Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review
Abstract
1. Introduction
1.1. Chronic Obstructive Pulmonary Disease
1.2. Polyunsaturated Fatty Acids (PUFAs)
2. Comorbid Conditions of Mood Disorders in COPD
3. Underlying Mechanisms Associated with Mood Disorders in COPD
| Mechanisms | References |
|---|---|
| Inflammation | |
| Elevated levels of inflammatory mediators such as CRP, fibrinogen, IL-6, IL-8, IL-13, MCP-1, TNF-α, and TNF-α receptor-1 were detected in the plasma of COPD patients. | [64,65,66,67,68,69] |
| Inflammatory cytokines such as CRP, IL-1, IL-6, IFN-γ, TNF-α, and TNF-α receptor 1 were associated with depressive symptoms in COPD. | [65,70,71,72,73] |
| Oxidative stress | |
| Decreased levels of reduced glutathione, as well as an increased amount of 8-isoprostane, were detected in COPD patients compared to controls. | [99] |
| Genetic polymorphisms associated with extracellular SOD as well as their expression in the sputum are frequently found in COPD patients. | [97,98] |
| Downregulation of Nrf2 and Nrf2-related, heme oxygenase-1, and glutamate-cysteine ligase catalytic subunit in the peripheral blood mononuclear cells were detected in COPD patients. | [99] |
| Reduced pulmonary expression of FOXO3a was reported in COPD patients. | [100] |
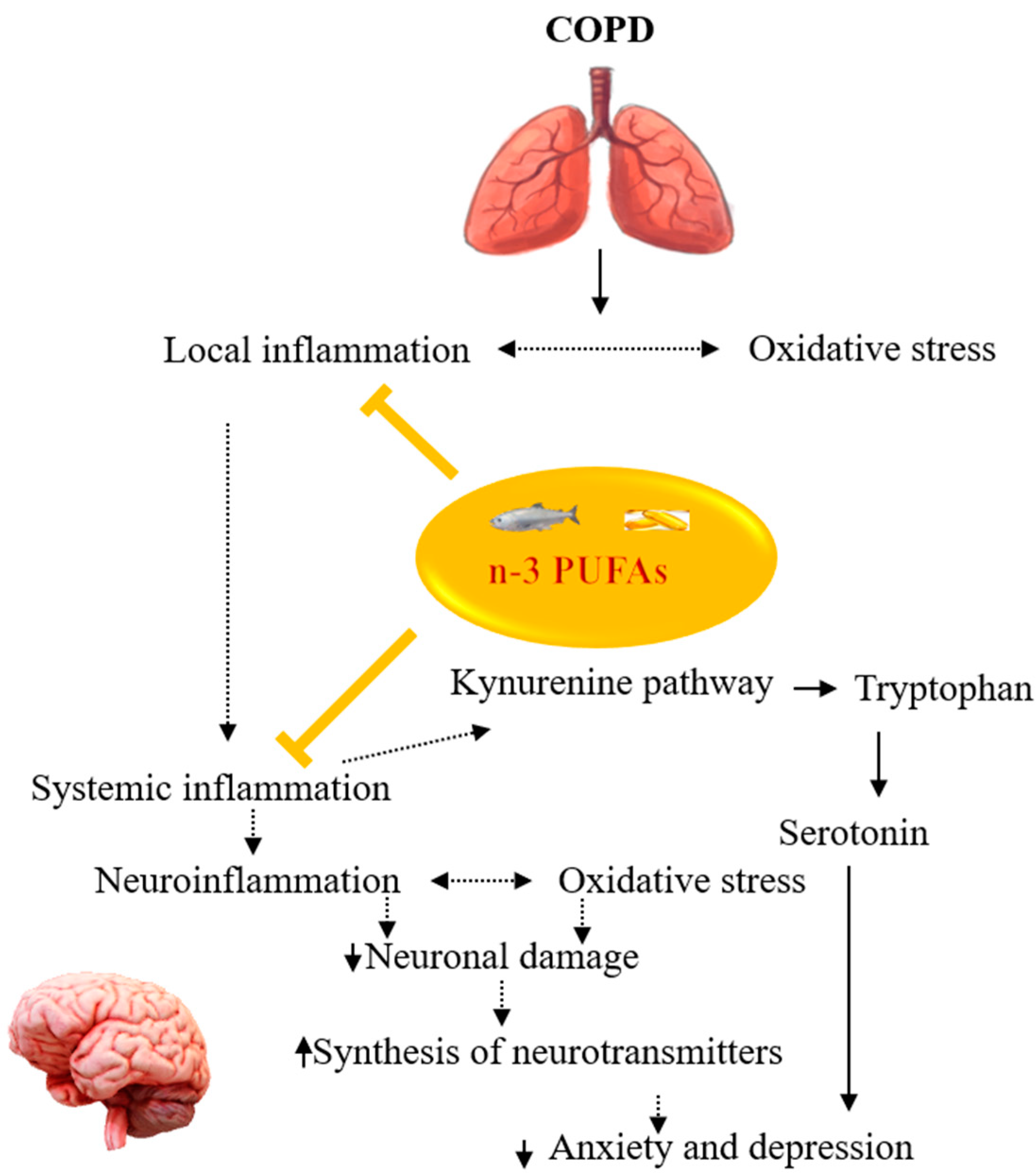
4. Potentials of n-3 PUFAs in Managing Comorbid Mood Disorders in COPD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Chronic Obstructive Pulmonary Disease (COPD); WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Hunt, J.M.; Tuder, R. Alpha 1 anti-trypsin: One protein, many functions. Curr. Mol. Med. 2012, 12, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Burney, P.G.J.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborín, R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef]
- Anderson, D.; Macnee, W. Targeted treatment in COPD: A multi-system approach for a multi-system disease. Int. J. Chronic Obs. Pulmon. Dis. 2009, 4, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Mahler, D.A.; Marcus, P.; Owen, C.A.; Yawn, B.; Rennard, S. Inflammation in COPD: Implications for management. Am. J. Med. 2012, 125, 1162–1170. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Ekbom, A.; Brandt, L.; Granath, F.; Löfdahl, C.G.; Egesten, A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung 2008, 186, 167–172. [Google Scholar] [CrossRef]
- Raj, A.A.; Birring, S.S.; Green, R.; Grant, A.; de Caestecker, J.; Pavord, I.D. Prevalence of inflammatory bowel disease in patients with airways disease. Respir. Med. 2008, 102, 780–785. [Google Scholar] [CrossRef]
- Cecere, L.M.; Littman, A.J.; Slatore, C.G.; Udris, E.M.; Bryson, C.L.; Boyko, E.J.; Pierson, D.J.; Au, D.H. Obesity and COPD: Associated symptoms, health-related quality of life, and medication use. Copd 2011, 8, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.; Backman, H.; Bossios, A.; Bjerg, A.; Hedman, L.; Lindberg, A.; Rönmark, E.; Lundbäck, B. Only severe COPD is associated with being underweight: Results from a population survey. ERJ Open Res. 2016, 2, 00051-2015. [Google Scholar] [CrossRef] [PubMed]
- Verberne, L.D.M.; Leemrijse, C.J.; Swinkels, I.C.S.; van Dijk, C.E.; de Bakker, D.H.; Nielen, M.M.J. Overweight in patients with chronic obstructive pulmonary disease needs more attention: A cross-sectional study in general practice. NPJ Prim. Care Respir. Med. 2017, 27, 63. [Google Scholar] [CrossRef]
- Park, Y.M.; Myers, M.; Vieira-Potter, V.J. Adipose tissue inflammation and metabolic dysfunction: Role of exercise. Mo. Med. 2014, 111, 65–72. [Google Scholar] [PubMed]
- Rutten, E.P.A.; Lenaerts, K.; Buurman, W.A.; Wouters, E.F.M. Disturbed intestinal integrity in patients with COPD: Effects of activities of daily living. Chest 2014, 145, 245–252. [Google Scholar] [CrossRef]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.S.; Marott, J.L.; Nordestgaard, B.G.; Lange, P.; Hallas, J.; Dahl, M.; Vestbo, J. Low Use and Adherence to Maintenance Medication in Chronic Obstructive Pulmonary Disease in the General Population. J. Gen. Intern. Med. 2015, 30, 51–59. [Google Scholar] [CrossRef]
