63) 30 min: 8 mm (0–22) | NC | - | Reduction at each time point (15, 30, 45, 60), exact value NR | <0.001 for reduction at each time point | |
| Nasr Isfahani et al., 2022 [58] | T | INF: 83.41 + 17.11), IN ketamine: 82.5 + 13.73, control: 85.85 + 16.73 | 5 min: 71.59 + 22.09 10 min: 64.5 + 22.87 15 min: 62.95 + 24.74 30 min: 64.32 + 24.72 40 min: 62.95 + 25.11 | For IN ketamine: 5 min: 61.5 + 20.45 10 min:55 + 21.96 15 min: 54.5 + 22.64 30 min: 57 + 23.56 40 min: 57.5 + 24.68, for control: 5 min: 72.44 + 22.11 10 min: 66.59 + 24.25 15 min: 67.8 + 27.88 30 min: 67.8 + 27.88 40 min: 67.32 + 27.48 | p > 0.05 for all comparisons except: 5 min: p = 0.03 for IN ketamine vs. control; p = 0.044 for IN ketamine vs. INF; 10 min: p = 0.047 for IN ketamine vs. control; p = 0.03 for IN ketamine vs. INF | Need for rescue analgesia: 3 (6.8), satisfaction with the administered drug: median 1.5 (min–max 1–10) | Need for rescue analgesia in IN ketamine group: 5 (12.5), need for rescue analgesia in control group: 7 (17.1), satisfaction with the administered drug for IN ketamine: median 4 (min-max 1–10), satisfaction with the administered drug for control: median 4 (min–max 1–10) | For the need of rescue analgesia: p = 0.336, for satisfaction: p = 0.047, for IN ketamine vs. control; p = 0.045 for IN ketamine vs. INF |
| Nazemian et al., 2020 [59] | M | INF group: 9.22 ± 0.62, IV fentanyl: 9.22 ± 0.51 | 10 min: 9.22 ± 0.52 20 min: 7.5 ± 0.97 30 min: 5.99 ± 1.09 40 min: 3.84 ± 0.62 60 min: 3 ± 0.4 | 10 min: 6.89 ± 1.05 20 min: 5.09 ± 1.19 30 min: 4.35 ± 0.8 40 min: 3.19 ± 1.1 60 min: 2.47 ± 1.06 | Baseline: p = 1; 10 min: <0.0001 20 min: <0.0001 30 min: 0.32 40 min: <0.0001 60 min: <0.0001 | Patients not satisfied: 14 (12.7%), satisfied: 71 (64.5%), very satisfied: 25 (22.7%) | Patients not satisfied: 10 (9.1), satisfied: 67 (60.9), very satisfied: 33 (30) | 0.38 |
| Author, Year of Publication | Cause of Pain * | Pain at Baseline | Time Point Evaluated, Pain after INF | Time Point Evaluated, Pain after Comparator | p Value | Other Efficacy Outcomes Evaluated after INF ^ | Other Efficacy Outcomes Evaluated after Comparator ^ | p Value |
|---|---|---|---|---|---|---|---|---|
| Prehospital emergency service setting | ||||||||
| Tanguay et al., 2020 [55] | A | NR | Exact value NR | Exact value NR | NR | Time to drug administration 9:36 ± 3:3 | Time to drug administration 9:30 ± 3:46 | 0.674 |
Appendix B. Table A4 and Table A5: Safety of INF in Children and Adults, Respectively
| Author, Year of Publication | Respiratory Depression, N (%) for INF | Respiratory Depression, N (%) for Comparator | p-Value | CV Depression, N (%) for INF | CV Depression, N (%) for Comparator | p-Value | CNS Depression, N (%) for INF | CNS Depression, N (%) for Comparator | p-Value | Other Adverse Events Evaluated for INF, N (%) | Other Adverse Events Evaluated for the Comparator, N (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prehospital | ||||||||||||
| Murphy et al., 2017 [38] | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - |
| Hospital | ||||||||||||
| Akinsola et al., 2018 [39] | 0 | 0 | NR | 0 | 0 | NR | 0 | 0 | NR | Nasal burning and irritation, NR | NR | NR |
| Anderson et al., 2022 [40] | 0 (0) | NC | - | 0 (0) | NC | - | NR | NC | - | Two events (NR type of event) due to higher dose administered | NC | - |
| Borland et al., 2011 [48] | 0 | 0 | NR | 0 | 0 | NR | 16 (16) | 22 (24) | 0.18 | Nausea: 4 (4), vomiting: 1 (1), itch: 5 (5), rash: 0, dizziness: 1 (1) | Nausea:6 (6.5), vomiting: 3 (3.2), itch: 9 (9), rash: 1, dizziness: 2 | Nausea: 0.442, vomiting: 0.27, itch: 0.21, rash: NR, dizziness: NR |
| Cole et al., 2009 [41] | 0 | NC | - | 0 | NC | - | 0 | NC | - | NR | NC | - |
| Crellin et al., 2010 [42] | 0 | NC | - | 0 | NC | - | 0 | NC | - | NR | NC | - |
