Role of Lower Esophageal Squamous Cell Carcinoma Margin Location on Abdominal Lymph Node Metastasis Risk
Abstract
1. Introduction
2. Method
2.1. Lymph Node Grouping
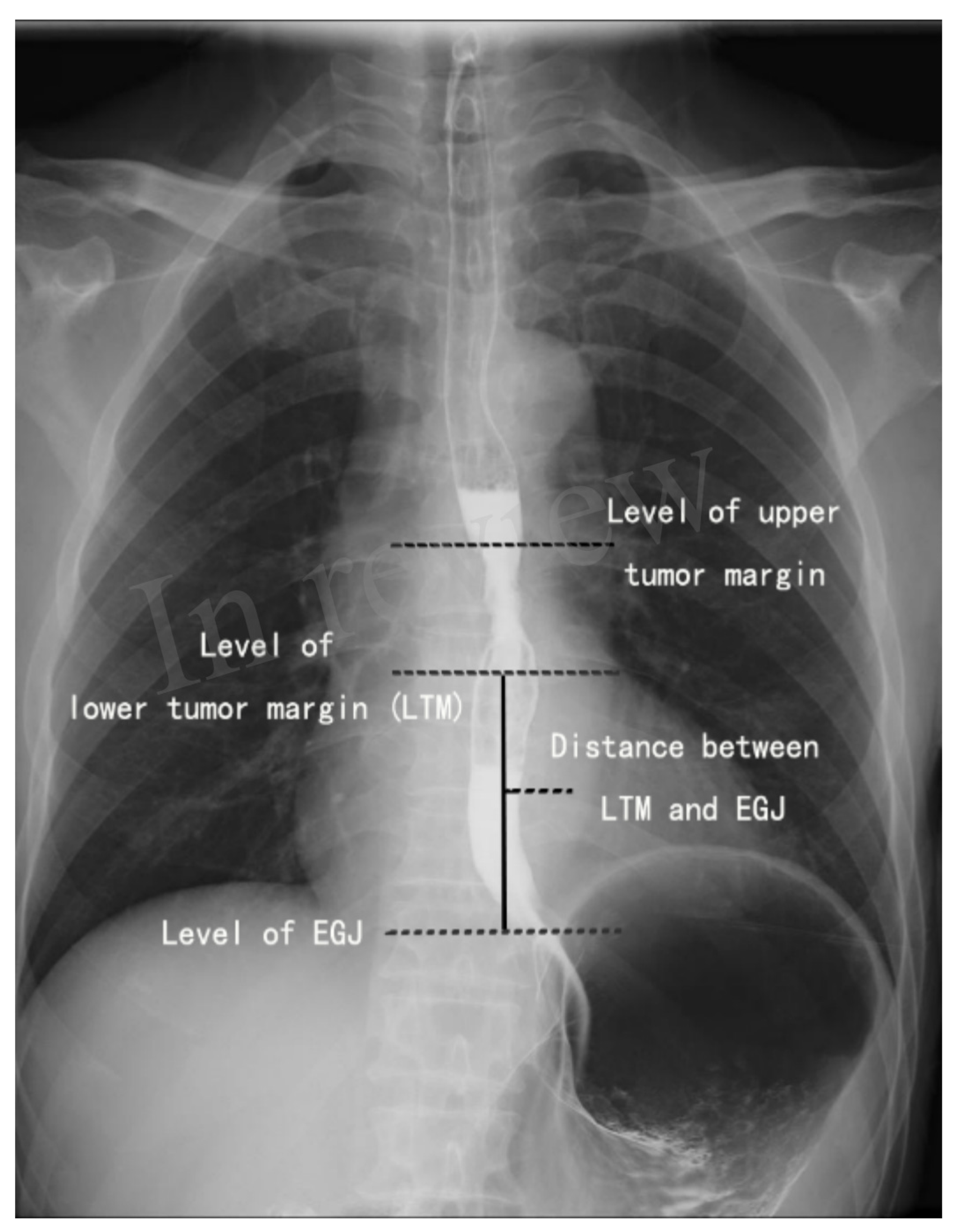
2.2. Localization of the Lower Tumor Margin on the Barium Esophagogram
2.3. Statistical Analysis
3. Result
3.1. Risk Factor for Abdominal LNM in Middle Thoracic ESCC
3.2. Risk Factor for Abdominal LNM in Lower Thoracic ESCC
3.3. Comparison of Abdominal LNM between Middle Thoracic ESCC with LED Less Than 10 cm and Lower Thoracic ESCC without EGJ Invasion
3.4. Abdomen LNM Risk Stratification for ESCC according to LTM
3.5. Abdominal LNM Prediction Model for ESCC
3.6. Long-Term Survival and Abdominal LNM in ESCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Di Pardo, B.J.; Bronson, N.W.; Diggs, B.S.; Thomas, C.R.; Hunter, J.G.; Dolan, J.P. The Global Burden of Esophageal Cancer: A Disability-Adjusted Life-Year Approach. World J. Surg. 2016, 40, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef] [PubMed]
- van Rijswijk, A.S.; Hagens, E.R.C.; van der Peet, D.L.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Differences in Esophageal Cancer Surgery in Terms of Surgical Approach and Extent of Lymphadenectomy: Findings of an International Survey. Ann. Surg. Oncol. 2019, 26, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Lu, L.; Fan, J.; Wang, S.; Chen, X. Lymph node metastatic patterns and its clinical significance for thoracic superficial esophageal squamous cell carcinoma. J. Cardiothorac. Surg. 2020, 15, 262. [Google Scholar] [CrossRef]
- Tachimori, Y.; Tachimori, Y.; Ozawa, S.; Numasaki, H.; Matsubara, H.; Shinoda, M.; Toh, Y. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Rice, T.W.; Blackstone, E.H.; Rusch, V.W. 7th edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann. Surg. Oncol. 2010, 17, 1721–1724. [Google Scholar] [CrossRef]
- Rice, T.W.; Ishwaran, H.; Hofstetter, W.L.; Kelsen, D.P.; Apperson-Hansen, C.; Blackstone, E.H.; Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis. Esophagus 2016, 29, 897–905. [Google Scholar] [CrossRef]
- Tachibana, M.; Kinugasa, S.; Hirahara, N.; Yoshimura, H. Lymph node classification of esophageal squamous cell carcinoma and adenocarcinoma. Eur. J. Cardiothorac. Surg. 2008, 34, 427–431. [Google Scholar] [CrossRef]