- Moradkhani, B.; Mollazadeh, S.; Niloofar, P.; Bashiri, A.; Oghazian, M.B. Association between medication adherence and health-related quality of life in patients with chronic obstructive pulmonary disease. J. Pharm. Health Care Sci. 2021, 7, 40. [Google Scholar] [CrossRef]
- Fogh-Andersen, I.S.; Farver-Vestergaard, I.; Tehrani, C.M.; Løkke, A. Examination and treatment of anxiety and depression in patients with COPD. Ugeskr. Laeger 2021, 183, V09200679. [Google Scholar]
- Laforest, L.; Denis, F.; Van Gansea, E.; Ritleng, C.; Saussier, C.; Passante, N.; Devouassoux, G.; Chattée, G.; Freymond, N.; Pacheco, Y. Correlates of adherence to respiratory drugs in COPD patients. Prim. Care Respir. J. 2010, 19, 148–154. [Google Scholar] [CrossRef][Green Version]
- Fritzsche, A.; Clamor, A.; von Leupoldt, A. Effects of medical and psychological treatment of depression in patients with COPD—A review. Respir. Med. 2011, 105, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.S.; Park, Y.; Hur, P.; Huang, T.-Y.; Harris, I.; Netzer, G.; Lehmann, S.W.; Langenberg, P.; Khokhar, B.; Wei, Y.-J. Adherence to maintenance medications among older adults with chronic obstructive pulmonary disease. The role of depression. Ann. Am. Thorac. Soc. 2016, 13, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef]
- Buydens-Branchey, L.; Branchey, M.; Hibbeln, J.R. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 568–575. [Google Scholar] [CrossRef]
- Amminger, G.P.; Schäfer, M.R.; Schlögelhofer, M.; Klier, C.M.; McGorry, P.D. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat. Commun. 2015, 6, 7934. [Google Scholar] [CrossRef]
- Hsu, M.-C.; Huang, Y.-S.; Ouyang, W.-C. Beneficial effects of omega-3 fatty acid supplementation in schizophrenia: Possible mechanisms. Lipids Health Dis. 2020, 19, 159. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharm. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.O.; Chaudhry, I.B.; Blakemore, A.; Shakoor, S.; Husain, M.A.; Lane, S.; Kiran, T.; Jafri, F.; Memon, R.; Panagioti, M.; et al. Prevalence of depression and anxiety in patients with chronic obstructive pulmonary disease and their association with psychosocial outcomes: A cross-sectional study from Pakistan. SAGE Open Med. 2021, 9, 20503121211032813. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Fahey, P.; Cochrane, B.; Smith, S. Bidirectional associations between clinically relevant depression or anxiety and COPD: A systematic review and meta-analysis. Chest 2013, 144, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, K.; Pengpid, S. Anxiety and depressive features in chronic disease patients in Cambodia, Myanmar and Vietnam. S. Afr. J. Psychiatr. 2016, 22, 940. [Google Scholar] [CrossRef]
- Hanania, N.A.; Müllerova, H.; Locantore, N.W.; Vestbo, J.; Watkins, M.L.; Wouters, E.F.; Rennard, S.I.; Sharafkhaneh, A. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am. J. Respir. Crit. Care Med. 2011, 183, 604–611. [Google Scholar] [CrossRef]
- Omachi, T.A.; Katz, P.P.; Yelin, E.H.; Gregorich, S.E.; Iribarren, C.; Blanc, P.D.; Eisner, M.D. Depression and health-related quality of life in chronic obstructive pulmonary disease. Am. J. Med. 2009, 122, 778.e9–778.e15. [Google Scholar] [CrossRef][Green Version]
- Schneider, C.; Jick, S.S.; Bothner, U.; Meier, C.R. COPD and the risk of depression. Chest 2010, 137, 341–347. [Google Scholar] [CrossRef]
- Hynninen, K.M.; Breitve, M.H.; Wiborg, A.B.; Pallesen, S.; Nordhus, I.H. Psychological characteristics of patients with chronic obstructive pulmonary disease: A review. J. Psychosom. Res. 2005, 59, 429–443. [Google Scholar] [CrossRef]
- Kunik, M.E.; Roundy, K.; Veazey, C.; Souchek, J.; Richardson, P.; Wray, N.P.; Stanley, M.A. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest 2005, 127, 1205–1211. [Google Scholar] [CrossRef]