| Fein et al., 2016 [49] | 3 (13) £ | 0 (0) | >0.05 | 2 (8) ££ | 2 (8) | >0.05 | 10 (42) £££ | 6 (24) | >0.05 | Headache: 4 (17), itching: 2 (8), nausea: 2 (8), vomiting: 0 (0), nasal pain: 3 (13), prolonged cough: 0 (0), prolonged gagging: 0 (0) | Headache: 0 (0), itching: 1 (4), nausea: 1 (4), vomiting: 0 (0), nasal pain: 0 (0), prolonged cough: 0 (0), prolonged gagging: 0 (0) | Headache: = 0.05, p > 0.05 for all adverse effects |
| Finn et al., 2010 [43] | 0 (0) | NC | - | NR | NC | - | 0 (0) | NC | - | Nausea: 0 (0), vomiting: 0 (0) | NC | - |
| Frey et al., 2019 [50] | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | UMSS score * 1: 9 (21), UMSS score * 2: 1 (2) | UMSS score * 1: 17 (39), UMSS score * 2: 4 (9) | >0.05 for all | Dizziness: 0, dysphoria: 0, unpleasant taste: 2, drowsiness: 10, nausea or vomiting: 0, itchiness: 0, vision changes: 0, headache: 1, rash: 1, light-headedness: 0, nystagmus: 0, total: 14 | Dizziness: 9, dysphoria: 1, unpleasant taste: 9, drowsiness: 21, nausea or vomiting: 3, itchiness: 1, vision changes: 2, headache: 0, rash: 0, light-headedness: 2, nystagmus: 1, total: 49 | NR |
| Graudins et al., 2015 [51] | NR | NR | - | NR | NR | - | Exact value NR | Exact value NR | Reported similar at each time point | Bad taste: 10 (42), drowsiness: 5 (21), dizziness: 4 (17), itchy nose: 3 (12), nausea: 1 (4), dysphoria: 1 (4), hallucinations: 0 (0), other: 0 (0), total: 15 (40) | Bad taste:17 (25), drowsiness: 11 (16), dizziness: 20 (30), itchy nose: 3 (4), nausea: 4 (6), dysphoria: 3 (4), hallucinations: 4 (6), other: 5 (7), total: 28 (78) | Total: difference 38 (95% CI: −58–16) |
| Kelly et al., 2018 [44] | NR | NR | - | NR | NR | - | NR | NR | - | NR | NR | - |
| Nemeth et al., 2019 [45] | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | UMSS score * = 1: 4 (21%), UMSS score * = 2: 1 (5%) | UMSS score * = 1: 13 (38), UMSS = 2 *: 6 (18) | > 0.05% | NR | Reported burning after IN midazolam in 5.1% of cases; vomiting, 1 episode, not specified group | NR- |
| Quinn et al., 2021 [52] | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 20 min: 0 (0) | 20 min: 7 (64%) | 0.004 | Dizziness: 9%, various adverse effects: 9% | Dizziness: 64%, various adverse effects: 73% | Dizziness: 0.02, various adverse effects: 0.002 |
| Reynolds et al., 2017 [53] | NR | NR | - | 1 (2) ££ | 0 (0) | Risk difference for IN ketamine vs. INF: −2% (95% CI: −7–2%) | 15 (37) £££ | 19 (46) £££ | Risk difference for IN ketamine vs. INF: 10% (95% CI: −11–31%) | Bad taste: 9 (22), dizziness: 6 (15), drowsiness: 3 (7), itchy nose: 9 (22), visual disturbance: 1 (2), mood change: 3 (7), nausea: 3 (7), funny dreams: 0 (0), other: 3 (7), any event: 25 (61) | Bad taste: 37 (90), dizziness: 30 (73), drowsiness: 6 (15), itchy nose: 10 (24), visual disturbance: 4 (10), mood change: 1 (2), nausea: 3 (7), funny dreams: 1 (2), other: 5 (12), any event: 41 (100%) | ** Bad taste: 68% (53–84%), dizziness: 59% (41–76%), drowsiness: 7% (−6–21%), itchy nose: 2% (−16–21%), visual disturbance: 7% (−3–2%), mood change: −2% (−11–6%), nausea: 0% (−11–11%), funny dreams: 2% (−2–7%), other: 5% (−8–18%), any event: 39% (24–54%) |
| Ruffin et al., 2022 [54] | 0 (0) | NR | - | 0 (0) | NR | - | No significant central nervous system depression. Definition not reported. | NR | - | Cry: 1 | NR | - |
| Saunders et al., 2010 [46] | 0 (0) | NC | - | 0 (0) | NC | - | NR | NC | - | Vomiting: 0 (0), rhinorrhoea: 0 (0), epistaxis (0), nasal complaints 0 (0) | NC | - |
| Schaefer et al., 2015 [47] | 0 (0) | 0 (0) | - | NR | NR | - | NR | NR | - | NR | Dizziness: 1 | |
| Younge et al., 1999 [30] | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | NR | NR | - | NR | NR | - |