- Ueda, Y.; Shiozaki, A.; Itoi, H.; Okamoto, K.; Fujiwara, H.; Ichikawa, D. The range of tumor extension should have precedence over the location of the deepest tumor center in determining the regional lymph node grouping for widely extending esophageal carcinomas. Jpn J. Clin. Oncol. 2006, 36, 775–782. [Google Scholar] [CrossRef]
- Castoro, C.; Scarpa, M.; Cagol, M.; Ruol, A.; Cavallin, F.; Alfieri, R.; Zanchettin, G.; Rugge, M.; Ancona, E. Nodal metastasis from locally advanced esophageal cancer: How neoadjuvant therapy modifies their frequency and distribution. Ann. Surg. Oncol. 2011, 18, 3743–3754. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Xia, W.; Wang, F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag. Res. 2018, 10, 6295–6303. [Google Scholar] [CrossRef] [PubMed]
- Kumakura, Y.; Yokobori, T.; Yoshida, T.; Hara, K.; Sakai, M.; Sohda, M.; Miyazaki, T.; Yokoo, H.; Handa, T.; Oyama, T.; et al. Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2018, 25, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, S.; Pan, J.; Zheng, X.; Zhu, K.; Zhu, J.; Xiao, J.; Ying, M. The pattern and prevalence of lymphatic spread in thoracic esophageal squamous cell carcinoma. Eur. J. Cardiothorac. Surg. 2009, 36, 480–486. [Google Scholar] [CrossRef]
- Hagens, E.R.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Distribution of Lymph Node Metastases in Esophageal Carcinoma Patients Undergoing Upfront Surgery: A Systematic Review. Cancers 2020, 12, 1592. [Google Scholar] [CrossRef]
- Shiomi, S.; Yajima, S.; Yoshimura, S.; Urabe, M.; Ri, M.; Okumura, Y.; Yagi, K.; Aikou, S.; Nomura, S.; Seto, Y. Optimal criteria for predicting lymph node metastasis in esophageal squamous cell carcinoma by anatomical location using preoperative computed tomography: A retrospective cohort study. Surg. Today 2022, 52, 1185–1193. [Google Scholar] [CrossRef]
- He, H.H.; Hao, Z.; Li, Z.; Cheng, F.; Fu, J.; Wang, W.; He, J.; Luo, J.; He, J. Significance of the dissection of common hepatic arterial lymph nodes in patients with oesophageal carcinoma: A multicentre retrospective study. BMJ Open 2022, 12, e050280. [Google Scholar] [CrossRef]
- Deng, X.M.; Zhu, T.Y.; Wang, G.J.; Gao, B.L.; Wang, J.T.; Li, R.X.; Zhang, Y.F.; Ding, H.X. Lymph node metastasis pattern and significance of left gastric artery lymph node dissection in esophagectomy for esophageal cancers. World J. Surg. Oncol. 