- Singh, G.; Zhang, W.; Kuo, Y.F.; Sharma, G. Association of Psychological Disorders With 30-Day Readmission Rates in Patients With COPD. Chest 2016, 149, 905–915. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Baldwin, R.C.; Connolly, M.J. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: Prevalence, and validation of the BASDEC screening questionnaire. Int. J. Geriatr. Psychiatry 2000, 15, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Matte, D.L.; Pizzichini, M.M.; Hoepers, A.T.; Diaz, A.P.; Karloh, M.; Dias, M.; Pizzichini, E. Prevalence of depression in COPD: A systematic review and meta-analysis of controlled studies. Respir. Med. 2016, 117, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kokturk, N.; Polatli, M.; Oguzulgen, I.K.; Saleemi, S.; Al Ghobain, M.; Khan, J.; Doble, A.; Tariq, L.; Aziz, F.; El Hasnaoui, A. Adherence to COPD treatment in Turkey and Saudi Arabia: Results of the ADCARE study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Zhu, Y.; Chen, P.; Zhang, P.; Yu, J.; Wang, Y.; Chen, N.; Zhang, L.; Wu, H.; Zhao, J. Interaction of depressive and anxiety symptoms on the mortality of patients with COPD: A preliminary study. Copd 2014, 11, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Müllerová, H.; Hanania, N.A.; Lavoie, K.; Tal-Singer, R.; Vestbo, J.; Rennard, S.I.; Wouters, E.F. Long-term Course of Depression Trajectories in Patients With COPD: A 3-Year Follow-up Analysis of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Cohort. Chest 2016, 149, 916–926. [Google Scholar] [CrossRef]
- Hilmarsen, C.W.; Wilke, S.; Engan, H.; Spruit, M.A.; Rodenburg, J.; Janssen, D.J.; Steinshamn, S.; Jones, P.W.; Wouters, E.F.; Oldervoll, L.; et al. Impact of symptoms of anxiety and depression on COPD Assessment Test scores. Eur. Respir. J. 2014, 43, 898–900. [Google Scholar] [CrossRef]
- Regvat, J.; Žmitek, A.; Vegnuti, M.; Košnik, M.; Šuškovič, S. Anxiety and depression during hospital treatment of exacerbation of chronic obstructive pulmonary disease. J. Int. Med. Res. 2011, 39, 1028–1038. [Google Scholar] [CrossRef]
- Pumar, M.I.; Gray, C.R.; Walsh, J.R.; Yang, I.A.; Rolls, T.A.; Ward, D.L. Anxiety and depression-Important psychological comorbidities of COPD. J. Thorac. Dis. 2014, 6, 1615–1631. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Alexopoulos, G.S. Pharmacological treatment of depression in older patients with chronic obstructive pulmonary disease: Impact on the course of the disease and health outcomes. Drugs Aging 2014, 31, 483–492. [Google Scholar] [CrossRef]
- Maurer, J.; Rebbapragada, V.; Borson, S.; Goldstein, R.; Kunik, M.E.; Yohannes, A.M.; Hanania, N.A. Anxiety and depression in COPD: Current understanding, unanswered questions, and research needs. Chest 2008, 134, 43s–56s. [Google Scholar] [CrossRef]
- Vozoris, N.T.; Wang, X.; Austin, P.C.; Stephenson, A.L.; O’Donnell, D.E.; Gershon, A.S.; Gill, S.S.; Rochon, P.A. Serotonergic antidepressant use and morbidity and mortality among older adults with COPD. Eur. Respir. J. 2018, 52, 1800475. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J. Neuroinflamm. 2014, 11, 151. [Google Scholar] [CrossRef]
- Miller, A.H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Colasanto, M.; Madigan, S.; Korczak, D.J. Depression and inflammation among children and adolescents: A meta-analysis. J. Affect. Disord. 2020, 277, 940–948. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Hepgul, N.; Pariante, C.M. Inflammation and depression. Curr. Top. Behav. Neurosci. 2013, 14, 135–151. [Google Scholar] [CrossRef]
- Chang, J.P.-C.; Lin, C.-Y.; Lin, P.-Y.; Shih, Y.-H.; Chiu, T.-H.; Ho, M.; Yang, H.-T.; Huang, S.-Y.; Gałecki, P.; Su, K.-P. Polyunsaturated fatty acids and inflammatory markers in major depressive episodes during pregnancy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 273–278. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- Wilson, C.J.; Finch, C.E.; Cohen, H.J. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 2002, 50, 2041–2056. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Su, K.P. Biological mechanism of antidepressant effect of omega-3 fatty acids: How does fish oil act as a ‘mind-body interface’? Neurosignals 2009, 17, 144–152. [Google Scholar] [CrossRef]