| Author, Year of Publication | Respiratory Depression, N (%) for INF | Respiratory Depression, N (%) for Comparator | p-Value | CV Depression, N (%) for INF | CV Depression, N (%) for Comparator | p-Value | CNS Depression, N (%) for INF | CNS Depression, N (%) for Comparator | p-Value | Other Adverse Events Evaluated for INF, N (%) | Other Adverse Events Evaluated for the Comparator, N (%) | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital: | ||||||||||||
| Assad et al., 2023 [56] | 0 (0) | 0 (0) | 1 | Hypotension: 0 (0), bradycardia 4 (12.9) $ | Hypotension 0 (0), bradycardia 5 (7.7) $ | Hypotension: 1, bradycardia: 0.2 | NR | NR | NR | NR | NR | NR |
| Belkouch et al., 2015 [57] | 0 (0) | 0 (0) | NR | NR | NR | NR | NR | NR | ||||
| Nasr Isfahani et al., 2022 [58] | 0 (0) | 0 (0) | >0.05 for difference in RR or SpO2% between all groups at each time point | 0 (0) | 0 (0) | - | 15 min: 2 (4.5) 30 min: 1 (2.3) $$ | For IN ketamine: 15 min:6 (15), 30 min: for 5 (12.5), control: 15 min: 2 (4.9), 30 min: 1 (2.4) $$ | >0.05 for all comparisons | General discomfort: 3 (6.8), headache: 0 (0), dizziness: 1 (2.3), feeling of unreality: 0 (0), nausea: 0 (0), fatigue: 0 (0), changes in hearing: 0 (0), mood change: 0 (0), hallucination: 0 (0) (at 15 min) | General discomfort: 5 (12.5) for IN ketamine; 2 (4.9) for control, headache: 0 (0), dizziness: 4 (10) for IN ketamine; 3 (7.3) for control, feeling of unreality: 0 (0), nausea: 9 (7.5) for IN ketamine, 1 (2.4) for control, fatigue:0 (0), changes in hearing: 0 (0), mood change: 7 (7.5) for IN ketamine, 0 (0) for control, hallucination: 1 (2.5) for IN ketamine, 0 (0) for control (at 15 min) | >0.05 for all comparisons, except for p = 0.038 for mood change in IN ketamine vs. control. |
| Nazemian et al., 2020 [59] | 1 (0.9) $$$ | 4 (3.6) $$$ | >0.05 | 0 (0) | 0 (0) | >0.05 | 0 (0) | 0 (0) | >0.05 | Nausea: 9 (8.2), dizziness: 3 (2.7), pruritus: 3 (2.7), bad taste: 12 (10.9), pharyngeal irritation: 9 (8.2) | Nausea: 10 (9.1), dizziness: 5 (4.5), pruritus: 8 (7.3), bad taste: 0 (0), pharyngeal irritation: 0 (0) | >0.05 for all comparisons |
References
- Alonso-Serra, H.M.; Wesley, K.; National Association of EMS Physicians Standards and Clinical Practices Committee. Prehospital pain management. Prehosp. Emerg. Care. 2003, 7, 482–488. [Google Scholar] [CrossRef]
- Cordell, W.H.; Keene, K.K.; Giles, B.K.; Jones, J.B.; Jones, J.H.; Brizendine, E.J. The high prevalence of pain in emergency medical care. Am. J. Emerg. Med. 2002, 20, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Galinski, M.; Picco, N.; Hennequin, B.; Raphael, V.; Ayachi, A.; Beruben, A.; Lapostolle, F.; Adnet, F. Out-of-hospital emergency medicine in pediatric patients: Prevalence and management of pain. Am. J. Emerg. Med. 2011, 29, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.C.; Gagnon, A.J.; Fullerton, L.; Common, C.; Ladores, M.; Forlini, S. One-week survey of pain intensity on admission to and discharge from the emergency department: A pilot study. J. Emerg. Med. 1998, 16, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Calil, A.M.; Pimenta, C.A.; Birolini, D. The “oligoanalgesia problem” in the emergency care. Clinics 2007, 62, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.E.; Pendleton, J.M. Oligoanalgesia in the emergency department. Am. J. Emerg. Med. 1989, 7, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Todd, K.H.; Sloan, E.P.; Chen, C.; Eder, S.; Wamstad, K. Survey of pain etiology, management practices and patient satisfaction in two urban emergency departments. CJEM 2002, 4, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Noble, J.; Zarling, B.; Geesey, T.; Smith, E.; Farooqi, A.; Yassir, W.; Sethuraman, U. Analgesia Use in Children with Acute Long Bone Fractures in the Pediatric Emergency Department. J. Emerg. Med. 2020, 58, 500–505. [Google Scholar] [CrossRef]