2021, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Zhuo, Z.G.; Song, T.N.; Alai, G.H.; Shen, X.; Yao, P.; Lin, Y.D. Role of nodal skip metastasis in patients with mid-thoracic oesophageal squamous cell carcinoma: A propensity score matching study. Eur. J. Cardiothorac. Surg. 2021, 59, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ge, X.; Wang, Z.; Weng, Y.; Yin, R.; You, Q. Clinical significance and prognosis of supraclavicular lymph node metastasis in patients with thoracic esophageal cancer. Ann. Transl. Med. 2020, 8, 90. [Google Scholar] [CrossRef]
- Shang, Q.X.; Yang, Y.S.; Xu, L.Y.; Yang, H.; Li, Y.; Li, Y.; Wu, Z.Y.; Fu, J.H.; Yao, X.D.; Xu, X.E.; et al. Prognostic Role of Nodal Skip Metastasis in Thoracic Esophageal Squamous Cell Carcinoma: A Large-Scale Multicenter Study. Ann. Surg. Oncol. 2021, 28, 6341–6352. [Google Scholar] [CrossRef]
- Harada, K.; Hwang, H.; Wang, X.; Abdelhakeem, A.; Iwatsuki, M.; Blum Murphy, M.A.; Maru, D.M.; Weston, B.; Lee, J.H.; Rogers, J.E.; et al. Frequency and Implications of Paratracheal Lymph Node Metastases in Resectable Esophageal or Gastroesophageal Junction Adenocarcinoma. Ann. Surg. 2021, 273, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yano, M.; Motoori, M.; Doki, Y.; Kishi, K.; Miyashiro, I.; Shingai, T.; Gotoh, K.; Noura, S.; Takahashi, H.; et al. The significance of abdominal para-aortic lymph node metastasis in patients with lower thoracic esophageal cancer. Dis. Esophagus 2012, 25, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, M.; Ben Safta, Y.; Kbir, G.H.; Mabrouk, A.; Ben Dhaou, A.; Daldoul, S.; Sayari, S.; Haouet, K.; Ben Moussa, M. Can we predict mediastinal lymph nodes metastasis in esophagogastric junction cancer? Results of a systematic review and meta-analysis. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, O.; Yasuda, T.; Kato, H.; Iwama, M.; Hiraki, Y.; Yasuda, A.; Shinkai, M.; Kimura, Y.; Imano, M. Risk Factors and Prognostic Impact of Mediastinal Lymph Node Metastases in Patients with Esophagogastric Junction Cancer. Ann. Surg. Oncol. 2020, 27, 4433–4440. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Noma, K.; Matsuda, T.; Maeda, N.; Tanabe, S.; Sakurama, K.; Shirakawa, Y.; Fujiwara, T. Risk factor of mediastinal lymph node metastasis of Siewert type I and II esophagogastric junction carcinomas. Langenbecks Arch. Surg. 2020, 405, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
| Variable | Middle ESCC with Abdomen LNM | Middle ESCC without LNM | p Value |
|---|---|---|---|
| Sex | 0.186 | ||