- Su, B.; Liu, T.; Fan, H.; Chen, F.; Ding, H.; Wu, Z.; Wang, H.; Hou, S. Inflammatory Markers and the Risk of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0150586. [Google Scholar] [CrossRef]
- Aaron, S.D.; Vandemheen, K.L.; Ramsay, T.; Zhang, C.; Avnur, Z.; Nikolcheva, T.; Quinn, A. Multi analyte profiling and variability of inflammatory markers in blood and induced sputum in patients with stable COPD. Respir. Res. 2010, 11, 41. [Google Scholar] [CrossRef]
- Eagan, T.M.; Ueland, T.; Wagner, P.D.; Hardie, J.A.; Mollnes, T.E.; Damås, J.K.; Aukrust, P.; Bakke, P.S. Systemic inflammatory markers in COPD: Results from the Bergen COPD Cohort Study. Eur. Respir. J. 2010, 35, 540–548. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Chen, P.; Wu, G.; Cai, S. Local inflammation occurs before systemic inflammation in patients with COPD. Respirology 2010, 15, 478–484. [Google Scholar] [CrossRef]
- Karadag, F.; Karul, A.B.; Cildag, O.; Yilmaz, M.; Ozcan, H. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung 2008, 186, 403–409. [Google Scholar] [CrossRef]
- Kersul, A.L.; Iglesias, A.; Ríos, Á.; Noguera, A.; Forteza, A.; Serra, E.; Agustí, A.; Cosío, B.G. Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch. Bronconeumol. 2011, 47, 176–183. [Google Scholar] [CrossRef]
- Eickmeier, O.; Huebner, M.; Herrmann, E.; Zissler, U.; Rosewich, M.; Baer, P.C.; Buhl, R.; Schmitt-Grohé, S.; Zielen, S.; Schubert, R. Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine 2010, 50, 152–157. [Google Scholar] [CrossRef]
- Strollo, H.C.; Nouraie, S.M.; Hoth, K.F.; Riley, C.M.; Karoleski, C.; Zhang, Y.; Hanania, N.A.; Bowler, R.P.; Bon, J.; Sciurba, F.C. Association of Systemic Inflammation with Depressive Symptoms in Individuals with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2515–2522. [Google Scholar] [CrossRef]
- Al-shair, K.; Kolsum, U.; Dockry, R.; Morris, J.; Singh, D.; Vestbo, J. Biomarkers of systemic inflammation and depression and fatigue in moderate clinically stable COPD. Respir. Res. 2011, 12, 3. [Google Scholar] [CrossRef]
- Du, Y.J.; Yang, C.J.; Li, B.; Wu, X.; Lv, Y.B.; Jin, H.L.; Cao, Y.X.; Sun, J.; Luo, Q.L.; Gong, W.Y.; et al. Association of pro-inflammatory cytokines, cortisol and depression in patients with chronic obstructive pulmonary disease. Psychoneuroendocrinology 2014, 46, 141–152. [Google Scholar] [CrossRef]
- Rybka, J.; Korte, S.M.; Czajkowska-Malinowska, M.; Wiese, M.; Kędziora-Kornatowska, K.; Kędziora, J. The links between chronic obstructive pulmonary disease and comorbid depressive symptoms: Role of IL-2 and IFN-γ. Clin. Exp. Med. 2016, 16, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Blakely, R.D.; Hewlett, W.A. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006, 31, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, O.; Sakai, N.; Obara, H.; Saito, N. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur. J. Pharm. 1998, 349, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Gulcev, M.; Reilly, C.; Griffin, T.J.; Broeckling, C.D.; Sandri, B.J.; Witthuhn, B.A.; Hodgson, S.W.; Woodruff, P.G.; Wendt, C.H. Tryptophan catabolism in acute exacerbations of chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2435–2446. [Google Scholar] [CrossRef]
- Meier, M.A.; Ottiger, M.; Vögeli, A.; Steuer, C.; Bernasconi, L.; Thomann, R.; Christ-Crain, M.; Henzen, C.; Hoess, C.; Zimmerli, W.; et al. Activation of the Serotonin Pathway is Associated with Poor Outcome in COPD Exacerbation: Results of a Long-Term Cohort Study. Lung 2017, 195, 303–311. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar] [CrossRef]
- Kumar, G.K. Hypoxia. 3. Hypoxia and neurotransmitter synthesis. Am. J. Physiol. Cell Physiol. 2011, 300, C743–C751. [Google Scholar] [CrossRef]
- Andelid, K.; Glader, P.; Yoshihara, S.; Andersson, A.; Ekberg-Jansson, A.; Lindén, A. Hypoxia associated with increased systemic concentrations of MPO and NE during exacerbations of COPD. Eur. Respir. J. 2015, 46, PA873. [Google Scholar] [CrossRef]