- Allione, A.; Pivetta, E.; Pizzolato, E.; Lorenzati, B.; Pomero, F.; Barutta, L.; Lauria, G.; Tartaglino, B. Determinants of inappropriate acute pain management in old people unable to communicate verbally in the emergency department. Turk. J. Emerg. Med. 2017, 17, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.S.; Johnson, K.; McNinch, M. Age as a risk factor for inadequate emergency department analgesia. Am. J. Emerg. Med. 1996, 14, 157–160. [Google Scholar] [CrossRef]
- Quattromani, E.; Normansell, D.; Storkan, M.; Gerdelman, G.; Krits, S.; Pennix, C.; Sprowls, D.; Armbrecht, E.; Dalawari, P. Oligoanalgesia in blunt geriatric trauma. J. Emerg. Med. 2015, 48, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Delaney, K.A. Inadequate analgesia in emergency medicine. Ann. Emerg. Med. 2004, 43, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Friedland, L.R.; Kilick, R.M. Emergency department analgesia use in pediatric trauma victims with fractures. Ann. Emerg. Med. 1994, 23, 203–207. [Google Scholar] [CrossRef]
- Izsak, E.; Moore, J.L.; Stringfellow, K.; Oswanski, M.F.; Lindstrom, D.A.; Stombaugh, H.A. Prehospital pain assessment in pediatric trauma. Prehospital Emerg. Care 2008, 12, 182–186. [Google Scholar] [CrossRef]
- Carter, D.; Sendziuk, P.; Eliott, J.A.; Braunack-Mayer, A. Why is Pain Still Under-Treated in the Emergency Department? Two New Hypotheses. Bioethics 2016, 30, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Prommer, E.; Thompson, L. Intranasal fentanyl for pain control: Current status with a focus on patient considerations. Patient Prefer. Adherence 2011, 5, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMartin, C.; Hurchinson, L.F.; Hyde, R.; Peters, G.E. Analysis of structure requirements for the absorption of drugs and macromol-ecules from the nasal cavity. J. Pharm. Sci. 1987, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Dale, O.; Hjortkjaer, R.; Kharasch, E.D. Nasal administration of opioids for pain management in adults. Acta Anaesthesiol. Scand. 2002, 46, 759–770. [Google Scholar] [CrossRef]
- Hussain, A.A. Mechanism of nasal absorption of drugs. Prog. Clin. Biol. Res. 1989, 292, 261–272. [Google Scholar]
- Harlos, M.S.; Stenekes, S.; Lambert, D.; Hohl, C.; Chochinov, H.M. Intranasal fentanyl in the palliative care of newborns and infants. J. Pain Symptom. Manag. 2013, 46, 265–274. [Google Scholar] [CrossRef]
- Mathison, S.; Nagilla, R.; Kompella, U.B. Nasal route for direct delivery of solutes to the central nervous system: Fact or fic- tion? J. Drug Target. 1998, 5, 415–441. [Google Scholar] [CrossRef] [PubMed]
- Sakane, T.; Akizuki, M.; Yoshida, M.; Yamashita, S.; Nadai, T.; Hashida, M.; Sezaki, H. Transport of cephalexin to the cerebro-spinal fluid directly from the nasal cavity. J. Pharm. Pharmacol. 1991, 436, 449–451. [Google Scholar]
- Banks, W.A.; During, M.J.; Niehoff, M.L. Brain uptake of the glucagon-like peptide-1 antagonist exendin after intranasal administration. J. Pharmacol. Exp. Ther. 2004, 309, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Striebel, H.W.; Krämer, J.; Luhmann, I.; Rohierse-Hohler, I.; Rieger, A. Pharmacokinetics of intranasal fentanyl. Schmerz 1993, 7, 122–125. (In German) [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, I.; Mystakidou, K. Intranasal fentanyl: From pharmacokinetics and bioavailability to current treatment applications. Expert Rev. Anticancer. Ther. 2010, 10, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Moksnes, K.; Fredheim, O.M.; Klepstad, P.; Kaasa, S.; Angelsen, A.; Nilsen, T.; Dale, O. Early pharmacokinetics of nasal fentanyl: Is there a significant arterio-venous difference? Eur. J. Clin. Pharmacol. 2008, 64, 497–502. [Google Scholar] [CrossRef]
- Foster, D.; Upton, R.; Christrup, L.; Popper, L. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain aNer oral surgery. Ann. Pharmacother. 2008, 42, 1380–1387. [Google Scholar] [CrossRef]
