Organic Nanodelivery Systems as a New Platform in the Management of Breast Cancer: A Comprehensive Review from Preclinical to Clinical Studies
Abstract
1. Introduction
1.1. Breast Cancer Background
1.2. Breast Cancer Pathogenesis
2. Breast Tumour Heterogeneity: Implications for Management Strategies
3. Challenges of Conventional Strategies for Management of Breast Cancer
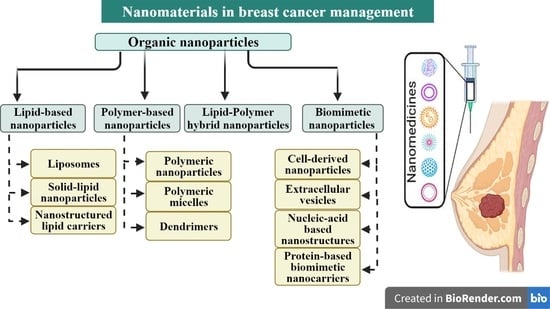
4. Breast Cancer in the Era of Nanomedicine
4.1. Lipid-Based Nanoparticles
4.1.1. Liposomes
4.1.2. Solid Lipid Nanoparticles
4.1.3. Nanostructured Lipid Carriers
4.2. Polymer-Based Nanoparticles
4.2.1. Polymeric Nanoparticles (PNPs)
4.2.2. Polymeric Micelles
4.2.3. Dendrimers
4.3. Lipid–Polymer Hybrid Nanoparticles
4.4. Biomimetic Nanoparticles in Breast Cancer
4.4.1. Cell-Derived Nanoparticles
| Name of Nanoparticle | Composition/Coating of Nanodelivery System | Size | Drug/Biomolecule | Cell Line/Animal Model | Targeting | Outcome | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Albumin nanoparticles | Human Serum Albumin (HAS) | NA | Methotrexate (MTX) and transforming growth factor-β1 antibody (TGF-β1) | MDA-MB-231 cell line | Active targeting by folate | Increased cellular uptake of folate-HSA-MTX and TGF antibody on nanoparticles scavenged extracellular TGFβ1 of cancer cells, reducing cell migration. | 2019 | [107] |
| Albumin nanoparticles | Human Serum Albumin | 246.5 nm | Curcumin (Cur) | MDA-MB-231, SK-BR-3, and MCF-7 cell lines | Active targeting by programmed death ligand 1 (PDL1) binding peptide for PDL1-overexpressing breast cancer cells | Peptide conjugation enhanced cellular uptake and cytotoxicity of HSA/Curcumin-loaded nanoparticles in PDL-1-overexpressing breast cancer cells. | 2020 | [108] |
| Albumin nanoparticles | Human Serum Albumin | 175 nm | Doxorubicin and MDR1 siRNA | MCF-7 and MCF-7/ADR cell lines. MCF7/ADR tumour-bearing mice model | Active targeting by cetuximab for epidermal growth factor receptor | Cetuximab-targeted doxorubicin/MDR1 siRNA-loaded nanoparticles inhibited MCF-7/ADR cells’ proliferation through promotion of apoptosis and superior tumour inhibition vs. doxorubicin alone. | 2021 | [109] |
| Reconstituted high-density lipoprotein (rHDL) nanoparticles | Lecithin, cholesterol, cholesteryl oleate, triglycerides | 101.3 nm | Paclitaxel (PTX) and HZ08 | MCF-7, MCF7/PTX resistant to paclitaxel, MCF 10A and MCF or MCF7/PTX tumour-bearing model in mice | Active targeting of cells overexpressing scavenger receptor class B type I (SR-BI) | Paclitaxel-HZ08-rHDL nanoparticles showed significant enhancement of anticancer efficacy in vitro demonstrated by higher cytotoxicity and induction of cell apoptosis against both paclitaxel-sensitive and -resistant cell lines; stronger antitumour activity using nanoparticles vs. equivalent dose of paclitaxel. | 2016 | [110] |
| Reconstituted high-density lipoprotein (rHDL) nanoparticles | (2,3-Dioleoyloxy-propyl)trimethylammo-nium chloride, phospholipids and cholesterol | 146–176 nm | Paclitaxel and siRNA VEGF (siVEGF) | MCF-7 cancer cell line/MCF-7 tumour-bearing mice model | Active targeting of cells overexpressing scavenger receptor class B type I (SR-BI) | In vitro results showed that rHDL/siVEGF-PTX caused a 14.96-fold increase in cytotoxicity compared to Taxol. In vivo studies demonstrated enhanced tumour growth inhibition. | 2017 | [111] |
| Reconstituted high-density lipoprotein (rHDL) nanoparticles | Dimyristoylphosphatidylcholine, Cholesterol oleate and ApoA-1 peptide | 16.2 nm | Lenvatinib and vadimezan | 4T1 cancer cell line/4T1 tumour-bearing mice | Active targeting of scavenger receptor class B type I (SR-BI) overexpressing 4T1 cells | In vivo results showed that LV-sHDL inhibited growth of 4T1 tumours, reduced lung metastasis and prolonged survival of animals. | 2022 | [112] |
| Human ferritin (HFn) nanocages | Heavy chain of human ferritin | 14.3 nm | Curcumin | MDA-MB-468 and MDA-MB231 cell lines | Active targeting of Transferrin receptor 1 (TfR1) | More effective compared to free drug by abrogating the activity of multidrug resistance transporters. | 2017 | [113] |
| Human ferritin (HFn) nanocages | Heavy chain of human ferritin | 15 nm | Paclitaxel | MDA-MB-231 cell line/MDAMB-231 tumour model in mice | Active targeting of Transferrin receptor 1 (TfR1) | HFtn-PTX nanoparticles showed significant cytotoxicity in vitro compared to free drug and had higher in vivo anticancer efficacy and lower systemic toxicity. | 2019 | [114] |
| Peptide-based nanoparticles | (C16-K(TPE)-GGGH-GFLGKPEG8, denoted as CTGP) | NA | Doxorubicin (DOX) | Cathepsin B-overexpressed MCF-7S and MCF-7R cell lines/MCF-7R tumour-bearing nude mice | Active targeting by cathepsin B-responsive peptide sequence (GlyPhe-Leu-Gly) | Efficient drug retention (46-fold of doxorubicin) and exceptional anti-MDR effect (50-fold of doxorubicin) in comparison to free drug as shown in in vitro and in vivo experiments. | 2018 | [115] |
| Peptide-based nanoparticles | Peptide amphiphile (PA) incorporating a TRAIL-mimetic peptide sequence | NA | Paclitaxel | MDA-MB-231 cells/tumour model in mice | Active targeting by binding of TRAIL-mimetic peptide sequence to death receptor 5 (DR5) overexpressed on cancer cells | High binding affinity to DR5-overexpressing cancer cells. When combined with paclitaxel, DR5-targeting nanoparticles showed potent antitumour activity in mice model. | 2019 | [116] |
| Nucleic acid-based nanoparticles | Staple DNA strands | NA | Doxorubicin and shPgp silencing P-glycoprotein and shSur silencing survivin | DOX-resistant human MCF7(MCF-7R) cell line/MCF-7R tumour-bearing mice | Active targeting by MUC1 aptamer | Results showed augmented synergistic antitumour effect against multidrug-resistant tumours both in vitro and in vivo. | 2018 | [117] |
| Nucleic acid-based nanoparticles | Branched DNA | 100–140 nm | sgRNA/Cas9/antisense complex targeting tumour-associated gene polo-like kinase 1 (PLK1) | MCF-7 cancer cell line/MCF tumour xenograft model | Active targeting by adamantine-conjugated aptamer | Data revealed efficient tumour growth inhibition with undetected systemic toxicity. | 2019 | [118] |
| Nucleic acid-based nanoparticles | Staple DNA strands | NA | Doxorubicin | MDA-MB-468 and MDA-MB231 cell lines | Active targeting by folate | Higher uptake for targeted nanoparticles compared to nontargeted nanoparticles and the doxorubicin dose required to kill cancer cells was ∼31-fold lower for folate-functionalized nanoparticles. | 2021 | [119] |
| Nucleic acid-based nanoparticles | RNA oligonucleotides | NA | Anti-miR-21 | MDA-MB-231 and MCF7/ADR cell lines | Active targeting by aptamer for epidermal growth factor receptor (EGFR) | RNA nanoparticles decreased cell viability and increased the sensitivity of breast cancer cells to doxorubicin in vitro. miR-21 inhibition by RNA nanoparticles caused the suppression of TNBC cell invasion, migration and colony formation. | 2021 | [120] |
| Cell-derived nanoparticles: (a) Cell membrane-coated nanoparticles 1. Cancer cell membrane (4T1 breast cancer cell membrane) | Polymeric nanoparticles Poly(caprolactone) (PCL) and pluronic copolymer F68) | 175 nm | Paclitaxel | 4T1 cells/4T1 tumour-bearing mice. | Active targeting binding of overexpressed VCAM1 adhesion molecule on cancer cells to monocytes expressing cell adhesion molecules such as α4β1 integrin | Higher cellular uptake of the cell membrane-coated nanoparticles and 36-fold greater cytotoxic efficacy compared to other groups. In the in vivo studies, cell membrane-coated nanoparticles exhibited the highest antitumour growth efficacy compared to the other treated groups. | 2016 | [121] |
| 2. Cancer cell membrane (MCF7 cell membrane) | PLGA nanoparticles | 202 nm | Curcumin and chlorin e6 (Ce6) | MCF-7 cell line/MCF-7 tumour-bearing mice model | Active targeting | Results showed significant cytotoxicity on MCF-7 cells of Cur/Ce6-cell membrane-coated NPs. In vivo data demonstrated prolonged circulation time, specific tumour accumulation and enhanced tumour growth inhibition compared to uncoated group. | 2021 | [122] |
| 3. Red blood cell (RBC) membrane | Polymeric nanoparticles | 148 nm | Paclitaxel | 4T1 cell line/4T1 tumour-bearing murine model | Active targeting by binding of iRGD to cells overexpressing αvβ3 integrin and neuropilin-1 | RBC-coated nanoparticles had a 5.8-fold higher elimination half time than that of the parental nanoparticles; nanoparticles significantly inhibited tumour growth and suppressed lung metastasis more efficiently than paclitaxel-loaded polymer nanoparticles alone or iRGD functionalized polymer nanoparticles. | 2016 | [123] |
| 4. Red blood cell (RBC) membrane | PLGA nanoparticles | 159 nm | Doxorubicin | MCF-7 epithelial cell adhesion molecule-positive (EpCAM+) cancer cell 2D and 3D spheroids | Active targeting by antiEpCAM antibodies | Results showed improved cytotoxic effect of targeted RBC NPs compared to nontargeted RBC NPs and free drug in both 2D and 3D in vitro breast cancer models. | 2022 | [124] |
| 5. Macrophage cell membrane | Polymeric nanoparticles | NA | Paclitaxel | MDA-MB-231 cell line/tumour-bearing mice | Active targeting by insulin-like growth factor 1 receptor (IGF1R) peptide | In vitro and in vivo studies demonstrated that macrophage membrane-coated NPs resulted in the most extensive cell apoptosis among treated groups. | 2018 | [125] |
| 6. Platelet membrane (PM) | PLGA nanoparticles | 121 nm | Tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and doxorubicin | MDA-MB-231 cell line/tumour-bearing mice | Active targeting by binding of P-selectin on platelet membrane and overexpressed CD44 receptors on cancer cells | Enhanced cytotoxicity compared with other treated groups at all studied TRAIL and doxorubicin concentrations, selective tumour targeting and higher tumour growth inhibition compared to doxorubicin. | 2015 | [126] |