- Van Den Borst, B.; Schols, A.M.; de Theije, C.; Boots, A.W.; Köhler, S.E.; Goossens, G.H.; Gosker, H.R. Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J. Appl. Physiol. 2013, 114, 1619–1628. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Zhang, X.; Xie, W.; Li, L.; Yang, D.; Heng, X.; Du, Y.; Doody, R.S.; Le, W. Prenatal hypoxia may aggravate the cognitive impairment and Alzheimer’s disease neuropathology in APPSwe/PS1A246E transgenic mice. Neurobiol. Aging 2013, 34, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, R.; Li, S.; Fu, Z.; Cheng, C.; Cai, H.; Le, W. Acute Hypoxia Induced an Imbalanced M1/M2 Activation of Microglia through NF-κB Signaling in Alzheimer’s Disease Mice and Wild-Type Littermates. Front. Aging Neurosci. 2017, 9, 282. [Google Scholar] [CrossRef]
- Sapin, E.; Peyron, C.; Roche, F.; Gay, N.; Carcenac, C.; Savasta, M.; Levy, P.; Dematteis, M. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep 2015, 38, 1537–1546. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Wang, Z.-Y.; Xie, J.-W.; Cai, J.-H.; Wang, T.; Xu, Y.; Wang, X.; An, L. CD36 upregulation mediated by intranasal LV-NRF2 treatment mitigates hypoxia-induced progression of Alzheimer’s-like pathogenesis. Antioxid. Redox Signal. 2014, 21, 2208–2230. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.L.; Vanover, K.E.; Zhu, H.; Miller, D.B.; O’Callaghan, J.P.; Tomesch, J.; Li, P.; Zhang, Q.; Krishnan, V.; Hendrick, J.P. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology 2015, 232, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Vaváková, M.; Ďuračková, Z.; Trebatická, J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxid. Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- Baek, D.; Park, Y. Association between erythrocyte n-3 polyunsaturated fatty acids and biomarkers of inflammation and oxidative stress in patients with and without depression. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 291–296. [Google Scholar] [CrossRef]
- Forlenza, M.J.; Miller, G.E. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Schaberg, T.; Klein, U.; Rau, M.; Eller, J.; Lode, H. Subpopulations of alveolar macrophages in smokers and nonsmokers: Relation to the expression of CD11/CD18 molecules and superoxide anion production. Am. J. Respir. Crit. Care Med. 1995, 151, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Drost, E.M.; Skwarski, K.M.; Sauleda, J.; Soler, N.; Roca, J.; Agusti, A.; MacNee, W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005, 60, 293–300. [Google Scholar] [CrossRef]
- Regan, E.A.; Mazur, W.; Meoni, E.; Toljamo, T.; Millar, J.; Vuopala, K.; Bowler, R.P.; Rahman, I.; Nicks, M.E.; Crapo, J.D.; et al. Smoking and COPD increase sputum levels of extracellular superoxide dismutase. Free Radic. Biol. Med. 2011, 51, 726–732. [Google Scholar] [CrossRef]
- Yao, H.; Arunachalam, G.; Hwang, J.W.; Chung, S.; Sundar, I.K.; Kinnula, V.L.; Crapo, J.D.; Rahman, I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc. Natl. Acad. Sci. USA 2010, 107, 15571–15576. [Google Scholar] [CrossRef]
- Fratta Pasini, A.M.; Stranieri, C.; Ferrari, M.; Garbin, U.; Cazzoletti, L.; Mozzini, C.; Spelta, F.; Peserico, D.; Cominacini, L. Oxidative stress and Nrf2 expression in peripheral blood mononuclear cells derived from COPD patients: An observational longitudinal study. Respir. Res. 2020, 21, 37. [Google Scholar] [CrossRef]
- Hwang, J.W.; Rajendrasozhan, S.; Yao, H.; Chung, S.; Sundar, I.K.; Huyck, H.L.; Pryhuber, G.S.; Kinnula, V.L.; Rahman, I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J. Immunol. 2011, 187, 987–998. [Google Scholar] [CrossRef]
- Arnau-Soler, A.; Macdonald-Dunlop, E.; Adams, M.J.; Clarke, T.K.; MacIntyre, D.J.; Milburn, K.; Navrady, L.; Hayward, C.; McIntosh, A.M.; Thomson, P.A. Genome-wide by environment interaction studies of depressive symptoms and psychosocial stress in UK Biobank and Generation Scotland. Transl. Psychiatry 2019, 9, 14. [Google Scholar] [CrossRef]