- Lim, S.; Paech, M.J.; Sunderland, V.B.; Roberts, M.J.; Banks, S.L.; Rucklidge, M.W.M. Pharmacokinetics of nasal fentanyl. J. Pharm. Pract. Res. 2003, 33, 59–63. [Google Scholar] [CrossRef]
- Paech, M.J.; Lim, C.B.; Banks, S.L.; Rucklidge, M.W.M.; Doherty, D.A. A new formulation of nasal fentanyl spray for postoperative analgesia: A pilot study. Anaesthesia 2003, 58, 740–744. [Google Scholar] [CrossRef]
- Younge, P.; Nicol, M.; Kendall, J.; Harrington, A.P. A prospective randomized pilot comparison of intranasal fentanyl and intramuscular morphine for analgesia in children presenting to the emergency department with clinical fractures. Emerg Med. 1999, 11, 90–94. [Google Scholar] [CrossRef]
- Borland, M.; Jacobs, I.; King, B.; O’Brien, D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann. Emerg. Med. 2007, 49, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Holdgate, A.; Cao, A.; Lo, K.M. The implementation of intranasal fentanyl for children in a mixed adult and pediatric emergency department reduces time to analgesic administration. Acad. Emerg. Med. 2010, 17, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.P.; Pedersen, D.M.; Trautner, S.; Dahl, J.B.; Hansen, M.S. Safety of intranasal fentanyl in the out-of-hospital setting: A prospective observational study. Ann. Emerg. Med. 2014, 63, 699–703. [Google Scholar] [CrossRef]
- Rickard, C.; O’Meara, P.; McGrail, M.; Garner, D.; McLean, A.; Le Lievre, P. A randomized controlled trial of intranasal fentanyl vs intravenous morphine for analgesia in the prehospital setting. Am. J. Emerg. Med. 2007, 25, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, P.M.; Simpson, P.; Sinclair, G.; Dobbins, T.; Math, B.; Bendall, A.J. Effectiveness of morphine, fentanyl, and methoxyflurane in the prehospital setting. Prehosp. Emerg. Care. 2010, 14, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, C.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Murphy, A.P.; Hughes, M.; Mccoy, S.; Crispino, G.; Wakai, A.; O’Sullivan, R. Intranasal fentanyl for the prehospital management of acute pain in children. Eur. J. Emerg. Med. 2017, 24, 450–454. [Google Scholar] [CrossRef]
- Akinsola, B.; Hagbom, R.; Zmitrovich, A.; Kavanagh, P.L.; Ashkouti, A.; Simon, H.K.; Fleming, A.; Jain, S.; Dampier, C.; Morris, C.R. Impact of Intranasal Fentanyl in Nurse Initiated Protocols for Sickle Cell Vaso-occlusive Pain Episodes in a Pediatric Emergency Department. Am. J. Hematol. 2018. ahead of print. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.; Harrell, C.; Snider, M.; Kink, R. The Safety of High-Dose Intranasal Fentanyl in the Pediatric Emergency Department. Pediatr. Emerg. Care. 2022, 38, e447–e450. [Google Scholar] [CrossRef]
- Cole, J.; Shepherd, M.; Young, P. Intranasal fentanyl in 1-3-year-olds: A prospective study of the effectiveness of intranasal fentanyl as acute analgesia. Emerg. Med. Australas. 2009, 21, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Crellin, D.; Ling, R.X.; Babl, F.E. Does the standard intravenous solution of fentanyl (50 microg/mL) administered intranasally have analgesic efficacy? Emerg. Med. Australas. 2010, 22, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.; Harris, D. Intranasal fentanyl for analgesia in the paediatric emergency department. Emerg. Med. J. 2010, 27, 300–301. [Google Scholar] [CrossRef]
- Kelly, G.S.; Stewart, R.W.; Strouse, J.J.; Anders, J.F. Intranasal fentanyl improves time to analgesic delivery in sickle cell pain crises. Am. J. Emerg. Med. 2018, 36, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, M.; Jacobsen, N.; Bantel, C.; Fieler, M.; Sümpelmann, R.; Eich, C. Intranasal Analgesia and Sedation in Pediatric Emergency Care-A Prospective Observational Study on the Implementation of an Institutional Protocol in a Tertiary Children’s Hospital. Pediatr Emerg Care 2019, 35, 89–95. [Google Scholar] [CrossRef]