| Male | 115 (80.4%) | 203 (74.6%) | |
| Female | 28 (19.6%) | 69 (25.4%) | |
| Age (years) | 61.99 ± 8.05 | 61.88 ± 8.01 | 0.897 |
| BMI (kg/m2) | 21.80 ± 2.63 | 22.33 ± 3.07 | 0.079 |
| Preoperative dysphagia duration (month) | 3.35 ± 2.88 | 3.22 ± 3.69 | 0.716 |
| Smoking | 0.193 | ||
| Yes | 94 (65.7%) | 161 (59.2%) | |
| No | 49 (34.3%) | 111 (40.8%) | |
| Drinking | 0.228 | ||
| Yes | 83 (58.0%) | 141 (51.8%) | |
| No | 60 (42.0%) | 131 (48.2%) | |
| Diabetes | 0.404 | ||
| Yes | 7 (4.9%) | 19 (7.0%) | |
| No | 136 (95.1%) | 253 (93.0%) | |
| Hypertension | 0.197 | ||
| Yes | 22 (15.4%) | 56 (20.6%) | |
| No | 121 (84.6%) | 216 (79.4%) | |
| Differentiation grade | <0.001 | ||
| High | 2 (1.4%) | 15 (5.5%) | |
| Moderate | 58 (40.6%) | 155 (57.0%) | |
| Low | 83 (58.0%) | 102 (37.5%) | |
| p-T stage | <0.001 | ||
| Tis | 0 (0.0%) | 6 (2.2%) | |
| T1 | 8 (5.6%) | 53 (19.5%) | |
| T2 | 24 (16.8%) | 64 (23.5%) | |
| T3 | 92 (64.3%) | 129 (47.4%) | |
| T4a | 19 (13.3%) | 20 (7.4%) | |
| LED (cm) | 7.55 ± 2.33 | 8.21 ± 2.65 | 0.012 |
| Variable | Wald c2 Value | OR | 95% CI | p Value |
|---|---|---|---|---|
| LED < 10 cm | 0.006 | |||
| No | ref | |||
| Yes | 7.574 | 2.250 | 1.263–4.007 | |
| BMI ≥ 24 kg/m2 | 0.276 | |||
| No | ref | |||
| Yes | 1.184 | 0.758 | 0.460–1.248 | |
| Differentiation grade | <0.001 | |||
| Moderate-high | Ref | |||
| Low | 15.466 | 2.372 | 1.542–3.647 | |
| p-T stage | <0.001 | |||
| Tis-T2 | Ref | |||
| T3-T4a | 18.122 | 2.807 | 1.745–4.515 |
| Variable | LED < 10 cm | LED > 10 cm | p Value |
|---|---|---|---|
| Sex | 0.027 | ||
| Male | 290 (75.3%) | 106 (84.8%) | |
| Female | 95 (24.7%) | 19 (15.2%) | |
| Age (years) | 61.79 ± 8.08 | 61.99 ± 8.29 | 0.812 |
| BMI (kg/m2) | 22.22 ± 2.98 | 22.31 ± 3.28 | 0.795 |
| Smoking | 0.388 | ||
| Yes | 236 (61.3%) | 82 (65.6%) | |
| No | 149 (38.7%) | 43 (34.4%) | |
| Drinking | 0.003 | ||
| Yes | 201 (52.2%) | 84 (67.2%) | |
| No | 184 (47.8%) | 41 (32.8%) | |
| Diabetes | 0.777 | ||
| Yes | 22 (5.7%) | 8 (6.4%) | |
| No | 363 (94.3%) | 117 (93.6%) | |
| Hypertension | 0.395 | ||
| Yes | 78 (20.3%) | 21 (16.8%) | |
| No | 307 (79.7%) | 104 (83.2%) | |
| differentiation | 0.869 | ||
| High | 12 (3.1%) | 5 (4.0%) | |
| Moderate | 191 (49.6%) | 60 (48.0%) | |
| Low | 182 (47.3%) | 60 (48.0%) | |
| p-T stage | 0.015 | ||
| Tis | 3 (0.8%) | 3 (2.4%) | |
| T1 | 40 (10.4%) | 25 (20.0%) | |
| T2 | 80 (20.8%) | 26 (20.8%) | |