- Ishii, T.; Wakabayashi, R.; Kurosaki, H.; Gemma, A.; Kida, K. Association of serotonin transporter gene variation with smoking, chronic obstructive pulmonary disease, and its depressive symptoms. J. Hum. Genet. 2011, 56, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, A.M.; Kohen, R.; Nguyen, H.Q.; Pike, K.C.; Borson, S.; Fan, V.S. Serotonin transporter gene polymorphisms and depressive symptoms in patients with chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2021, 15, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Chen, P.; Zhang, P.; Yu, J.; Wang, Y.; Chen, N.; Zhang, L.; Wu, H.; Zhao, J. Interaction of Depression and Nicotine Addiction on the Severity of Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study. Iran. J. Public Health 2016, 45, 146–157. [Google Scholar]
- Melro, H.; Gomes, J.; Moura, G.; Marques, A. Genetic profile and patient-reported outcomes in chronic obstructive pulmonary disease: A systematic review. PLoS ONE 2018, 13, e0198920. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Motegi, T.; Kamio, K.; Gemma, A.; Kida, K. Association of group component genetic variations in COPD and COPD exacerbation in a Japanese population. Respirology 2014, 19, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, J.M.; Ha, J.H.; Kang, H.H.; Rhee, C.K.; Kim, J.W.; Moon, H.S.; Baek, K.H.; Lee, S.H. Association between vitamin D receptor polymorphisms and osteoporosis in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1809–1817. [Google Scholar] [CrossRef]
- Chen, X.; Guan, X.J.; Peng, X.H.; Cui, Z.L.; Luan, C.Y.; Guo, X.J. Acetylation of lysine 9 on histone H3 is associated with increased pro-inflammatory cytokine release in a cigarette smoke-induced rat model through HDAC1 depression. Inflamm. Res. 2015, 64, 513–526. [Google Scholar] [CrossRef]
- Monick, M.M.; Beach, S.R.; Plume, J.; Sears, R.; Gerrard, M.; Brody, G.H.; Philibert, R.A. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159, 141–151. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry 2003, 160, 1554–1565. [Google Scholar] [CrossRef]
- Deng, X.; Fu, J.; Song, Y.; Xu, B.; Ji, Z.; Guo, Q.; Ma, S. Glucocorticoid receptor dysfunction orchestrates inflammasome effects on chronic obstructive pulmonary disease-induced depression: A potential mechanism underlying the cross talk between lung and brain. Brain Behav. Immun. 2019, 79, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Pietras, T.; Szemraj, J.; Panek, M.; Witusik, A.; Banasiak, M.; Antczak, A.; Górski, P. Functional polymorphism of cyclooxygenase-2 gene (G–765C) in chronic obstructive pulmonary disease patients. Mol. Biol. Rep. 2012, 39, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Szemraj, J.; Bliźniewska, K.; Maes, M.; Berk, M.; Su, K.P.; Gałecki, P. An immune gate of depression—Early neuroimmune development in the formation of the underlying depressive disorder. Pharm. Rep. 2019, 71, 1299–1307. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Gil, A. Omega 3 fatty acids in cardiovascular disease risk factors: An updated systematic review of randomised clinical trials. Clin. Nutr. 2018, 37, 72–77. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. Eclinicalmedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef]
- Su, K.P.; Lai, H.C.; Yang, H.T.; Su, W.P.; Peng, C.Y.; Chang, J.P.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol. Psychiatry 2014, 76, 559–566. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, T.H.; Huang, K.C.; Huang, C.L.; Chang, H.C.; Pariante, C.M. Omega-3 fatty acids for major depressive disorder during pregnancy: Results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2008, 69, 644–651. [Google Scholar] [CrossRef]
- Chang, J.P.; Chang, S.S.; Yang, H.T.; Chen, H.T.; Chien, Y.C.; Yang, B.; Su, H.; Su, K.P. Omega-3 polyunsaturated fatty acids in cardiovascular diseases comorbid major depressive disorder—Results from a randomized controlled trial. Brain Behav. Immun. 2020, 85, 14–20. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef]