- Saunders, M.; Adelgais, K.; Nelson, D. Use of intranasal fentanyl for the relief of pediatric orthopedic trauma pain. Acad. Emerg. Med. 2010, 17, 1155–1161. [Google Scholar] [CrossRef]
- Schaefer, J.A.; Mlekoday, T.J. Time to opioid administration after implementation of an intranasal fentanyl protocol. Am. J. Emerg. Med. 2015, 33, 1805–1807. [Google Scholar] [CrossRef]
- Borland, M.; Milsom, S.; Esson, A. Equivalency of two concentrations of fentanyl administered by the intranasal route for acute analgesia in children in a paediatric emergency department: A randomized controlled trial. Emerg. Med. Australas. 2011, 23, 202–208. [Google Scholar] [CrossRef]
- Fein, D.M.; Avner, J.R.; Scharbach, K.; Manwani, D.; Khine, H. Intranasal fentanyl for initial treatment of vaso-occlusive crisis in sickle cell disease. Pediatr. Blood Cancer 2017, 64, e26332. [Google Scholar] [CrossRef]
- Frey, T.M.; Florin, T.A.; Caruso, M.; Zhang, N.; Zhang, Y.; Mittiga, M.R. Effect of Intranasal Ketamine vs Fentanyl on Pain Reduction for Extremity Injuries in Children: The PRIME Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Graudins, A.; Meek, R.; Egerton-Warburton, D.; Oakley, E.; Seith, R. The PICHFORK (Pain in Children Fentanyl or Ketamine) trial: A randomized controlled trial comparing intranasal ketamine and fentanyl for the relief of moderate to severe pain in children with limb injuries. Ann. Emerg. Med. 2015, 65, 248–254.e1. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.; Kriss, S.; Drapkin, J.; Likourezos, A.; Pushkar, I.; Brady, J.; Yasavolian, M.; Chitnis, S.S.; Motov, S.; Fromm, C. Analgesic Efficacy of Intranasal Ketamine Versus Intranasal Fentanyl for Moderate to Severe Pain in Children: A Prospective, Randomized, Double-Blind Study. Pediatr. Emerg. Care 2021, 37, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, S.L.; Bryant, K.K.; Studnek, J.R.; Hogg, M.; Dunn, C.; Templin, M.A.; Moore, C.; Young, J.R.; Walker, K.R.; Runyon, M.S. Randomized Controlled Feasibility Trial of Intranasal Ketamine Compared to Intranasal Fentanyl for Analgesia in Children with Suspected Extremity Fractures. Acad. Emerg. Med. 2017, 24, 1430–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffin, T.B., Jr.; Salinero, E.; Papa, L.M.; Cramm, K.; Florez, C.; Chen, J.G.M.; Ramirez, J. Intranasal Fentanyl to Reduce Pain and Improve Oral Intake in the Management of Children With Painful Infectious Mouth Lesions. Pediatr. Emerg. Care 2022, 38, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, A.; Lebon, J.; Hébert, D.; Bégin, F. Intranasal Fentanyl versus Subcutaneous Fentanyl for Pain Management in Prehospital Patients with Acute Pain: A Retrospective Analysis. Prehosp. Emerg. Care 2020, 24, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Assad, O.; Zamora, R.; Brown, K.; Melnitsky, L.; Moses, J.; Sherman, V. IF IM in a crisis: Intranasal fentanyl versus intravenous morphine in adult vaso-occlusive crisis. Am. J. Emerg. Med. 2023, 64, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Belkouch, A.; Zidouh, S.; Rafai, M.; Chouaib, N.; Sirbou, R.; Elbouti, A.; Bakkali, H.; Belyamani, L. Does intranasal fentanyl provide efficient analgesia for renal colic in adults? Pan. Afr. Med. J. 2015, 20, 407. [Google Scholar] [CrossRef] [PubMed]
- Nasr Isfahani, M.; Shokoohi, O.; Golshani, K. Intranasal ketamine versus intranasal fentanyl on pain management in isolated traumatic patients. J. Res. Med. Sci. 2022, 27, 1. [Google Scholar] [CrossRef]
- Nazemian, N.; Torabi, M.; Mirzaee, M. Atomized intranasal vs intravenous fentanyl in severe renal colic pain management: A randomized single-blinded clinical trial. Am. J. Emerg. Med. 2020, 38, 1635–1640. [Google Scholar] [CrossRef]