| T3 | 215 (55.8%) | 63 (50.4%) | |
| T4a | 47 (12.2%) | 8 (6.4%) | |
| p-N stage | 0.337 | ||
| N0 | 198 (51.4%) | 74 (59.2%) | |
| N1 | 99 (25.7%) | 31 (24.8%) | |
| N2 | 66 (17.1%) | 16 (12.8%) | |
| N3 | 22 (5.7%) | 4 (3.2%) | |
| Abdomen LNM | <0.001 | ||
| Yes | 124 (32.2%) | 19 (15.2%) | |
| No | 261 (67.8%) | 106 (84.8%) |
| Variable | Lower ESCC with Abdomen LNM | Lower ESCC without LNM | p Value |
|---|---|---|---|
| Sex | 0.292 | ||
| Male | 93 (82.3%) | 109 (87.2%) | |
| Female | 20 (17.7%) | 16 (12.8%) | |
| Age (year) | 60.96 ± 9.67 | 62.87 ± 7.80 | 0.097 |
| BMI (kg/m2) | 22.08 ± 2.95 | 22.80 ± 2.99 | 0.062 |
| Smoking | 0.837 | ||
| Yes | 80 (70.8%) | 90 (72.0%) | |
| No | 33 (29.2%) | 35 (28.0%) | |
| Drinking | 0.214 | ||
| Yes | 80 (70.8%) | 79 (63.2%) | |
| No | 33 (29.2%) | 46 (36.8%) | |
| Diabetes | 0.418 | ||
| Yes | 6 (5.3%) | 4 (3.2%) | |
| No | 107 (94.7%) | 121 (96.8%) | |
| Hypertension | 0.878 | ||
| Yes | 29 (25.7%) | 31 (24.8%) | |
| No | 84 (74.3%) | 94 (75.2%) | |
| Differentiation grade | 0.009 | ||
| High | 0 (0.0%) | 10 (8.0%) | |
| Moderate | 59 (52.2%) | 62 (49.6%) | |
| Low | 54 (47.8%) | 53 (42.4%) | |
| p-T stage | <0.001 | ||
| Tis | 0 (0.0%) | 3 (2.4%) | |
| T1 | 4 (3.5%) | 24 (19.2%) | |
| T2 | 21 (18.6%) | 30 (24.0%) | |
| T3 | 83 (73.5%) | 67 (53.6%) | |
| T4a | 5 (4.4%) | 1 (0.8%) | |
| LED (cm) | 2.17 ± 2.32 | 2.79 ± 2.33 | 0.041 |
| LED = 0 cm | 0.001 | ||
| Yes | 47 (41.6%) | 28 (22.4%) | |
| No | 66 (58.4%) | 97 (77.6%) |
| Variable | Wald c2 Value | OR | 95% CI | p Value |
|---|---|---|---|---|
| LED = 0 cm | 0.004 | |||
| No | Ref | |||
| Yes | 8.143 | 2.326 | 1.303–4.155 | |
| Age | 0.339 | |||
| ≤65 | ref | |||
| >65 | 0.915 | 0.764 | 0.441–1.326 | |
| BMI (kg/m2) | 0.898 | |||
| <24 | ref | |||
| ≥24 | 0.016 | 0.961 | 0.525–1.761 | |
| Differentiation grade | 0.491 | |||
| Low | Ref | |||
| Moderate-high | 0.473 | 0.828 | 0.483–1.419 | |
| p-T stage | <0.001 | |||
| Tis-T2 | Ref | |||
| T3-T4a | 12.479 | 2.850 | 1.594–5.097 |
| Variable | Middle ESCC with LED < 10 cm | Lower ESCC with LED > 10 cm | p Value |
|---|---|---|---|
| Sex | 0.055 | ||
| Male | 290 (75.3%) | 155 (82.4%) | |
| Female | 95 (24.7%) | 33 (17.6%) | |
| Age (year) | 61.79 ± 8.08 | 61.98 ± 8.54 | 0.799 |
| BMI (kg/m2) | 22.39 ± 2.94 | 22.22 ± 2.98 | 0.527 |
| Smoking | 0.027 | ||
| Yes | 236 (61.3%) | 133 (70.7%) | |
| No | 149 (38.7%) | 55 (29.3%) | |