- Stoodley, I.; Garg, M.; Scott, H.; Macdonald-Wicks, L.; Berthon, B.; Wood, L. Higher Omega-3 Index Is Associated with Better Asthma Control and Lower Medication Dose: A Cross-Sectional Study. Nutrients 2019, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Lin, A.H.; Lu, S.H.; Peng, R.Y.; Lee, T.S.; Kou, Y.R. Eicosapentaenoic acid attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Front. Physiol. 2014, 5, 440. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Baines, K.J.; Smart, J.M.; Gibson, P.G.; Wood, L.G. Rosuvastatin, lycopene and omega-3 fatty acids: A potential treatment for systemic inflammation in COPD; a pilot study. J. Nutr. Intermed. Metab. 2016, 5, 86–95. [Google Scholar] [CrossRef]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachexia Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Su, K.-P. A Meta-Analytic Review of Double-Blind, Placebo-Controlled Trials of Antidepressant Efficacy of Omega3 Fatty Acids. J. Clin. Psychiatry 2007, 68, 1056–1061. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Mischoulon, D.; Freeman, M.P.; Matsuoka, Y.; Hibbeln, J.; Belmaker, R.H.; Su, K.-P. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. (letter). Mol. Psychiatry 2012, 17, 1161–1163. [Google Scholar] [CrossRef]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Arakawa, M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: Ryukyus Child Health Study. Pediatrics 2010, 126, e623–e630. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Mamplekou, E.; Pitsavos, C.; Kalogeropoulos, N.; Kastorini, C.M.; Papageorgiou, C.; Papadimitriou, G.N.; Stefanadis, C. Fatty acids intake and depressive symptomatology in a Greek sample: An epidemiological analysis. J. Am. Coll. Nutr. 2010, 29, 586–594. [Google Scholar] [CrossRef]
- Maes, M.; Smith, R.; Christophe, A.; Cosyns, P.; Desnyder, R.; Meltzer, H. Fatty acid composition in major depression: Decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. J. Affect. Disord. 1996, 38, 35–46. [Google Scholar] [CrossRef]
- Adams, P.B.; Lawson, S.; Sanigorski, A.; Sinclair, A.J. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids 1996, 31, S157–S161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, L.; Pan, D.; Xu, D.; Lu, Y.; Yin, S.; Wang, S.; Xia, H.; Liao, W.; Sun, G. Effect of High Ratio of n-6/n-3 PUFAs on Depression: A Meta-Analysis of Prospective Studies. Front. Nutr. 2022, 9, 1026. [Google Scholar] [CrossRef] [PubMed]
- Conklin, S.M.; Gianaros, P.J.; Brown, S.M.; Yao, J.K.; Hariri, A.R.; Manuck, S.B.; Muldoon, M.F. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci. Lett. 2007, 421, 209–212. [Google Scholar] [CrossRef]
- Pelgrim, C.E.; Peterson, J.D.; Gosker, H.R.; Schols, A.; van Helvoort, A.; Garssen, J.; Folkerts, G.; Kraneveld, A.D. Psychological co-morbidities in COPD: Targeting systemic inflammation, a benefit for both? Eur. J. Pharm. 2019, 842, 99–110. [Google Scholar] [CrossRef]
- Peiffer, G.; Underner, M.; Perriot, J.; Fond, G. COPD, anxiety-depression and cognitive disorders: Does inflammation play a major role? Rev. Mal. Respir. 2021, 38, 357–371. [Google Scholar] [CrossRef]
- de Batlle, J.; Sauleda, J.; Balcells, E.; Gómez, F.P.; Méndez, M.; Rodriguez, E.; Barreiro, E.; Ferrer, J.J.; Romieu, I.; Gea, J.; et al. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J. Nutr. Biochem. 2012, 23, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Takahashi, H.; Kasai, C.; Kiyokawa, N.; Watanabe, T.; Fujii, S.; Kashiwagura, T.; Honma, M.; Satake, M.; Shioya, T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir. Med. 2010, 104, 1883–1889. [Google Scholar] [CrossRef]
- Yu, H.; Su, X.; Lei, T.; Zhang, C.; Zhang, M.; Wang, Y.; Zhu, L.; Liu, J. Effect of Omega-3 Fatty Acids on Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 2677–2686. [Google Scholar] [CrossRef]
- Berthon, B.S.; Wood, L.G. Nutrition and respiratory health—feature review. Nutrients 2015, 7, 1618–1643. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592s–599s. [Google Scholar] [CrossRef]