- Khanna, A.K.; Bergese, S.D.; Jungquist, C.R.; Morimatsu, H.; Uezono, S.; Lee, S.; Ti, L.K.; Urman, R.D.; McIntyre, R., Jr.; Tornero, C.; et al. Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth Analg. 2020, 131, 1012–1024. [Google Scholar] [CrossRef]
- Gupta, K.; Prasad, A.; Nagappa, M.; Wong, J.; Abrahamyan, L.; Chung, F.F. Risk factors for opioid-induced respiratory depression and failure to rescue: A review. Curr. Opin. Anaesthesiol. 2018, 31, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.R.; Tuckett, R.P.; Song, C.W. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J. Pain 2008, 9, 122–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumley, M.A.; Cohen, J.L.; Borszcz, G.S.; Cano, A.; Radcliffe, A.M.; Porter, L.S.; Schubiner, H.; Keefe, F.J. Pain and emotion: A biopsychosocial review of recent research. J. Clin. Psychol. 2011, 67, 942–968. [Google Scholar] [CrossRef] [Green Version]
- Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T. Route of placebo administration: Robust placebo effects in laboratory and clinical settings. Neurosci. Biobehav. Rev. 2017, 83, 451–457. [Google Scholar] [CrossRef]
- Schwartz, N.A.; Turturro, M.A.; Istvan, D.J.; Larkin, G.L. Patients’ perceptions of route of nonsteroidal anti-inflammatory drug administration and its effect on analgesia. Acad. Emerg. Med. 2000, 7, 857–861. [Google Scholar] [CrossRef]
- Swerts, D.B.; Benedetti, F.; Peres, M.F.P. Different routes of administration in chronic migraine prevention lead to different placebo responses: A meta-analysis. Pain 2022, 163, 415–424. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H.; Fainsinger, R.; Aass, N.; Kaasa, S. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Rizzo, R.R.N.; Lee, H.; Cashin, A.G.; Costa, L.O.P.; Gustin, S.M.; McAuley, J.H. The mediating effect of pain catastrophizing on pain intensity: The influence of the timing of assessments. Eur. J. Pain 2021, 25, 1938–1947. [Google Scholar] [CrossRef]
- Jensen, M.P.; Tomé-Pires, C.; de la Vega, R.; Galán, S.; Solé, E.; Miró, J. What Determines Whether a Pain is Rated as Mild, Moderate, or Severe? The Importance of Pain Beliefs and Pain Interference. Clin. J. Pain 2017, 33, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Hunsinger, M.; McKeown, A.; Parkhurst, M.; Allen, R.; Kopko, S.; Lu, Y.; Wilson, H.D.; Burke, L.B.; Desjardins, P.; et al. Quality of pain intensity assessment reporting: ACTTION systematic review and recommendations. J. Pain 2015, 16, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Mathiesen, O.; Trautner, S.; Dahl, J.B. Intranasal fentanyl in the treatment of acute pain--a systematic review. Acta Anaesthesiol. Scand. 2012, 56, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; O’Sullivan, R.; Wakai, A.; Grant, T.S.; Barrett, M.J.; Cronin, J.; McCoy, S.C.; Hom, J.; Kandamany, N. Intranasal fentanyl for the management of acute pain in children. Cochrane Database Syst. Rev. 2014, 2014, CD009942. [Google Scholar] [CrossRef]
- Setlur, A.; Friedland, H. Treatment of pain with intranasal fentanyl in pediatric patients in an acute care setting: A systematic review. Pain Manag. 2018, 8, 341–352. [Google Scholar] [CrossRef]
- Abebe, Y.; Hetmann, F.; Sumera, K.; Holland, M.; Staff, T. The effectiveness and safety of paediatric prehospital pain management: A systematic review. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 170. [Google Scholar] [CrossRef]
- Savoia, G. Italian Intersociety Recommendations on pain management in the emergency setting (SIAARTI, SIMEU, SIS 118, AISD, SIARED, SICUT, IRC). Minerva Anestesiol. 2015, 81, 205–225. [Google Scholar]
| Author, Year of Publication | Risk of Bias Arising from the Randomisation Process | Risk of Bias Due to Deviations from the Intended Interventions (Effect of Assignment to Intervention) | Risk of Bias Due to Deviations from the Intended Interventions (Effect of Adhering to Intervention) | Risk of Bias Due to Missing Outcome Data | Risk of Bias in the Measurement of the Outcome | Risk of Bias in Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Children, emergency department setting: | |||||||