| Drinking | 0.003 | ||
| Yes | 201 (52.2%) | 123 (65.4%) | |
| No | 184 (47.8%) | 65 (34.6%) | |
| Diabetes | 0.307 | ||
| Yes | 22 (5.7%) | 7 (3.7%) | |
| No | 363 (94.3%) | 181 (96.3%) | |
| Hypertension | 0.388 | ||
| Yes | 78 (20.3%) | 44 (23.4%) | |
| No | 307 (79.7%) | 144 (76.6%) | |
| Differentiation grade | 0.780 | ||
| High | 12 (3.1%) | 8 (4.3%) | |
| Moderate | 191 (49.6%) | 93 (49.5%) | |
| Low | 182 (47.3%) | 87 (46.3%) | |
| p-T stage | 0.826 | ||
| Tis | 3 (0.8%) | 3 (1.6%) | |
| T1 | 40 (10.4%) | 26 (13.8%) | |
| T2 | 80 (20.8%) | 38 (20.2%) | |
| T3 | 215 (55.8%) | 114 (60.6%) | |
| T4a | 47 (12.2%) | 7 (3.7%) | |
| p-N stage | 0.015 | ||
| N0 | 198 (51.4%) | 97 (51.6%) | |
| N1 | 99 (25.7%) | 53 (28.2%) | |
| N2 | 66 (17.1%) | 27 (14.4%) | |
| N3 | 22 (5.7%) | 11 (5.9%) | |
| Abdomen LNM | 0.489 | ||
| Yes | 124 (32.2%) | 66 (35.1%) | |
| No | 261 (67.8%) | 122 (64.9%) |
| Location of Lower Tumor Margin | Abdomen LNM Rate | Abdomen LNM Risk |
|---|---|---|
| Upper esophagus | 2.9% | Very low |
| Middle esophagus with LED more than 10 cm | 15.2% | Low |
| Middle esophagus with LED less than 10 cm | 32.2% | |
| Lower esophagus without invading EGJ | 35.1% | Moderate |
| Lower esophagus with invading EGJ | 60.3% | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Tu, X.-H.; A-Lai, G.-H.; Zhuo, Z.-G.; Yao, P.; Zhang, Y.; Xu, Z.-J.; Lin, Y.-D. Role of Lower Esophageal Squamous Cell Carcinoma Margin Location on Abdominal Lymph Node Metastasis Risk. J. Clin. Med. 2023, 12, 2657. https://doi.org/10.3390/jcm12072657
Zhong X, Tu X-H, A-Lai G-H, Zhuo Z-G, Yao P, Zhang Y, Xu Z-J, Lin Y-D. Role of Lower Esophageal Squamous Cell Carcinoma Margin Location on Abdominal Lymph Node Metastasis Risk. Journal of Clinical Medicine. 2023; 12(7):2657. https://doi.org/10.3390/jcm12072657
Chicago/Turabian StyleZhong, Xia, Xue-Hua Tu, Gu-Ha A-Lai, Ze-Guo Zhuo, Peng Yao, Ying Zhang, Zhi-Jie Xu, and Yi-Dan Lin. 2023. "Role of Lower Esophageal Squamous Cell Carcinoma Margin Location on Abdominal Lymph Node Metastasis Risk" Journal of Clinical Medicine 12, no. 7: 2657. https://doi.org/10.3390/jcm12072657
APA StyleZhong, X., Tu, X.-H., A-Lai, G.-H., Zhuo, Z.-G., Yao, P., Zhang, Y., Xu, Z.-J., & Lin, Y.-D. (2023). Role of Lower Esophageal Squamous Cell Carcinoma Margin Location on Abdominal Lymph Node Metastasis Risk. Journal of Clinical Medicine, 12(7), 2657. https://doi.org/10.3390/jcm12072657