- Cesario, A.; Rocca, B.; Rutella, S. The Interplay between Indoleamine 2,3-Dioxygenase 1 (IDO1) and Cyclooxygenase (COX)-2 In Chronic Inflammation and Cancer. Curr. Med. Chem. 2011, 18, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. Cell survival matters: Docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Ertley, R.; Lee, H.; DeMar, J.; Arnold, J.; Rapoport, S.; Bazinet, R. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol. Psychiatry 2007, 12, 36–46. [Google Scholar] [CrossRef]
- Beltz, B.S.; Tlusty, M.F.; Benton, J.L.; Sandeman, D.C. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci. Lett. 2007, 415, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: A road to remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef]
- Castrén, E.; Hen, R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- van der Does, A.M.; Heijink, M.; Mayboroda, O.A.; Persson, L.J.; Aanerud, M.; Bakke, P.; Eagan, T.M.; Hiemstra, P.S.; Giera, M. Dynamic differences in dietary polyunsaturated fatty acid metabolism in sputum of COPD patients and controls. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 224–233. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Wyatt, T.A.; Poole, J.A.; LeVan, T.D.; Cerutis, D.R.; Romberger, D.J. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir. Res. 2013, 14, 51. [Google Scholar] [CrossRef]
- Katrenčíková, B.; Vaváková, M.; Paduchová, Z.; Nagyová, Z.; Garaiova, I.; Muchová, J.; Ďuračková, Z.; Trebatická, J. Oxidative Stress Markers and Antioxidant Enzymes in Children and Adolescents with Depressive Disorder and Impact of Omega-3 Fatty Acids in Randomised Clinical Trial. Antioxidants 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Bigornia, S.J.; Harris, W.S.; Falcón, L.M.; Ordovás, J.M.; Lai, C.Q.; Tucker, K.L. The Omega-3 Index Is Inversely Associated with Depressive Symptoms among Individuals with Elevated Oxidative Stress Biomarkers. J. Nutr. 2016, 146, 758–766. [Google Scholar] [CrossRef]
- Khajehnasiri, F.; Akhondzadeh, S.; Mortazavi, S.B.; Allameh, A.; Sotoudeh, G.; Khavanin, A.; Zamanian, Z. Are Supplementation of Omega-3 and Ascorbic Acid Effective in Reducing Oxidative Stress and Depression among Depressed Shift Workers? Int. J. Vitam. Nutr. Res. 2015, 85, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Zgórzyńska, E.; Dziedzic, B.; Gorzkiewicz, A.; Stulczewski, D.; Bielawska, K.; Su, K.P.; Walczewska, A. Omega-3 polyunsaturated fatty acids improve the antioxidative defense in rat astrocytes via an Nrf2-dependent mechanism. Pharm. Rep. 2017, 69, 935–942. [Google Scholar] [CrossRef] [PubMed]
- van de Bool, C.; Rutten, E.P.A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A. A randomized clinical trial investigating the efficacy of targeted nutrition as an adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef]



| Fatty Acid | Important Sources |
|---|---|
| ALA | flaxseed, canola, and soybean |
| EPA | Fish and fish oils |
| DHA | Fish oil and brown algae |
| LA | Corn, safflower, sunflower |
| ALA | Dairy products, eggs, and meats |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zailani, H.; Satyanarayanan, S.K.; Liao, W.-C.; Liao, H.-F.; Huang, S.-Y.; Gałecki, P.; Su, K.-P.; Chang, J.P.-C. Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. J. Clin. Med. 2023, 12, 2653. https://doi.org/10.3390/jcm12072653
Zailani H, Satyanarayanan SK, Liao W-C, Liao H-F, Huang S-Y, Gałecki P, Su K-P, Chang JP-C. Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. Journal of Clinical Medicine. 2023; 12(7):2653. https://doi.org/10.3390/jcm12072653
Chicago/Turabian StyleZailani, Halliru, Senthil Kumaran Satyanarayanan, Wei-Chih Liao, Hsien-Feng Liao, Shih-Yi Huang, Piotr Gałecki, Kuan-Pin Su, and Jane Pei-Chen Chang. 2023. "Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review" Journal of Clinical Medicine 12, no. 7: 2653. https://doi.org/10.3390/jcm12072653
APA StyleZailani, H., Satyanarayanan, S. K., Liao, W.-C., Liao, H.-F., Huang, S.-Y., Gałecki, P., Su, K.-P., & Chang, J. P.-C. (2023). Omega-3 Polyunsaturated Fatty Acids in Managing Comorbid Mood Disorders in Chronic Obstructive Pulmonary Disease (COPD): A Review. Journal of Clinical Medicine, 12(7), 2653. https://doi.org/10.3390/jcm12072653