| 7. Platelet membrane (PM) | PLGA nanoparticles | 293–300 nm | Doxorubicin and IR780 iodide | 4T1 cell line/4T1 tumour model in mice | Active targeting by binding of P-selectin on platelet membrane and overexpressed CD44 receptors on cancer cells | Platelet membrane-coated NPs enhanced cancer cell killing in vitro and in vivo studies, showed accumulation at tumour site and enhanced tumour cell death upon NIR irradiation. | 2020 | [127] |
| Cell-derived nanoparticles (b) Extracellular vesicles (Exosomes) | Human bone marrow-derived mesenchymal stem cell (MSC) exosomes | 128 nm | Paclitaxel | MDA-MB-231, MCF-7 cell lines/MDA-MB-231 tumour model in mice | Passive targeting | Reduced the viability of MDAMB-231 cells in vitro; significant tumour growth inhibition compared to control and/or MSC-EMs in vivo. | 2018 | [128] |
| Extracellular vesicles (Exosomes) | Cancer-derived exosome, HER2-positive SKBR-3 and EFM-192A cells and HER2-negative MCF-7 cells | 30–300 nm | Trastuzumab emtansine (T-DM1) | HER2-positive SKBR-3 and HER2-negative MCF-7 cell lines | Active targeting by binding of trastuzumab to HER-2-positive breast cancer cells | T-DM1 enhanced binding to HER2-positive cancer cell-derived exosomes but not to exosomes derived from HER2-negative MCF-7 cells. Treatment of SKBR-3 and EFM-192A cells with T-DM1 containing exosomes caused tumour growth inhibition and activation of caspases 3 and/or 7 | 2018 | [129] |
| Extracellular vesicles (Exosomes) | Monocyte-derived macrophages (THp1) exosomes | 179 nm | Doxorubicin and miR159 | MDA-MB-231); xenograft-breast tumour model in mice | Active targeting by binding of disintegrin and metalloproteinase 15 (A15) expressed on exosomal membranes to integrin αvβ3 on cancer cells | Co-loading of doxorubicin and miR159 to A15-Exo generated synergistic therapeutic effects in vitro. Delivery of miR159 and doxorubicin effectively silenced the TCF-7 gene, resulting in better anticancer effects without noticeable side effects. | 2019 | [130] |
| Extracellular vesicles (Exosomes) | Adipose-derived mesenchymal stem cells | 40–100 nm | miR-381 | MDA-MB-231 cell line | Passive targeting | miR-381-loaded exosomes significantly downregulated expression of epithelial to mesenchymal transition (EMT)-related genes and proteins, which led to inhibition of proliferation and migration of MDA-MB-231 cells in vitro. | 2021 | [131] |
| Extracellular vesicles (Exosomes) | Cancer-derived exosomes (SKBR-3 and MCF-7 cells) | 65 nm | Recombinant P53 protein | SKBR-3, MCF-7 cell lines/4T1 tumour model in mice | Active targeting of mitochondria by triphenylphosphonium (TPP) | Findings revealed successful targeting of PP/P53 to mitochondria of breast cancer cells. In vivo results showed good tumour accumulation and destruction without apparent systemic toxicity. | 2022 | [132] |
| Nanoparticle | Properties |
|---|---|
| Liposomes | Biocompatible |
| Biodegradable and can be stored in lyophilised form or as an aqueous suspension | |
| Versatile carrier of neutral, hydrophobic and hydrophilic agents | |
| Improved pharmacokinetics using PEGylation renders liposomes suitable for | |
| passive targeting; active targeting via surface ligands | |
| Polymeric nanoparticles/micelles | Biocompatible and generally biodegradable |
| Nanocarriers for hydrophobic agents, but less suitable for hydrophilic agents | |
| Can offer high drug loading capacity (copolymer micelles) | |
| Solid lipid nanoparticles | Biocompatible |
| Biodegradable but low-temperature storage required with current formulations | |
| Incorporation of agent within solid lipid matrix affords protection against | |
| degradation, e.g., hydrolysis | |
| Dendrimers | Branched, well-defined polymeric structure that can accommodate hydrophobic |
| agents within or surface-conjugated hydrophilic agents | |
| More complicated structurally than other polymeric nanocarriers; therefore, | |
| synthesis is more expensive, and only limited or no biodegradability | |
| Suitable for passive (large dendrimers) and active targeting | |
| Biomimetic nanoparticles (e.g., exosomes) | Biodegradable and versatile nanocarriers |
| Very low immunogenicity with ability to traverse physiological barriers | |
| Scaled-up production of biomimetic exosomes is difficult and long-term storage | |
| requires low temperatures |
4.4.2. Extracellular Vesicles (EVs)
4.4.3. Nucleic-Acid-Based Nanostructures
4.4.4. Protein-Based Biomimetic Nanocarriers
Serum Albumin Fabricated Nanoparticles
Ferritin-Based Nanocarriers
Lipoprotein-Based Nanocarriers
Peptide-Based Nanocarriers
4.5. Multifunctional Nanostructures and Tumour-Targeted Delivery
4.5.1. Tumour-Targeted Delivery
Targeting the Extracellular Matrix
Targeting Breast Cancer Cells
Targeting Breast Cancer Subcellular Organelles
4.5.2. Stimuli-Responsive Nanoparticles
pH-Responsive Nanoparticles
Hypoxia-Responsive Nanoparticles
Redox-Responsive Nanoparticles
Thermosensitive Nanoparticles
Near-Infrared (NIR) Stimuli-Responsive Nanoparticles
Multi-Stimuli Nanocarriers for Breast Cancer Therapy
5. Clinical Status of Nanomedicine for Breast Cancer Treatment
5.1. Clinically Approved Nanoformulated Drugs
5.2. Nanoformulated Drugs in Clinical Trials
6. Environmental Hazards of Nanoparticles
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Institute of Medicine. Breast Cancer and the Environment: A Life Course Approach; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 19, 23–28. [Google Scholar] [CrossRef]
- Kong, D.; Hughes, C.J.; Ford, H.L. Cellular Plasticity in Breast Cancer Progression and Therapy. Front. Mol. Biosci. 2020, 7, 72. [Google Scholar] [CrossRef]
- Koren, S.; Bentires-Alj, M. Breast Tumor Heterogeneity: Source of Fitness, Hurdle for Therapy. Mol. Cell. 2015, 60, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Molecular Subtypes of Breast Cancer, from Breastcancer.org. 2022. Available online: https://www.breastcancer.org/symptoms/types/molecular-subtypes (accessed on 6 July 2022).
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Garattini, S.; Fuso Nerini, I.; D’Incalci, M. Not only tumor but also therapy heterogeneity. Ann. Oncol. 2018, 29, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Morosi, L.; Zucchetti, M.; D’Incalci, M.; Davoli, E. Imaging mass spectrometry: Challenges in visualization of drug distribution in solid tumors. Curr. Opin. Pharmacol. 2013, 13, 807–812. [Google Scholar] [CrossRef]
- Dobosz, M.; Ntziachristos, V.; Scheuer, W.; Strobel, S. Multispectral fluorescence ultramicroscopy: Three-dimensional visualization and automatic quantification of tumor morphology, drug penetration, and antiangiogenic treatment response. Neoplasia 2014, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, I.H.; Prideaux, B.; Krings, G.; Wilmes, L.; Lee, P.R.E.; Bo, P.; Hann, B.; Coppé, J.P.; Heditsian, D.; Swigart-Brown, L.; et al. Heterogeneous drug penetrance of veliparib and carboplatin measured in triple negative breast tumors. Breast Cancer Res. 2017, 19, 107. [Google Scholar] [CrossRef]
- Giordano, S.; Morosi, L.; Veglianese, P.; Licandro, S.A.; Frapolli, R.; Zucchetti, M.; Cappelletti, G.; Falciola, L.; Pifferi, V.; Visentin, S.; et al. 3D mass spectrometry imaging reveals a very heterogeneous drug distribution in tumors. Sci. Rep. 2016, 6, 37027. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Martin, J.D.; Snuderl, M.; Mpekris, F.; Jain, S.R.; Jain, R.K. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: Implications for vascular collapse. Cancer Res. 2013, 73, 3833–3841. [Google Scholar] [CrossRef]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Tuasha, N.; Petros, B. Heterogeneity of Tumors in Breast Cancer: Implications and Prospects for Prognosis and Therapeutics. Scientifica 2020, 2020, 4736091. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020, 370, m2836. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. New clinical and biological insights from the international TARGIT-A randomised trial of targeted intraoperative radiotherapy during lumpectomy for breast cancer. Br. J. Cancer 2021, 125, 380–389. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Loprinzi, C.L.; Haddad, T.C. Updates in the Evaluation and Management of Breast Cancer. Mayo Clin. Proc. 2018, 93, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Masoud, V.; Pagès, G. Targeted therapies in breast cancer: New challenges to fight against resistance. World J. Clin. Oncol. 2017, 8, 120–134. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Tobias, J.S.; on behalf of the TARGIT-A trial authors. Single-dose intraoperative radiotherapy during lumpectomy for breast cancer: An innovative patient-centred treatment. Br. J. Cancer 2021, 124, 1469–1474. [Google Scholar] [CrossRef]
- Yadav, P.; Shankar, B.S. Radio resistance in breast cancer cells is mediated through TGF-β signalling, hybrid epithelial-mesenchymal phenotype and cancer stem cells. Biomed. Pharmacother. 2019, 111, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Muley, H.; Fadó, R.; Rodríguez-Rodríguez, R.; Casals, N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem. Pharmacol. 2020, 177, 113959. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N.D.; Bauer, J. Nanomedicine: Next generation modality of breast cancer therapeutics. In Nanomedicines for Breast Cancer Theranostics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 8, pp. 3–14. [Google Scholar]
- Mirza, Z.; Karim, S. Nanoparticles-based drug delivery and gene therapy for breast cancer: Recent advancements and future challenges. Semin. Cancer Biol. 2021, 69, 226–237. [Google Scholar] [CrossRef]