| Borland et al., 2011 [48] | L | L | L | L | L | L | L |
| Fein et al., 2016 [49] | L | L | L | L | L | L | L |
| Frey et al., 2018 [50] | L | L | L | L | L | L | L |
| Graudins et al., 2015 [51] | L | L | L | L | L | L | L |
| Quinn et al., 2018 [52] | L | L | L | L | L | L | L |
| Reynolds et al., 2017 [53] | L | L | L | L | L | L | L |
| Ruffin et al., 2022 [54] | L | L | L | L | H | L | H |
| Younge et al., 1999 [30] | L | L | L | L | H | L | H |
| Adult, emergency department setting: | |||||||
| Nasr Isfahani et al., 2022 [58] | L | L | L | L | L | L | L |
| Nazemian et al., 2019 [59] | SC | H | H | L | H | L | H |
| Author, Year of Publication | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children, prehospital emergency service: | |||||||||||||
| Murphy et al., 2017 [38] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | NA | NA | NA | NA | 13 |
| Children, emergency department | |||||||||||||
| Akinsola et al., 2018 [39] | 2 | 2 | 1 | 2 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 0 | 14 |
| Anderson et al., 2022 [40] | 2 | 2 | 1 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | NA | 13 |
| Cole et al., 2009 [41] | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | NA | NA | NA | NA | 16 |
| Crelin et al., 2010 [42] | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | NA | NA | NA | NA | 12 |
| Finn et al., 2010 [43] | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 10 |
| Kelly et al., 2017 [44] | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 1 | 2 | 17 |
| Nemeth et al., 2017 [45] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 0 | 2 | 2 | 16 |
| Saunders et al., 2010 [46] | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | NA | 14 |
| Schaefer et al., 2015 [47] | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 15 |
| Adult, prehospital emergency service setting | |||||||||||||
| Tanguay et al., 2020 [55] | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 20 |
| Adult, emergency department setting | |||||||||||||
| Assad et al., 2022 [56] | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 19 |
| Belkouch et al., 2015 [57] | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, S.; Spampinato, M.D.; Riccardi, A.; Guarino, M.; Pavasini, R.; Fabbri, A.; De Iaco, F. Intranasal Fentanyl for Acute Pain Management in Children, Adults and Elderly Patients in the Prehospital Emergency Service and in the Emergency Department: A Systematic Review. J. Clin. Med. 2023, 12, 2609. https://doi.org/10.3390/jcm12072609
Serra S, Spampinato MD, Riccardi A, Guarino M, Pavasini R, Fabbri A, De Iaco F. Intranasal Fentanyl for Acute Pain Management in Children, Adults and Elderly Patients in the Prehospital Emergency Service and in the Emergency Department: A Systematic Review. Journal of Clinical Medicine. 2023; 12(7):2609. https://doi.org/10.3390/jcm12072609
Chicago/Turabian StyleSerra, Sossio, Michele Domenico Spampinato, Alessandro Riccardi, Mario Guarino, Rita Pavasini, Andrea Fabbri, and Fabio De Iaco. 2023. "Intranasal Fentanyl for Acute Pain Management in Children, Adults and Elderly Patients in the Prehospital Emergency Service and in the Emergency Department: A Systematic Review" Journal of Clinical Medicine 12, no. 7: 2609. https://doi.org/10.3390/jcm12072609
APA StyleSerra, S., Spampinato, M. D., Riccardi, A., Guarino, M., Pavasini, R., Fabbri, A., & De Iaco, F. (2023). Intranasal Fentanyl for Acute Pain Management in Children, Adults and Elderly Patients in the Prehospital Emergency Service and in the Emergency Department: A Systematic Review. Journal of Clinical Medicine, 12(7), 2609. https://doi.org/10.3390/jcm12072609