- Mi, Y.; Shao, Z.; Vang, J.; Kaidar-Person, O.; Wang, A.Z. Application of nanotechnology to cancer radiotherapy. Cancer Nanotechnol. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Parvanian, S.; Mostafavi, S.M.; Aghashiri, M. Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens. Bio-Sens. Res. 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef]
- Papa, A.L.; Sidiqui, A.; Balasubramanian, S.U.; Sarangi, S.; Luchette, M.; Sengupta, S.; Harfouche, R. PEGylated liposomal Gemcitabine: Insights into a potential breast cancer therapeutic. Cell. Oncol. 2013, 36, 449–457. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Dai, D.D.; Lu, C.T.; Chen, L.J.; Lin, M.; Shen, X.T.; Li, X.K.; Zhang, M.; Jiang, X.; Jin, R.R.; et al. Epirubicin loaded with propylene glycol liposomes significantly overcomes multidrug resistance in breast cancer. Cancer Lett. 2013, 330, 74–83. [Google Scholar] [CrossRef]
- Ju, R.J.; Cheng, L.; Peng, X.M.; Wang, T.; Li, C.Q.; Song, X.L.; Liu, S.; Chao, J.P.; Li, X.T. Octreotide-modified liposomes containing daunorubicin and dihydroartemisinin for treatment of invasive breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 616–628. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Zhao, W.; Zeng, C.; Li, W.; Li, B.; Luo, X.; Li, J.; Jiang, J.; Deng, B.; et al. Chemotherapy drugs derived nanoparticles encapsulating mRNA encoding tumor suppressor proteins to treat triple-negative breast cancer. Nano Res. 2019, 12, 855–861. [Google Scholar] [CrossRef]
- Ghafari, M.; Haghiralsadat, F.; Khanamani, S.; Pour, F.; Reza, J.Z. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J. Cell. Biochem. 2020, 121, 3584–3592. [Google Scholar] [CrossRef]
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.J.; Mandal, T.; Dash, S.; Kundu, A. Targeted delivery of doxorubicin liposomes for HER-2+ breast cancer treatment. AAPS Pharm. SciTech. 2020, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, N.; Torrieri, G.; Figueiredo, P.; Celia, C.; Paolino, D.; Correia, A.; Moslova, K.; Teesalu, T.; Fresta, M.; Santos, H.A. LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy. Int. J. Pharm. 2021, 597, 120346. [Google Scholar] [CrossRef]
- Swami, R.; Kumar, Y.; Chaudhari, D.; Katiyar, S.S.; Kuche, K.; Katare, P.B.; Banerjee, S.K.; Jain, S. pH sensitive liposomes assisted specific and improved breast cancer therapy using co-delivery of SIRT1 shRNA and Docetaxel. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111664. [Google Scholar] [CrossRef]
- Chen, T.; Chen, H.; Jiang, Y.; Yan, Q.; Zheng, S.; Wu, M. Co-Delivery of 5-Fluorouracil and Paclitaxel in Mitochondria-Targeted KLA-Modified Liposomes to Improve Triple-Negative Breast Cancer Treatment. Pharmaceuticals 2022, 15, 881. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Chun, M.K.; Kim, O.; Subedi, R.K.; Aln, S.-G.; Yoon, J.-H.; Choi, H.-K. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy. Nanomedicine 2010, 6, 210–213. [Google Scholar] [CrossRef]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: Enhanced in vitro anticancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Md, S.; Alhakamy, N.A.; Fahmy, U.A.; Kesharwani, P. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290. [Google Scholar] [CrossRef]
- Xu, W.; Bae, E.J.; Lee, M.K. Enhanced anticancer activity and intracellular uptake of paclitaxel-containing solid lipid nanoparticles in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2018, 13, 7549–7563. [Google Scholar] [CrossRef]
- Pindiprolu, S.K.S.S.; Chintamaneni, P.K.; Krishnamurthy, P.T.; Ratna Sree Ganapathineedi, K. Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev. Ind. Pharm. 2019, 45, 304–313. [Google Scholar] [CrossRef]
- Darabi, F.; Saidijam, M.; Nouri, F.; Mahjub, R.; Soleimani, M. Anti-CD44 and EGFR Dual-Targeted Solid Lipid Nanoparticles for Delivery of Doxorubicin to Triple-Negative Breast Cancer Cell Line: Preparation, Statistical Optimization, and In Vitro Characterization. BioMed Res. Int. 2022, 2022, 6253978. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Lima-Sousa, R.; Alves, C.G.; de Melo-Diogo, D.; Pinheiro, M.; Sousa, C.T.; Correia, I.J.; Reis, S. Mitoxantrone-loaded lipid nanoparticles for breast cancer therapy-Quality-by-design approach and efficacy assessment in 2D and 3D in vitro cancer models. Int. J. Pharm. 2021, 607, 121044. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Nie, S.; Pan, X.; Zhang, R.; Fan, Z.; Wang, S. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf. B Biointerfaces 2014, 113, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mahboubeh, R.; Jaber, E.; Farshid, H.; Hojjat, S.; Mahboubeh, R.; Hamid, M. Targeted Nanostructured Lipid Carriers for Delivery of Paclitaxel to Cancer Cells: Preparation, Characterization, and Cell Toxicity. Curr. Drug Deliv. 2017, 14, 1189–1200. [Google Scholar]
- Li, X.; Jia, X.; Niu, H. Nanostructured lipid carriers co-delivering lapachone and doxorubicin for overcoming multidrug resistance in breast cancer therapy. Int. J. Nanomed. 2018, 13, 4107–4119. [Google Scholar] [CrossRef]
- Poonia, N.; Kaur Narang, J.; Lather, V.; Beg, S.; Sharma, T.; Singh, B.; Pandita, D. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: Systematic development, characterization and pharmacokinetic evaluation. Colloids Surf B. Biointerfaces 2019, 181, 756–766. [Google Scholar] [CrossRef]
- Zhao, Y.; Huan, M.l.; Liu, M.; Cheng, Y.; Sun, Y.; Cui, H.; Liu, D.Z.; Mei, Q.B.; Zhou, S.Y. Doxorubicin and resveratrol co-delivery nanoparticle to overcome doxorubicin resistance. Sci. Rep. 2016, 6, 35267. [Google Scholar] [CrossRef]
- Majidi Zolbanin, N.; Jafari, R.; Majidi, J.; Atyabi, F.; Yousefi, M.; Jadidi-Niaragh, F.; Aghebati-Maleki, L.; Shanehbandi, D.; Soltani Zangbar, M.S.; Nayebi, A.M. Targeted Co-Delivery of Docetaxel and cMET siRNA for Treatment of Mucin1 Overexpressing Breast Cancer Cells. Adv. Pharm. Bull. 2018, 8, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Nieto-Jiménez, C.; Noblejas-López, M.D.M.; Bravo, I.; Castro-Osma, J.A.; Cruz-Martínez, F.; Buchaca, M.M.S.; Posadas, I.; Canales-Vázquez, J.; Lara-Sanchez, A.; et al. Poly (Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy. Nanomaterials 2019, 9, 1208. [Google Scholar] [CrossRef]
- Ghassami, E.; Varshosaz, J.; Mirian, M.; Jahanian-Najafabadi, A. HER-2 aptamer-targeted Ecoflex® nanoparticles loaded with docetaxel promote breast cancer cells apoptosis and anti-metastatic effect. IET Nanobiotechnol. 2019, 13, 428–434. [Google Scholar] [CrossRef]
- Akbari, E.; Mousazadeh, H.; Sabet, Z.; Fattahi, T.; Dehnad, A.; Akbarzadeh, A.; Alizadeh, E. Dual drug delivery of trapoxin A and methotrexate from biocompatible PLGA-PEG polymeric nanoparticles enhanced antitumor activity in breast cancer cell line. J. Drug Deliv. Sci. Technol. 2020, 61, 102294. [Google Scholar] [CrossRef]
- Behl, A.; Sarwalia, P.; Kumar, S.; Behera, C.; Mintoo, M.J.; Datta, T.K.; Gupta, P.N.; Chhillar, A.K. Codelivery of Gemcitabine and MUC1 Inhibitor Using PEG-PCL Nanoparticles for Breast Cancer Therapy. Mol. Pharm. 2022, 19, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Tortorella, S.; Agnello, L.; Spanu, C.; d’Argenio, A.; Nilo, R.; Zannetti, A.; Locatelli, E.; Fedele, M.; Comes Franchini, M.; et al. Aptamer-Functionalized Nanoparticles Mediate PD-L1 siRNA Delivery for Effective Gene Silencing in Triple-Negative Breast Cancer Cells. Pharmaceutics 2022, 14, 2225. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, S.; Wang, F.; Zou, A.; Zhang, S.; Xiong, Y.; Cao, S.; Zhang, Q.; Wang, Y.; Jiang, X. A novel combined micellar system of lapatinib and Paclitaxel with enhanced antineoplastic effect against human epidermal growth factor receptor-2 positive breast tumor in vitro. J. Pharm. Sci. 2015, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Qiu, K.; Yu, X.; Chen, C.; Qin, F.; Shi, Y.; Ou, J.; Zhang, T.; Zhu, H.; Wu, J.; et al. Amphiphilic Copolymeric Micelles for Doxorubicin and Curcumin Co-Delivery to Reverse Multidrug Resistance in Breast Cancer. J. Biomed. Nanotechnol. 2016, 12, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.W.; Ma, B.Y.; Zhao, Q.; Zhang, L.; Chen, L.J.; Peng, J.R.; Qian, Z.Y. Toxicity Evaluation and Anti-Tumor Study of Docetaxel Loaded mPEG-Polyester Micelles for Breast Cancer Therapy. J. Biomed. Nanotechnol. 2017, 13, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, A.; Yaghoobi, E.; Azizollahi, H.; Shojaee, S.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Design and synthesis of a star-like polymeric micelle modified with AS1411 aptamer for targeted delivery of camptothecin for cancer therapy. Int. J. Pharm. 2022, 611, 121346. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, C.; Chen, F.; Pan, W.; Zhang, L. Hyaluronan-Based Theranostic Nanomicelles for Breast Cancer-Targeting and Anticancer Drug Delivery. Mater. Des. 2023, 225, 111551. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; HuangFu, M.; Xiao, Y.; Zhang, T.; Han, M.; Xu, D.; Li, F.; Ling, D.; Jin, Y.; et al. Pluronic-attached polyamidoamine dendrimer conjugates overcome drug resistance in breast cancer. Nanomedicine 2016, 11, 2917–2934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, Q.; Wang, C.; Xu, J.; Wu, J.P.; Kirk, T.B.; Ma, D.; Xue, W. Star-Shaped Amphiphilic Hyperbranched Polyglycerol Conjugated with Dendritic Poly(l-lysine) for the Codelivery of Docetaxel and MMP-9 siRNA in Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 12609–12619. [Google Scholar] [CrossRef]
- Chittasupho, C.; Anuchapreeda, S.; Sarisuta, N. CXCR4 targeted dendrimer for anti-cancer drug delivery and breast cancer cell migration inhibition. Eur. J. Pharm. Biopharm. 2017, 119, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Stanczyk, M.; Janaszewska, A.; Sobierajska, E.; Chworos, A.; Klajnert-Maculewicz, B. Multicomponent Conjugates of Anticancer Drugs and Monoclonal Antibody with PAMAM Dendrimers to Increase Efficacy of HER-2 Positive Breast Cancer Therapy. Pharm. Res. 2019, 36, 154. [Google Scholar] [CrossRef] [PubMed]
- Abedi Gaballu, F.; Cho, W.C.-S.; Dehghan, G.; Zarebkohan, A.; Baradaran, B.; Mansoori, B.; Abbaspour-Ravasjani, S.; Mohammadi, A.; Sheibani, N.; Aghanejad, A.; et al. Silencing of HMGA2 by siRNA Loaded Methotrexate Functionalized Polyamidoamine Dendrimer for Human Breast Cancer Cell Therapy. Genes 2021, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, D.; Chen, J.; Ye, L.; Xie, Y.; Zhao, X.; Zou, H.; Chen, X.; Pu, J.; Liu, P. A targeted nanoplatform co-delivery of pooled siRNA and doxorubicin for reversing of multidrug resistance in breast cancer. Nano Res. 2022, 15, 6306–6314. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, W.; Luo, X.; Zhang, X.; Zhang, C.; Li, H.; Gao, D.; Luo, H.; Jiang, Q.; Liu, J. Dual-Ligand Modified Polymer-Lipid Hybrid Nanoparticles for Docetaxel Targeting Delivery to Her2/neu Overexpressed Human Breast Cancer Cells. J. Biomed. Nanotechnol. 2015, 11, 1401–1417. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Kushwah, V.; Garg, N.K.; Kesharwani, P.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Jain, S.; Katare, O.P. Methotrexate and beta-carotene loaded-lipid polymer hybrid nanoparticles: A preclinical study for breast cancer. Nanomedicine 2017, 12, 1851–1872. [Google Scholar] [CrossRef]
- Jadon, R.S.; Sharma, M. Docetaxel-loaded lipid-polymer hybrid nanoparticles for breast cancer therapeutics. J. Drug Deliv. Sci. Technol. 2019, 51, 475–484. [Google Scholar] [CrossRef]
- Yalcin, T.E.; Ilbasmis-Tamer, S.; Takka, S. Antitumor activity of gemcitabine hydrochloride loaded lipid polymer hybrid nanoparticles (LPHNs): In vitro and in vivo. Int. J. Pharm. 2020, 580, 119246. [Google Scholar] [CrossRef]
- Khodaei, M.; Rostamizadeh, K.; Taromchi, A.H.; Monirinasab, H.; Fathi, M. DDAB cationic lipid-mPEG, PCL copolymer hybrid nano-carrier synthesis and application for delivery of siRNA targeting IGF-1R into breast cancer cells. Clin. Transl. Oncol. 2021, 23, 1167–1178. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Aldawsari, M.F.; Alalaiwe, A.; Alali, A.S.; Alharthi, A.I.; Kalam, M.A. Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach. Polymers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Yang, B.; Song, B.P.; Shankar, S.; Guller, A.; Deng, W. Recent advances in liposome formulations for breast cancer therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.S.; Shaker, M.A.; Hanafy, M.S. Cellular uptake, cytotoxicity and in-vivo evaluation of Tamoxifen citrate loaded niosomes. Int. J. Pharm. 2015, 493, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Roque, M.C.; de Barros, A.L.B.; de Oliveira Silva, J.; Cassali, G.D.; Oliveira, M.C. Investigation of the antitumor activity and toxicity of long-circulating and fusogenic liposomes co-encapsulating paclitaxel and doxorubicin in a murine breast cancer animal model. Biomed. Pharmacother. 2019, 109, 1728–1739. [Google Scholar] [CrossRef]
- Coscoa, D.; Paolinoa, D.; Cilurzoa, F.; Casalea, F.; Frestaa, M. Gemcitabine and tamoxifen-loaded liposomes as multidrug carriers for the treatment of breast cancer diseases. Int. J. Pharm. 2012, 422, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Chiu, G.N. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model. Nanomedicine 2011, 7, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Acevedo-Morantes, C.Y.; Acevedo-Morantes, M.T.; Suleiman-Rosado, D.; Ramírez-Vick, J.E. Evaluation of the cytotoxic effect of camptothecin solid lipid nanoparticles on MCF7 cells. Drug Deliv. 2013, 20, 338–348. [Google Scholar] [CrossRef]
- Yuan, Q.; Han, J.; Cong, W.; Ge, Y.; Ma, D.; Dai, Z.; Li, Y.; Bi, X. Docetaxel-loaded solid lipid nanoparticles suppress breast cancer cells growth with reduced myelosuppression toxicity. Int. J. Nanomed. 2014, 9, 4829–4846. [Google Scholar] [CrossRef]
- da Rocha, M.C.O.; da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; de Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef]
- Wenrui, W.; Mengyang, Z.; Yang, X.; Wei, P.; Shiwen, Z.; Rongjie, L.; Han, Z.; Hui, Z.; Shumin, C.; Youjing, W.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef]
- Fang, C.L.; Al-Suwayeh, S.A.; Fang, J.Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef]
- Du, M.; Ouyang, Y.; Meng, F.; Ma, Q.; Liu, H.; Zhuang, Y.; Pang, M.; Cai, T.; Cai, Y. Nanotargeted agents: An emerging therapeutic strategy for breast cancer. Nanomedicine 2019, 14, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Wu, H.; Gao, Y.; Li, W.; Zou, D.; Dong, C. Doxorubicin- and cisplatin-loaded nanostructured lipid carriers for breast cancer combination chemotherapy. Drug Dev. Ind. Pharm. 2016, 42, 2038–2043. [Google Scholar] [CrossRef]
- de Sousa Marcial, S.P.; Carneiro, G.; Leite, E.A. Lipid-based nanoparticles as drug delivery system for paclitaxel in breast cancer treatment. J. Nanopart. Res. 2017, 19, 340. [Google Scholar] [CrossRef]
- Makeen, H.A.; Mohan, S.; Al-Kasim, M.A.; Sultan, M.H.; Albarraq, A.A.; Ahmed, R.A.; Alhazmi, H.A.; Alam, M.I. Preparation, Characterization, and Anti-Cancer Activity of Nanostructured Lipid Carriers Containing Imatinib. Pharmaceutics 2021, 13, 1086. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Seabra, H.A.; Oliveira, M.S.; Carregal, V.M.; Vilela, J.M.C.; Andrade, M.S.; Townsend, D.M.; Colletti, P.M.; Leite, E.A.; et al. α-Tocopherol succinate loaded nano-structed lipid carriers improves antitumor activity of doxorubicin in breast cancer models in vivo. Biomed. Pharmacother. 2018, 103, 1348–1354. [Google Scholar] [CrossRef]
- Kandekar, U.; Pujari, R. Nanocarriers for Breast Cancer: Advanced Perspective. Hacet. Univ. J. Fac. Pharm. 2021, 41, 177–193. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Ann. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Ye, Y.J.; Wang, Y.; Lou, K.Y.; Chen, Y.Z.; Chen, R.; Gao, F. The preparation, characterization, and pharmacokinetic studies of chitosan nanoparticles loaded with paclitaxel/dimethyl-β-cyclodextrin inclusion complexes. Int. J. Nanomed. 2015, 10, 4309–4319. [Google Scholar] [CrossRef][Green Version]
- DeVeaux, S.D.; Gomillion, C.T. Assessing the potential of chitosan/polylactide nanoparticles for delivery of therapeutics for triple-negative breast cancer treatment. Regen. Eng. Transl. Med. 2019, 5, 61–73. [Google Scholar] [CrossRef]
- Tran, P.; Nguyen, T.N.; Lee, Y.; Tran, P.N.; Park, J.S. Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells. Korean J. Physiol. Pharmacol. 2021, 25, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.; Zhang, P.; Zhang, W.; Sun, J.; Meng, X.; Qin, Y.; Deng, Y.; He, Z. Development of novel self-assembled DS-PLGA hybrid nanoparticles for improving oral bioavailability of vincristine sulfate by P-gp inhibition. J. Control Release 2010, 148, 241–248. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, Y.; Wen, Q.; Luo, J.; Lu, Y.; Wu, Z.; Wang, B.; Chen, Y.; Zhao, L.; Fu, S. Co-delivery of paclitaxel and curcumin by biodegradable polymeric nanoparticles for breast cancer chemotherapy. Int. J. Pharm. 2020, 589, 119875. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Kang, X.X.; Wang, Y.Q.; Zhang, X.J.; Li, C.J.; Liu, Y.; Du, L.B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019, 84, 367–377. [Google Scholar] [CrossRef]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.-B.; Li, F.; Jiang, L.-H.; Kang, C.-S.; Yao, Z. Suppression of breast cancer cells in vitro by polyamidoamine-dendrimer-mediated 5-fluorouracil chemotherapy combined with antisense micro-RNA 21 gene therapy. J. Appl. Polym. Sci. 2009, 114, 3760–3766. [Google Scholar] [CrossRef]
- Bochicchio, S.; Lamberti, G.; Barba, A.A. Polymer-Lipid Pharmaceutical Nanocarriers: Innovations by New Formulations and Production Technologies. Pharmaceutics 2021, 13, 198. [Google Scholar] [CrossRef]
- Zhang, R.X.; Cai, P.; Zhang, T.; Chen, K.; Li, J.; Cheng, J.; Pang, K.S.; Adissu, H.A.; Rauth, A.M.; Wu, X.Y. Polymer-lipid hybrid nanoparticles synchronize pharmacokinetics of co-encapsulated doxorubicin-mitomycin C and enable their spatiotemporal co-delivery and local bioavailability in breast tumor. Nanomedicine 2016, 12, 1279–1290. [Google Scholar] [CrossRef]
- Monirinasab, H.; Asadi, H.; Rostamizadeh, K.; Esmaeilzadeh, A.; Khodaei, M.; Fathi, M. Novel lipid-polymer hybrid nanoparticles for siRNA delivery and IGF-1R gene silencing in breast cancer cells. J. Drug Deliv. Sci. Technol. 2018, 48, 96–105. [Google Scholar] [CrossRef]
- Jiang, T.; Mo, R.; Bellotti, A.; Zhou, J.; Gu, Z. Gel–Liposome-Mediated Co-Delivery of Anticancer Membrane-Associated Proteins and Small-Molecule Drugs for Enhanced Therapeutic Efficacy. Adv. Funct. Mater. 2014, 24, 2295–2304. [Google Scholar] [CrossRef]
- Li, B.; Wang, F.; Gui, L.; He, Q.; Yao, Y.; Chen, H. The potential of biomimetic nanoparticles for tumor-targeted drug delivery. Nanomedicine 2018, 13, 2099–2118. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhao, Y.; Li, Y.; Jiang, L.; Gu, Y.; Liu, J. Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles. Drug Deliv. 2021, 28, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Human serum albumin: A nanomedicine platform targeting breast cancer cells. J. Drug Deliv. Sci. Technol. 2019, 52, 652–659. [Google Scholar] [CrossRef]
- Hasanpoor, Z.; Mostafaie, A.; Nikokar, I.; Hassan, Z.M. Curcumin-human serum albumin nanoparticles decorated with PDL1binding peptide for targeting PDL1-expressing breast cancer cells. Int. J. Biol. Macromol. 2020, 159, 137–153. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Chen, S.; Zhang, S.; Cui, C. Cetuximab-Modified Human Serum Albumin Nanoparticles Co-Loaded with Doxorubicin and MDR1 siRNA for the Treatment of Drug-Resistant Breast Tumors. Int. J. Nanomed. 2021, 16, 7051–7069. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xu, X.; Li, M.; Zhou, J.; Wang, W. Reconstituted high-density lipoprotein mediated targeted co-delivery of HZ08 and paclitaxel enhances the efficacy of paclitaxel in multidrug-resistant MCF-7 breast cancer cells. Eur. J. Pharm. Sci. 2016, 92, 11–21. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Z.; Han, Y.; Hu, S.; Opoku-Damoah, Y.; Zhou, J.; Yin, L.; Ding, Y. Natural Particulates Inspired Specific-Targeted Codelivery of siRNA and Paclitaxel for Collaborative Antitumor Therapy. Mol. Pharm. 2017, 14, 2999–3012. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, W.; Wang, J.; Zhai, Y.; Xiong, F.; Cai, Y.; Gong, X.; Zhu, B.; Zhu, H.H.; Wang, H.; et al. Lenvatinib- and vadimezan-loaded synthetic high-density lipoprotein for combinational immunochemotherapy of metastatic triple-negative breast cancer. Acta Pharm. Sin. B 2022, 12, 3726–3738. [Google Scholar] [CrossRef]
- Pandolfi, L.; Bellini, M.; Vanna, R.; Morasso, C.; Zago, A.; Carcano, S.; Avvakumova, S.; Bertolini, J.A.; Rizzuto, M.A.; Colombo, M.; et al. H-Ferritin Enriches the Curcumin Uptake and Improves the Therapeutic Efficacy in Triple Negative Breast Cancer Cells. Biomacromolecules 2017, 18, 3318–3330. [Google Scholar] [CrossRef]
- Li, R.; Ma, Y.; Dong, Y.; Zhao, Z.; You, C.; Huang, S.; Li, X.; Wang, F.; Zhang, Y. Novel Paclitaxel-Loaded Nanoparticles Based on Human H Chain Ferritin for Tumor-Targeted Delivery. ACS Biomater. Sci. Eng. 2019, 5, 6645–6654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.H.; Qiu, W.X.; Zhang, Y.H.; Song, W.; Zhang, L.; Wang, S.B.; Zhang, X.Z. A Transformable Chimeric Peptide for Cell Encapsulation to Overcome Multidrug Resistance. Small 2018, 14, e1703321. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Chen, F.; Toft, D.J.; Ruff, Y.; Cryns, V.L.; Stupp, S.I. Self-assembled peptide nanostructures targeting death receptor 5 and encapsulating paclitaxel as a multifunctional cancer therapy. ACS Biomater. Sci. Eng. 2019, 5, 6046–6053. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, L.; Liu, S.; Zhao, S.; Jiang, Q.; Ding, B. A Tailored DNA Nanoplatform for Synergistic RNAi-/Chemotherapy of Multidrug-Resistant Tumors. Angew. Chem. Int. Ed. Engl. 2018, 57, 15486–15490. [Google Scholar] [CrossRef]
- Liu, J.; Wu, T.; Lu, X.; Wu, X.; Liu, S.; Zhao, S.; Xu, X.; Ding, B. A Self-Assembled Platform Based on Branched DNA for sgRNA/Cas9/Antisense Delivery. J. Am. Chem. Soc. 2019, 141, 19032–19037. [Google Scholar] [CrossRef]
- Pal, S.; Rakshit, T. Folate-Functionalized DNA Origami for Targeted Delivery of Doxorubicin to Triple-Negative Breast Cancer. Front. Chem. 2021, 9, 721105. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Y.; Yang, D.; Wu, C.; Li, H. Preparation, characterization, and in vitro tumor-suppressive effect of anti-miR-21-equipped RNA nanoparticles. Biochem. Biophys. Res. Commun. 2021, 558, 107–113. [Google Scholar] [CrossRef]
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Li, Y.; Xia, Q.; Li, Z.; Hou, X.; Feng, N. Tumor cell membrane-derived nano-Trojan horses encapsulating phototherapy and chemotherapy are accepted by homologous tumor cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111670. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Yin, Q.; Tang, S.; Zhang, P.; Chen, Y.; Zhang, Z.; Yu, H.; Li, Y. Long Circulation Red-Blood-Cell-Mimetic Nanoparticles with Peptide-Enhanced Tumor Penetration for Simultaneously Inhibiting Growth and Lung Metastasis of Breast Cancer. Adv. Funct. Mater. 2016, 26, 1243–1252. [Google Scholar] [CrossRef]
- Marshall, S.K.; Angsantikul, P.; Pang, Z.; Nasongkla, N.; Hussen, R.S.D.; Thamphiwatana, S.D. Biomimetic Targeted Theranostic Nanoparticles for Breast Cancer Treatment. Molecules 2022, 27, 6473. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated Nanoparticles for Tumor-Targeted Chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer Platelet-Mimicking Nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef]
- Pei, W.; Huang, B.; Chen, S.; Wang, L.; Xu, Y.; Niu, C. Platelet-Mimicking Drug Delivery Nanoparticles for Enhanced Chemo-Photothermal Therapy of Breast Cancer. Int. J. Nanomed. 2020, 15, 10151–10167. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.W.; et al. A New Approach for Loading Anticancer Drugs into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef]
- Barok, M.; Puhka, M.; Vereb, G.; Szollosi, J.; Isola, J.; Joensuu, H. Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer 2018, 18, 504. [Google Scholar] [CrossRef]
- Gong, C.; Tian, J.; Wang, Z.; Gao, Y.; Ding, X.; Qiang, L.; Li, G.; Han, Z.; Yuan, Y.; Gao, S. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Sharifi, K.; Salehi, M.; Mohammadi-Yeganeh, S. Delivery of miR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem Cell Rev. Rep. 2021, 17, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tang, Y.; Li, Y.; Liu, C.; He, J.; Zhang, L.K.; Guan, Y.-Q. Tumor cell-derived extracellular vesicles for breast cancer specific delivery of therapeutic P53. J. Control Release 2022, 349, 606–616. [Google Scholar] [CrossRef]
- Yaman, S.; Chintapula, U.; Rodriguez, E.; Ramachandramoorthy, H.; Nguyen, K.T. Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy. Cancer Drug Resist. 2020, 3, 879–911. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Lu, J.; Jiao, D. Stem cell membrane vesicle-coated nanoparticles for efficient tumor-targeted therapy of orthotopic breast cancer. Polym. Adv. Technol. 2019, 30, 1051–1060. [Google Scholar] [CrossRef]
- Kang, T.; Zhu, Q.; Wei, D.; Feng, J.; Yao, J.; Jiang, T.; Song, Q.; Wei, X.; Chen, H.; Gao, X.; et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano 2017, 11, 1397–1411. [Google Scholar] [CrossRef]
- Pan, V.; Siva, P.N.; Modery-Pawlowski, C.L.; Sekhon, U.D.S.; Gupta, A.S. Targeted killing of metastatic cells using a platelet-inspired drug delivery system. RSC Adv. 2015, 5, 46218–46228. [Google Scholar] [CrossRef]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target Ther. 2021, 6, 225. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cai, G.; Li, Q. Exosomes as Actively Targeted Nanocarriers for Cancer Therapy. Int. J. Nanomed. 2020, 15, 4257–4273. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Shaw, A.; Zeng, X.; Benson, E.; Nyström, A.M.; Högberg, B. DNA origami delivery system for cancer therapy with tunable release properties. ACS Nano 2012, 6, 8684–8691. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.; Liu, Q.; Song, L.; Wang, J.; Li, Y.; Tian, J.; Ding, B.; et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 22, 6633–6643. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, F.; Liu, S.; Wu, T.; Shang, Y.; Tian, R.; Liu, J.; Wang, Z.G.; Jiang, Q.; Ding, B. Efficient Intracellular Delivery of RNase A Using DNA Origami Carriers. ACS Appl. Mater. Interfaces 2019, 11, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, S.; Zhang, D.; Bai, X.; Hecht, S.M.; Chen, S. DNA-affibody nanoparticles for inhibiting breast cancer cells overexpressing HER2. Chem. Commun. 2017, 53, 573–576. [Google Scholar] [CrossRef]
- Jasinski, D.; Haque, F.; Binzel, D.W.; Guo, P. Advancement of the Emerging Field of RNA Nanotechnology. ACS Nano 2017, 11, 1142–1164. [Google Scholar] [CrossRef]
- Shu, D.; Li, H.; Shu, Y.; Xiong, G.; Carson, W.E., 3rd; Haque, F.; Xu, R.; Guo, P. Systemic Delivery of Anti-miRNA for Suppression of Triple Negative Breast Cancer Utilizing RNA Nanotechnology. ACS Nano 2015, 9, 9731–9740. [Google Scholar] [CrossRef]
- Guo, S.; Vieweger, M.; Zhang, K.; Yin, H.; Wang, H.; Li, X.; Li, S.; Hu, S.; Sparreboom, A.; Evers, B.M.; et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat. Commun. 2020, 11, 972. [Google Scholar] [CrossRef]
- Li, A.; Zhao, J.; Fu, J.; Cai, J.; Zhang, P. Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor. Asian J. Pharm. Sci. 2021, 16, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.Y.; Prajnamitra, R.P.; Chen, L.L.; Hsieh, P.C. Advances in Biomimetic Nanoparticles for Targeted Cancer Therapy and Diagnosis. Molecules 2021, 26, 5052. [Google Scholar] [CrossRef]
- Wan, X.; Zheng, X.; Pang, X.; Pang, Z.; Zhao, J.; Zhang, Z.; Jiang, T.; Xu, W.; Zhang, Q.; Jiang, X. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 2016, 7, 34038–34051. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Lian, X.; Dong, J.; Wan, Z.; Xia, C.; Song, X.; Fu, Y.; Gong, T.; Zhang, Z. Co-delivery of Pirarubicin and Paclitaxel by Human Serum Albumin Nanoparticles to Enhance Antitumor Effect and Reduce Systemic Toxicity in Breast Cancers. Mol. Pharm. 2015, 12, 4085–4098. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef]
- Dong, Y.; Ma, Y.; Li, X.; Wang, F.; Zhang, Y. ERK-peptide-inhibitor-modified ferritin enhanced the therapeutic effects of paclitaxel in cancer cells and spheroids. Mol. Pharm. 2021, 18, 3365–3377. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Truffi, M.; Baccarini, F.; Beretta, M.; Sorrentino, L.; Bellini, M.; Rizzuto, M.A.; Ottria, R.; Ravelli, A.; Ciuffreda, P.; et al. H-Ferritin-nanocaged olaparib: A promising choice for both BRCA-mutated and sporadic triple negative breast cancer. Sci. Rep. 2017, 7, 7505. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, Q.; Zhao, Q.; Zheng, J.; Li, Y.; Wang, S.; Yuan, Y. Development of synthetic high-density lipoprotein-based ApoA-I mimetic peptide-loaded docetaxel as a drug delivery nanocarrier for breast cancer chemotherapy. Drug Deliv. 2019, 6, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, X.; Teng, B.; Wang, Z.; Li, F.; Zhao, Y.; Guo, Y.; Zeng, Q. Peptide-Targeted High-Density Lipoprotein Nanoparticles for Combinatorial Treatment against Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 35248–35265. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-assembly of organic nanomaterials and biomaterials: The bottom-up approach for functional nanostructures formation and advanced applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yu, X.; Shen, B.; Sun, L. Peptide Self-assembled nanostructures for drug delivery applications. J. Nanomater. 2017, 2017, 4562474. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Sun, L.; Huang, Y.; Lenaghan, S.C.; Zhang, M. Doxorubicin-loaded cyclic peptide nanotube bundles overcome chemoresistance in breast cancer cells. J. Biomed. Nanotechnol. 2014, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zhao, Y.; Ding, Y.; Wang, J.; Zhao, R.; Lang, J.; Qin, H.; Liu, X.; Shi, J.; Tao, N.; et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew. Chem. Int. Ed. Engl. 2016, 55, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef]
- Li, X.; Qin, F.; Yang, L.; Mo, L.; Li, L.; Hou, L. Sulfatide-containing lipid perfluorooctylbromide nanoparticles as paclitaxel vehicles targeting breast carcinoma. Int. J. Nanomed. 2014, 9, 3971–3985. [Google Scholar] [CrossRef]
- Jiang, K.; Song, X.; Yang, L.; Li, L.; Wan, Z.; Sun, X.; Gong, T.; Lin, Q.; Zhang, Z. Enhanced antitumor and anti-metastasis efficacy against aggressive breast cancer with a fibronectin-targeting liposomal doxorubicin. J. Control Release 2018, 271, 21–30. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Chen, K.G.; Yu, X.Y.; Zhao, G.; Shen, S.; Cao, Z.T.; Luo, Y.L.; Wang, Y.C.; Wang, J. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-β signaling pathway inhibition. Biomaterials 2016, 82, 48–59. [Google Scholar] [CrossRef]
- Sorolla, A.; Sorolla, M.A.; Wang, E.; Ceña, V. Peptides, proteins and nanotechnology: A promising synergy for breast cancer targeting and treatment. Expert Opin. Drug Deliv. 2020, 17, 1597–1613. [Google Scholar] [CrossRef]
- Dai, W.; Yang, F.; Ma, L.; Fan, Y.; He, B.; He, Q.; Wang, X.; Zhang, H.; Zhang, Q. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin α3 in triple-negative breast cancer. Biomaterials 2014, 35, 5347–5358. [Google Scholar] [CrossRef]
- Ma, Y.; He, P.; Tian, X.; Liu, G.; Zeng, X.; Pan, G. Mussel-Derived, Cancer-Targeting Peptide as pH-Sensitive Prodrug Nanocarrier. ACS Appl. Mater. Interfaces 2019, 11, 23948–23956. [Google Scholar] [CrossRef]
- Niza, E.; Noblejas-López, M.D.M.; Bravo, I.; Nieto-Jiménez, C.; Castro-Osma, J.A.; Canales-Vázquez, J.; Lara-Sanchez, A.; Galán Moya, E.M.; Burgos, M.; Ocaña, A.; et al. Trastuzumab-Targeted Biodegradable Nanoparticles for Enhanced Delivery of Dasatinib in HER2+ Metastasic Breast Cancer. Nanomaterials 2019, 9, 1793. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Li, X.; Li, F.; Lee, R.J.; Sun, F.; Li, Y.; Liu, Z.; Teng, L. Trastuzumab-coated nanoparticles loaded with docetaxel for breast cancer therapy. Dose Response 2019, 17, 1559325819872583. [Google Scholar] [CrossRef]
- Roncato, F.; Rruga, F.; Porcù, E.; Casarin, E.; Ronca, R.; Maccarinelli, F.; Realdon, N.; Basso, G.; Alon, R.; Viola, G.; et al. Improvement and extension of anti-EGFR targeting in breast cancer therapy by integration with the Avidin-Nucleic-Acid-Nano-Assemblies. Nat. Commun. 2018, 9, 4070. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.C.; Allen, C. Dual-Targeted Delivery of Nanoparticles Encapsulating Paclitaxel and Everolimus: A Novel Strategy to Overcome Breast Cancer Receptor Heterogeneity. Pharm. Res. 2020, 37, 39. [Google Scholar] [CrossRef]
- Nag, O.K.; Delehanty, J.B. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics 2019, 11, 543. [Google Scholar] [CrossRef]
- Arafaa, K.K.; Smyth, H.D.C.; El-Sherbiny, I.M. Mitotropic triphenylphosphonium doxorubicin-loaded core-shell nanoparticles for cellular and mitochondrial sequential targeting of breast cancer. Int. J. Pharm. 2021, 606, 120936. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Jia, X.; Han, Q.; Qian, Y.; Li, Q.; Xiang, J.; Wang, Q.; Hu, Z.; Wang, W. MMP-2-Controlled Transforming Micelles for Heterogeneic Targeting and Programmable Cancer Therapy. Theranostics 2019, 9, 1728–1740. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharmacother. 2019, 117, 109072. [Google Scholar] [CrossRef]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Zhou, Z.; Badkas, A.; Stevenson, M.; Lee, J.Y.; Leung, Y.K. Herceptin conjugated PLGA-PHis-PEG pH sensitive nanoparticles for targeted and controlled drug delivery. Int. J. Pharm. 2015, 487, 81–90. [Google Scholar] [CrossRef]
- Guo, Z.; Sui, J.; Ma, M.; Hu, J.; Sun, Y.; Yang, L.; Fan, Y.; Zhang, X. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J. Control Release 2020, 326, 350–364. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, H.; Shen, W.; Liu, W.; Chen, L.; Xiao, C. Hypoxia-Responsive Polypeptide Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 13, 2167–2174. [Google Scholar] [CrossRef]
- Oshiro-Júnior, J.A.; Rodero, C.; Hanck-Silva, G.; Sato, M.R.; Alves, R.C.; Eloy, J.O.; Chorilli, M. Stimuli-responsive Drug Delivery Nanocarriers in the Treatment of Breast Cancer. Curr. Med. Chem. 2020, 27, 2494–2513. [Google Scholar] [CrossRef]
- Li, J.; Huo, M.; Wang, J.; Zhou, J.; Mohammad, J.M.; Zhang, Y.; Zhu, Q.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef]
- Hu, C.; Fan, F.; Qin, Y.; Huang, C.; Zhang, Z.; Guo, Q.; Zhang, L.; Pang, X.; Ou-Yang, W.; Zhao, K.; et al. Redox-Sensitive Folate-Conjugated Polymeric Nanoparticles for Combined Chemotherapy and Photothermal Therapy against Breast Cancer. J. Biomed. Nanotechnol. 2018, 14, 2018–2030. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Lai, C.Y.; Tam, S.M.; Mahakian, L.M.; Ingham, E.S.; Watson, K.D.; Ferrara, K.W. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J. Control Release 2013, 172, 266–273. [Google Scholar] [CrossRef]
- Lyon, P.C.; Gray, M.D.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.F.; Scott, S.; Anderson, M.; Goldin, R.; et al. Safety and feasibility of ultrasound-triggered targeted drug delivery of doxorubicin from thermosensitive liposomes in liver tumours (TARDOX): A single-centre, open-label, phase 1 trial. Lancet Oncol. 2018, 19, 1027–1039. [Google Scholar] [CrossRef]
- Yildiz, T.; Gu, R.; Zauscher, S.; Betancourt, T. Doxorubicin-loaded protease-activated near-infrared fluorescent polymeric nanoparticles for imaging and therapy of cancer. Int. J. Nanomed. 2018, 13, 6961–6986. [Google Scholar] [CrossRef]
- Su, S.; Tian, Y.; Li, Y.; Ding, Y.; Ji, T.; Wu, M.; Wu, Y.; Nie, G. “Triple-punch” strategy for triple negative breast cancer therapy with minimized drug dosage and improved antitumor efficacy. ACS Nano 2015, 9, 1367–1378. [Google Scholar] [CrossRef]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Li, H.; Li, C.; Ding, H.; Zhang, M.; Guo, Y.; Sun, M. Near-infrared light triggered liposomes combining photodynamic and chemotherapy for synergistic breast tumor therapy. Colloids Surf. B Biointerfaces 2019, 173, 564–570. [Google Scholar] [CrossRef]
- Tewari, K.M.; Dondi, R.; Yaghini, E.; Pourzand, C.; MacRobert, A.J.; Eggleston, I.M. Peptide-targeted dendrimeric prodrug of 5-aminolevulinic acid: A novel approach towards enhanced accumulation of protophorphyrin IX for photodynamic therapy. Bioorg. Chem. 2021, 109, 104667. [Google Scholar] [CrossRef]
- Verma, N.K.; Purohit, M.P.; Equbal, D.; Dhiman, N.; Singh, A.; Kar, A.K.; Shankar, J.; Tehlan, S.; Patnaik, S. Targeted Smart pH and Thermoresponsive N,O-Carboxymethyl Chitosan Conjugated Nanogels for Enhanced Therapeutic Efficacy of Doxorubicin in MCF-7 Breast Cancer Cells. Bioconjug. Chem. 2016, 27, 2605–2619. [Google Scholar] [CrossRef]
- Yang, H.; Shen, W.; Liu, W.; Chen, L.; Zhang, P.; Xiao, C.; Chen, X. PEGylated Poly (α-lipoic acid) Loaded with Doxorubicin as a pH and Reduction Dual Responsive Nanomedicine for Breast Cancer Therapy. Biomacromolecules 2018, 19, 4492–4503. [Google Scholar] [CrossRef]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X.; et al. pH- and NIR Light-Responsive Micelles with Hyperthermia-Triggered Tumor Penetration and Cytoplasm Drug Release to Reverse Doxorubicin Resistance in Breast Cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N.D.; Bauer, J. Breast cancer nanomedicine market update and other industrial perspectives of nanomedicine. In Nanomedicines for Breast Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–395. [Google Scholar]
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449. [Google Scholar] [CrossRef]
- Harris, L.; Batist, G.; Belt, R.; Rovira, D.; Navari, R.; Azarnia, N.; Welles, L.; Winer, E.; TLC D-99 Study Group. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002, 94, 25–36. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K.; et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Sparreboom, A.; Scripture, C.D.; Trieu, V.; Williams, P.J.; De, T.; Yang, A.; Beals, B.; Figg, W.D.; Hawkins, M.; Desai, N. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol). Clin. Cancer Res. 2005, 11, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Dieci, M.V.; Conte, P. Enhancing intracellular taxane delivery: Current role and perspectives of nanoparticle albumin-bound paclitaxel in the treatment of advanced breast cancer. Expert Opin. Pharmacother. 2012, 13, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kümmel, S.; Hilfrich, J.; et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): A randomised, phase 3 trial. Lancet Oncol. 2016, 17, 345–356. [Google Scholar] [CrossRef]
- Celsion, ClinicalTrial.org Identifier: NCT00826085. 2009. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00826085 (accessed on 4 March 2022).
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Wang, Z.-G.; Liu, S.-L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 17, 5607. [Google Scholar] [CrossRef]
- Sachdev, J.C.; Munster, P.; Northfelt, D.W.; Han, H.S.; Ma, C.; Maxwell, F.; Wang, T.; Belanger, B.; Zhang, B.; Moore, Y.; et al. Phase I study of liposomal irinotecan in patients with metastatic breast cancer: Findings from the expansion phase. Breast Cancer Res. Treat. 2021, 185, 759–771. [Google Scholar] [CrossRef]
- Malakar, A.; Kanel, S.R.; Ray, C.; Snow, D.D.; Nadagouda, M.N. Nanomaterials in the environment, human exposure pathway, and health effects: A review. Sci. Total Environ. 2021, 759, 143470. [Google Scholar] [CrossRef]
- Hauser, M.; Li, G.Y.; Nowack, B. Environmental hazard assessment for polymeric and inorganic nanobiomaterials used in drug delivery. J. Nanobiotechnol. 2019, 17, 56. [Google Scholar] [CrossRef]
- Shatkin, J.A. Nanotechnology: Health and Environmental Risks; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kabir, E.; Kumar, V.; Kim, K.H.; Yip, A.C.K.; Sohn, J.R. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]



| Name of Nanoparticle | Composition/Coating of Nanodelivery System | Size | Drug/Biomolecule | Cell Line/Animal Model | Targeting | Outcome | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Liposomes | Phosphatidylcholine, cholesterol and DSPE-PEG2000, dichloromethane | NA | Gemcitabine | MDA-MB-231 and 4T1 cell lines/4T1 tumour-bearing mice | Passive targeting | Gemcitabine-loaded liposomes significantly inhibited cell viability and induced apoptosis compared to free drug, irrespective of cell sensitivity, both in vitro and in vivo. | 2013 | [30] |
| Liposomes | Hydrogenated phosphatidylcholine (HPC), cholesterol, Propylene glycol (PG), tween-80, 5% trehalose solution | 182 nm | Epirubicin (EPI) | MDA-MB 435, (MDA-MB 435/ADR) and chemically induced tumour model | Passive targeting | Effective growth inhibition in MDA-MB-435 cells, as well as in the resistant variant MDA-MB 435/ADR cells. | 2013 | [31] |
| Liposomes | EPC/cholesterol/DSPE-PEG2000 | 100 nm | OCT-modified daunorubicin plus dihydroartemisinin | MCF-7 cells and MDA-MB-435S cells/DA-MB-435S cell xenografts nude mice | Active targeting of somatostatin receptors | Enhanced cytotoxicity and cellular uptake; specific tumour accumulation and antitumour efficacy. | 2018 | [32] |
| Liposomes | 1,2-dioleoylsn-glycero-3-phosphoethanolamine (DOPE), cholesterol (Chol), 1,2-dimyristoyl-rac-glycero-3-methylpolyoxyethylene (DMG-PEG2000, PEG) | 163 nm | Paclitaxel, camptothecin and P53 mRNA | MDA-MB-231 cell line, orthotopic TNBC model in nude mice | Passive targeting | Nanoparticles displayed synergetic cytotoxicity of paclitaxel and P53 mRNA both in vitro and in vivo. | 2019 | [33] |
| Liposomes | DSPE-mPEG2000 (distearoyl phosphoethanolamine- polyethylene glycol) and SPC (soybean phospholipids with 75% phosphatidylcholine and cholesterol. | 120 nm | Cisplatin | MCF-7 cells | Passive targeting | Liposome-loaded cisplatin had greater uptake and cytotoxicity compared with cisplatin alone. | 2019 | [34] |
| Liposomes | (Soy) (HSPC), (POPC), (DOPC), (DPPC), (DSPE-PEG-2000), (Mal-PEG-2000), Cholesterol (CHO), (DOTAP) and (DDAB) | 99 to 181 nm | Doxorubicin | Her-2+ MCF-7 and SKBR-3 cells | Active targeting of HER2+ by aptamer A6 | Aptamer-labelled liposomes elicited higher uptake by more than 60% into Her-2+ MCF-7 and SKBR-3 cells compared to non-targeted nanoparticles. | 2020 | [35] |
| Liposomes | DPPC, CHO, GANGLIOSIDE, DSPEmPEG2000-maleimmide | 142–150 nm | Doxorubicin (DOX) and sorafenib (SRF) | MCF-7 and MDA-MB-231 cell lines, 2D and 3D spheroid models | Active targeting of p32 by LinTT1 peptide | LinTT1-functionalized liposomes enhanced therapeutic efficacy of both drugs in 2D culture and in 3D spheroids of MDA-MB-231 cells. | 2021 | [36] |
| Liposomes | DOTAP, DOPE, Cholesterol, PC | 200 nm | Docetaxel and SIRT1 shRNA | MCF-7, MDA-MB-231 cells and chemically induced animal breast cancer model | Passive targeting | Co-loaded NPs resulted in the highest apoptotic profile in vitro and ~52% reduction in tumour burden in animal models compared to docetaxel liposomes and free docetaxel. | 2021 | [37] |
| Liposomes | DSPE-PEG2000, SPC, CHO | 127–134 nm | 5-fluorouracil and paclitaxel | MDA-MB-231 cell line and tumour-bearing mouse model | Mitochondri a-targeted KLA | In vitro and in vivo studies showed that combined drug-loaded KLA-conjugated liposomes exhibited the highest apoptosis of breast cancer cell line and the highest tumour growth inhibition compared to other groups. | 2022 | [38] |
| Solid lipid nanoparticles (SLN) | Curdlan, glyceryl caprate and polyethylene glycol (PEG) 660 hydroxystearate | NA | Doxorubicin (DOX) | MCF-7 cell line and its adriamycin-resistant variant (ADR) | Passive targeting | SLN-DOX showed significant cytotoxicity as a result of doxorubicin accumulation in the cells overcoming chemoresistance. | 2010 | [39] |
| Solid lipid nanoparticles (SLN) | Hydrogenated soya phosphatidylcholine (HSPC), distearoyl phosphatidyl ethanolamine (DSPE) and cholesterol | NA | Curcumin | MCF-7 cell line | Active targeting of transferrin (Tf) receptors | Conjugated curcumin nanoparticles resulted in better delivery and enhanced cytotoxicity with active targeting against MCF-7 cells. | 2010 | [40] |
| Solid lipid nanoparticles (SLN) | Glyceryl palmitostearate, Polyoxyl 35 and Polysorbate 80 | 216 nm | Tamoxifen | MCF-7 cell line | Active targeting of transferrin (Tf) receptors | Tamofixen-loaded solid lipid nanoparticles induced significantly higher cytotoxicity vs. free drug | 2020 | [41] |
| Solid lipid nanoparticles (SLN) | l-α-phosphatidylcholine (PC) and DSPE–methyl (polyethylene glycol)-2000 (mPEG2,000) | NA | Paclitaxel | MCF-7 and MCF-7/ADR (ADR) cell lines | Passive targeting | Significant increase in the intracellular uptake of paclitaxel and improved anticancer activity in MCF-7/ADR cells. | 2018 | [42] |
| Solid lipid nanoparticles (SLN) | Stearylamine, Pluronic F-68 | 112.18 nm | Niclosamide | MDA-MB231 cell line | Passive targeting | In vitro studies of the Niclo SLNs showed better cytotoxicity than the naïve Niclo, and significant higher cell uptake after 24 h exposure. | 2019 | [43] |
| Solid lipid nanoparticles (SLN) | glycerol monostearate, soy lecithin, dichloromethane | 88–114 nm | Doxorubicin | MDA-MB-468 cell line | Active targeting by anti-EGFR/CD4 4 dual-RNA aptamers | SLNs/DOX/Dexa/CD44/EGF R resulted in a significant reduction in cell viability compared to other treated groups. | 2022 | [44] |
| Solid lipid nanoparticles (SLN) | Disteroylphosphatidylet hanolamine-poly(ethylene glycol) | 224–232 nm | mitoxantrone | MCF-7 breast cancer cell line | Active targeting by folic acid | Results showed high cellular uptake of folate conjugated NPs compared to untargeted NPs and improved cytotoxicity of mitoxantrone against MCF-7 cells. | 2022 | [45] |
| Nanostructured lipid carriers | Glyceryl tridecanoate, glyceryl tripalmitate, phosphocholine (NBD-PC) | 32 nm | Quercetin | MCF-7 and MDA-MB-231 cell lines | Passive targeting | The solubility of Quercetin improved 1000-fold, and the cytotoxicity and apoptosis increased in a dose-dependent manner. | 2014 | [46] |
| Nanostructured lipid carriers | Cholesterol, α-tocopherol, lecithin and Poloxamer and polyethylene glycol (PEG) | 154.6 nm | Paclitaxel (PTX) | MCF-7 cell line | Active targeting by folate | Cytotoxicity of paclitaxel-loaded, folic acid-PEG-modified nanoparticles was significantly enhanced compared to free paclitaxel and other drug-loaded modified nanoparticles. | 2017 | [47] |
| Nanostructured lipid carriers | PEG-SA, soybean phosphatidylcholine (S100), oleic acid, glycerin monostearate and Compritol® 888 ATO | 100 nm | Doxorubicin and Lapachone | MCF-7 ADR cell line/BALB/c nude mice | Passive targeting | In vitro experiments and in vivo anti-cancer assays on MCF-7 ADR mice model showed that combined drugs loaded on the nanocarrier had significant anticancer efficacy, confirming synergistic effects. | 2018 | [48] |
| Nanostructured lipid carriers | Stearic acid, oleic acid, Phospho-Lipon® 90 G | 82–88 nm | Resveratrol | MCF-7 breast cancer cell line | Active targeting by folate | In vitro studies showed a 2.5-fold increase in cytotoxicity of MCF-7 cells using targeted nanocarrier compared to free drug. | 2019 | [49] |
| Name of Nanoparticle | Composition/Coating of Nanodelivery System | Size | Drug/Biomolecule | Cell Line/Animal Model | Targeting | Outcome | Year | Reference |
|---|---|---|---|---|---|---|---|---|
| Polymeric nanoparticles | Poly (lactic-co-glycolic acid) | 170 nm | Doxorubicin and resveratrol (RES) | MDA-MB231/ADR and MCF-7/ADR cells and BALB/c nude mice tumour model | Passive targeting | Dual drug-loaded nanoparticles exhibited significant cytotoxicity on cells. In mice, nanoparticles delivered both drugs mainly to tumours with significantly inhibited growth compared with free doxorubicin. | 2016 | [50] |
| Polymeric nanoparticles | Chitosan | <150 nm | Docetaxel and cMET siRNA | Mucin1+ SK-BR-3 vs. mucin1CHO cells | Active targeting by mucin1 aptamer | Higher cellular uptake of aptamer-conjugated nanoparticles in cells overexpressing mucin1. cMET gene silencing was confirmed by significantly reduced expression of the genes involved in tumourigenicity, metastasis and angiogenesis. | 2018 | [51] |
| Polymeric nanoparticles | Poly (Cyclohexene Phthalate) | 100 nm | Dasatinib | MDA-MB-231 and BT549 cell lines | Passive targeting | Dasatinib-loaded polymeric nanoparticles showed higher efficacy compared to free drug. | 2019 | [52] |
| Polymeric nanoparticles | Polybutyleneadipate-co-butylene terephthalate | NA | Docetaxel | BT-474 (HER-2-positive) and MDA-B-468 (HER-2-negative) cell lines | Active targeting by HER-2 aptamer | Significantly higher uptake in BT-474 cells compared to MDA-B-468 and higher cytotoxicity compared to free docetaxel. Lower relative migration suggested improved inhibition of migration by nanoparticles. | 2019 | [53] |
| Polymeric nanoparticles | Poly-lactic-co-glycolic acid (PLGA-PEG) | 107 nm | Methotrexate (MTX) and trapoxin (TPX) | MCF-7 cell line | Passive targeting | TPX/MTX-co-loaded PEG-PLGA nanoparticles caused a significant dose-dependent decrease in cell viability with synergistic antitumour effects and activation of the mitochondrial apoptosis pathway. | 2021 | [54] |
| Polymeric nanoparticles | polyethylene glycol-polycaprolactone (PEG-PCL) | 106–152 nm | Gemcitabine and MUC1 inhibitor | MCF-7 and MDAMB-231 cell lines, Ehrlich ascites carcinoma (EAC) tumour-bearing animal model | Active targeting | Gem-MUC1 Inhibitor NPs showed sustained drug release in vitro, and tumour-targeting ability with enhanced effectiveness of anticancer drugs in vitro and in vivo. | 2022 | [55] |
| Polymeric nanoparticles | PLGA-PEG | 98.1 nm | PD-L1 siRNA | MDA-MB-231, BT-549 and BT474 cell lines | Active targeting by sTN145 RNA aptamer | In vitro findings revealed specific uptake of aptamer-linked NPs by TNBC MDA-MB-231 and BT-549 cells with almost complete suppression of PD-L1 expression. | 2022 | [56] |
| Polymeric micelles | MPEG2000-PDLLA2000 | 22.83–25.8 nm | Paclitaxel and Lapatinib (LP) | SKBr-3 (HER-2-positive) and MDA-MB-231 (HER-2-negative) cell lines | Active targeting of HER2 | LP co-loaded polymeric micelles showed significantly greater cytotoxicity to SKBr-3 cells and almost no significantly different cytotoxicity to MDA-MB-231 cells compared to paclitaxel-loaded micelles. | 2015 | [57] |
| Polymeric micelles | Poly (ethylene glycol)-bock-poly(lactide) (PEG2k-PLA5k) | 102.5–110 nm | Doxorubicin and curcumin | MCF-7 and MCF7/ADR cell lines/BALB/c nude mice bearing MCF7/ADR tumours | Passive targeting | In vitro studies showed that co-loaded micelles were superior to free doxorubicin, free combination (doxorubicin curcumin) and doxorubicin-loaded micelles in the inhibition of proliferation of resistant cells. Dual-loaded micelles showed enhanced tumour accumulation and strong tumour growth inhibition. | 2016 | [58] |
| Polymeric micelles | Monomethoxy poly (ethylene glycol)poly(ε-caprolactone) (mPEG-PCL) and monomethoxy poly (ethylene glycol)-poly (D, L-lactic acid) (mPEG-PLA) | 24–26 nm | Docetaxel | 4T1 cells and MCF-7 cell lines/4T1 tumour model in mice | Passive targeting | Encapsulation of docetaxel in micelles enhanced its water solubility with high encapsulation efficiency and cytotoxicity on 4T1 and MCF-7 cells in vitro. Anti-cancer activity on 4T1 tumour model in vivo suggested the excellent efficacy of DTX micelles. | 2017 | [59] |
| Polymeric micelles | β-cyclodextrin-{poly(ε-caprolactone)poly(2-aminoethylmethacrylate)}21 | 150 nm | Camptothecin | MCF-7 and 4T1 cell lines/4T1 tumour model in mice | Active targeting by nucleolin aptamer (AS1411) | Aptamer-functionalized NPs showed higher internalization and cytotoxic effects in cancer cells compared to normal cells in vitro. In vivo experiments demonstrated significant tumour growth inhibition. | 2022 | [60] |
| Polymeric micelles | b-poly(ε-caprolactone), tocopheryl polyethylene glycol succinate (TPGS) | 117–137 nm | Curcumin | 4T1 cell lines/4T1 tumour model in mice | Active targeting of CD44 by hyaluronan | In vivo experiments showed high tumour uptake and antitumour efficacy. | 2023 | [61] |
| Dendrimers | Pluronic F68 (PF68)-conjugated polyamidoamine (PAMAM) | 200–400 nm | Doxorubicin | MCF-7/ADR cell line, MCF-7/ADR tumour spheroids and MCF-7/ADR tumour-bearing nude mice MCF-7 cell line | Passive targeting | Increased antitumour activity of the doxorubicin-loaded dendrimers was demonstrated in vitro and in vivo. | 2016 | [62] |
| Dendrimers | Hyperbranched polyglycerol derivative (HPG-C18) and dendritic poly(L-lysine) (PLLD) | 100 nm | Docetaxel and MMP-9 siRNA | MCF-7 murine tumour model | Passive targeting | In vitro studies showed that the combined drug-loaded dendrimers caused significant apoptosis compared to docetaxel or MMP-9 alone, and stronger tumour inhibition. | 2016 | [63] |
| Dendrimers | Pluronic F68 (PF68)-conjugated polyamidoamine (PAMAM) | NA | Doxorubicin | T47D cell line | Active targeting by antiCXCR4 antibody, which binds to CXCR4 receptors on cancer cells | AntiCXCR4 doxorubicin-loaded dendrimers induced a significant increase in in vitro cytotoxicity compared to non-targeted dendrimers. They also showed a remarkable reduction in the migration of BT-549-Luc breast cancer cells. | 2017 | [64] |
| Dendrimers | Amine terminated pluronic F68 (PF68)-conjugated polyamidoamine (PAMAM) G4 | NA | Docetaxel/Paclitaxel | MCF-7 (HER-2-negative) cell line and SKBR-3 (HER-2-positive) cell line | Active targeting of HER2 by trastuzumab | Drug-loaded trastuzumab-conjugated PAMAM dendrimers exhibited remarkably high toxicity on SKBR-3 cells and very low toxicity on MCF-7 cells. | 2019 | [65] |
| Dendrimers | polyamidoamine (PAMAM) G4 | 113.3–206.7 nm | Methotrexate and high-mobility group protein A2 (HMGA2) siRNA | MCF-7 and MDAMB231 cancer cell lines | Active targeting of folate receptor | G4/MTX-siRNA showed strong internalization, resulting in significant apoptosis-mediated cell death by specific downregulation of HMGA2 expression. | 2021 | [66] |
| Dendrimers | poly-lysine dendrimers | 180 nm | Doxorubicin and P-gp siRNA, Bcl-2 siRNA | MCF-7 and MCF7/ADR cancer cell lines and orthotopic breast tumour model in mice | Active targeting of cells overexpressing sialic acid by phenylboronic acid | In vitro studies demonstrated that targeted NPs were capable of suppressing the proliferation of MCF-7/ADR cancer cells, and in vivo studies revealed significant tumour growth inhibition. | 2022 | [67] |
| Lipid–polymer hybrid nanoparticles | Carboxylic modified latex (CML) polystyrene nanoparticles, phospholipid functionalized liposomes composed of DSPC:Cholesterol:POPG | 120 nm | Doxorubicin and siRNA for multidrug resistance protein 1 (MDR-1) | MDA-MB-468 cell line/MDA-MB468 cells xenograft animal model | Active targeting of CD44 receptors by hyaluronic acid | A single dose significantly lowered the expression of MDR-1 in tumours by almost 80%. Combining siRNA resulted in a four-fold improvement of the efficacy of doxorubicin in vitro and up to an eight-fold reduction in tumour volume compared to the control treatments. | 2013 | [68] |
| Lipid–polymer hybrid nanoparticles | (DSPE-PEG 2000), (DSPE), NBD-PE c) Poly(lactide-co-glycolide) (PLGA) | 230 nm | Docetaxel (DTX) | SK-BR-3 cells (Her2-positive cell line) and MDAMB-435 | Active targeting by antiHER2/neu peptide (AHNP) and modified HIV-1 Tat (mTAT) for cell membrane penetration | Docetaxel-loaded dual ligand hybrid nanoparticles exhibited gradual sustained drug release. They were also substantially more potent against SK-BR-3 cancer cells than other nanoparticle formulations and free drug. | 2015 | [69] |
| Lipid–polymer hybrid nanoparticles | PLA, DSPE-PEG, SA (stearyl amine) | 71 nm | Methotrexate and beta carotene | MCF-7 cell line/tumour model in rats | Active targeting by fructose | Fructose-methotrexate-beta carotene-loaded nanoparticles had the highest cytotoxic effect against MCF-7 cells and the greatest tumour growth suppression in the animal model. | 2017 | [70] |
| Lipid–polymer hybrid nanoparticles (LPHNP) | DSPE-PEG (2000)NH2, PLGA | 143 nm | Docetaxel (DTX) | MDA-MB-231 cell line/MDA-MB231 cells tumour model in mice | Passive targeting | Enhanced cytotoxicity and greater cellular uptake of docetaxel in breast cancer cells, and better in vivo pharmacokinetic profile. | 2019 | [71] |
| Lipid–polymer hybrid nanoparticles (LPHNP) | SPC, DSPE-PEG2000, PLGA | ~200 nm | Gemcitabine | MCF-7 and MDAMB-231 cell lines/NMU-induced breast tumour model in rats | Passive targeting | GEM-loaded LPHNs significantly reduced cell viability of both cancer cell lines in vitro and showed enhanced antitumour efficacy in rats. | 2020 | [72] |
| Lipid–polymer hybrid nanoparticles (LPHNP) | Dimethyldioctadecylammonium bromidemethoxy poly (ethylene glycol)-poly (εcaprolactone) | 80–90 nm | Insulin-like growth factor type I (IGF-1R) siRNA | MCF-7 human breast cancer cell line | Passive targeting | IGF-1RsiRNA-loaded LPHNs caused significant cytotoxicity of MCF-7 cells with a significant decrease in IGF-1R mRNA expression compared to free IGF-1RsiRNA. | 2021 | [73] |
| Lipid–polymer hybrid nanoparticles (LPHNP) | Lipoid-90H and chitosan | 218–439 nm | Sunitinib | MCF-7 breast cancer cell line | Passive targeting | In vitro studies showed potent cytotoxicity against MCF-7 breast cancer cells. | 2022 | [74] |
| Name of Product | Type of Nanoparticle | Drug/Biomolecule | Specific Indication and Confirmed Benefit | Approval/Clinical trial Status |
|---|---|---|---|---|
| EndoTAG-1 | Liposome | Paclitaxel | HER2-negative relapsed or metastatic TNBC | Phase III |
| LEP-ETU (Liposomal Entrapped Paclitaxel-Easy To Use) | Liposome | Paclitaxel | Metastatic breast cancer | Phase II |
| LIPUSU® | Liposome | Paclitaxel | Metastatic breast cancer | Phase IV |
| nal-IRI | Liposome | Irinotecan | Metastatic breast cancer | Phase I |
| Doxil® (US) Caelyx® (Europe) | PEGylated Liposome | Doxorubicin hydrochloride | Metastatic and advanced breast cancer—reduced cardiac toxicity | Approved |
| Myocet | Non-PEGylated Liposome | Doxorubicin citrate | Metastatic breast cancer Reduced cardiac toxicity | Approved in Europe and Canada/Phase III US |
| MM-302 | HER2-targetingLiposome | Doxorubicin | HER2-positive, locally advanced/metastatic BC | Phase III |
| 2B3-101 | GSH PEG-liposome | Doxorubicin | Metastatic | Phase II |
| Lipolatin (regulon Inc.) | PEGylated liposome | Cisplatin | Metastatic | Phase III |
| Mitoxantrone HCL Liposome | Liposome | Mitoxantrone | Advanced recurrent/metastatic breast cancer | Phase II |
| ThermoDox® | Heat-activated liposome | Doxorubicin | Refractory chest wall breast cancer | Phase I/II |
| Narekt-102 | PEGylated liposome | Irinotecan | Metastatic | Phase III |
| SPI-077 | Stealth liposomal | Cisplatin | Advanced breast cancer | Phase I/II |
| DEP® docetaxel | PEGylated dendrimer | Docetaxel | Locally advanced or metastatic breast cancer | Phase II |
| NK-105 | mPEG-b-poly (asparticacid) micelles | Paclitaxel | Metastatic or recurrent breast cancer | Phase III |
| Nanoxel M® | PEG-poly (D, L-lactide) | Docetaxel | Metastatic | Phase I |
| Genexol®-PM | Polymeric micelle PEG-poly (D, L-lactide) | Paclitaxel | Metastatic breast cancer. Improved progression-free survival only and not overall survival | Phase III US/(Approved in Europe and South Korea) |
| Abraxane/Nab-paclitaxel | Human serum albumin | Paclitaxel | Metastatic breast cancer. Improved progression-free survival only and not overall survival | Approved by US FDA and EuropeanMedicine Agency in 2005 |
| ABI-008 | Human serum albumin | Docetaxel | Metastatic breast cancer | Phase II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafik, S.T.; Vaidya, J.S.; MacRobert, A.J.; Yaghini, E. Organic Nanodelivery Systems as a New Platform in the Management of Breast Cancer: A Comprehensive Review from Preclinical to Clinical Studies. J. Clin. Med. 2023, 12, 2648. https://doi.org/10.3390/jcm12072648
Rafik ST, Vaidya JS, MacRobert AJ, Yaghini E. Organic Nanodelivery Systems as a New Platform in the Management of Breast Cancer: A Comprehensive Review from Preclinical to Clinical Studies. Journal of Clinical Medicine. 2023; 12(7):2648. https://doi.org/10.3390/jcm12072648
Chicago/Turabian StyleRafik, Salma T., Jayant S. Vaidya, Alexander J. MacRobert, and Elnaz Yaghini. 2023. "Organic Nanodelivery Systems as a New Platform in the Management of Breast Cancer: A Comprehensive Review from Preclinical to Clinical Studies" Journal of Clinical Medicine 12, no. 7: 2648. https://doi.org/10.3390/jcm12072648
APA StyleRafik, S. T., Vaidya, J. S., MacRobert, A. J., & Yaghini, E. (2023). Organic Nanodelivery Systems as a New Platform in the Management of Breast Cancer: A Comprehensive Review from Preclinical to Clinical Studies. Journal of Clinical Medicine, 12(7), 2648. https://doi.org/10.3390/jcm12072648