Fifteen-Year Nationwide Trend in Antiplatelet Treatment among Drug-Eluting Stent Recipients in Korea: Many Patients Receive Very Prolonged Dual-Antiplatelet Treatment, and Newer Drugs Are Replacing the Older Ones
Abstract
:1. Introduction
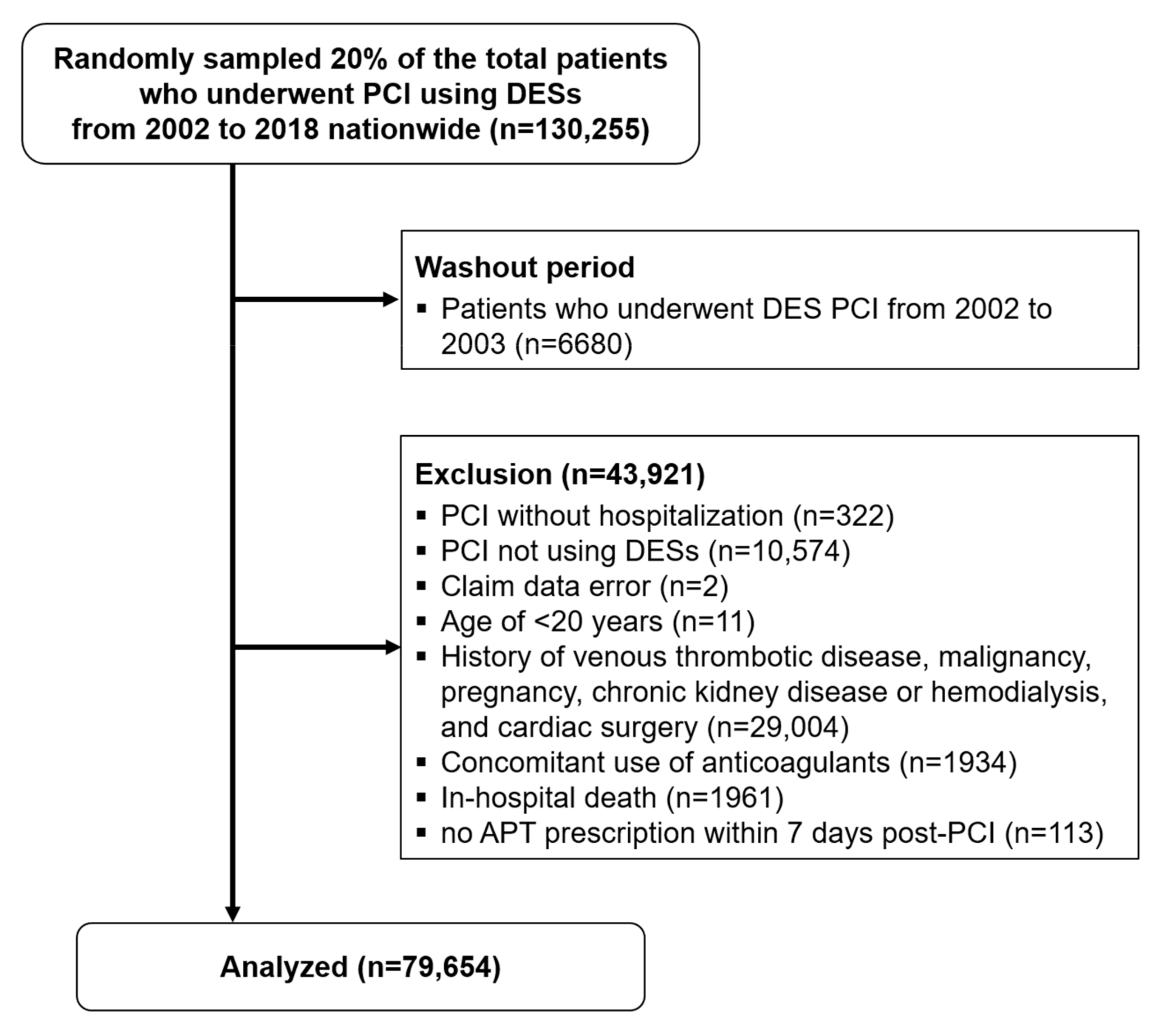
2. Materials and Methods
2.1. Study Population and Data Sources
2.2. APT Patterns and 15-Year Temporal Trends
2.3. Statistical Methods
3. Results
3.1. Study Population
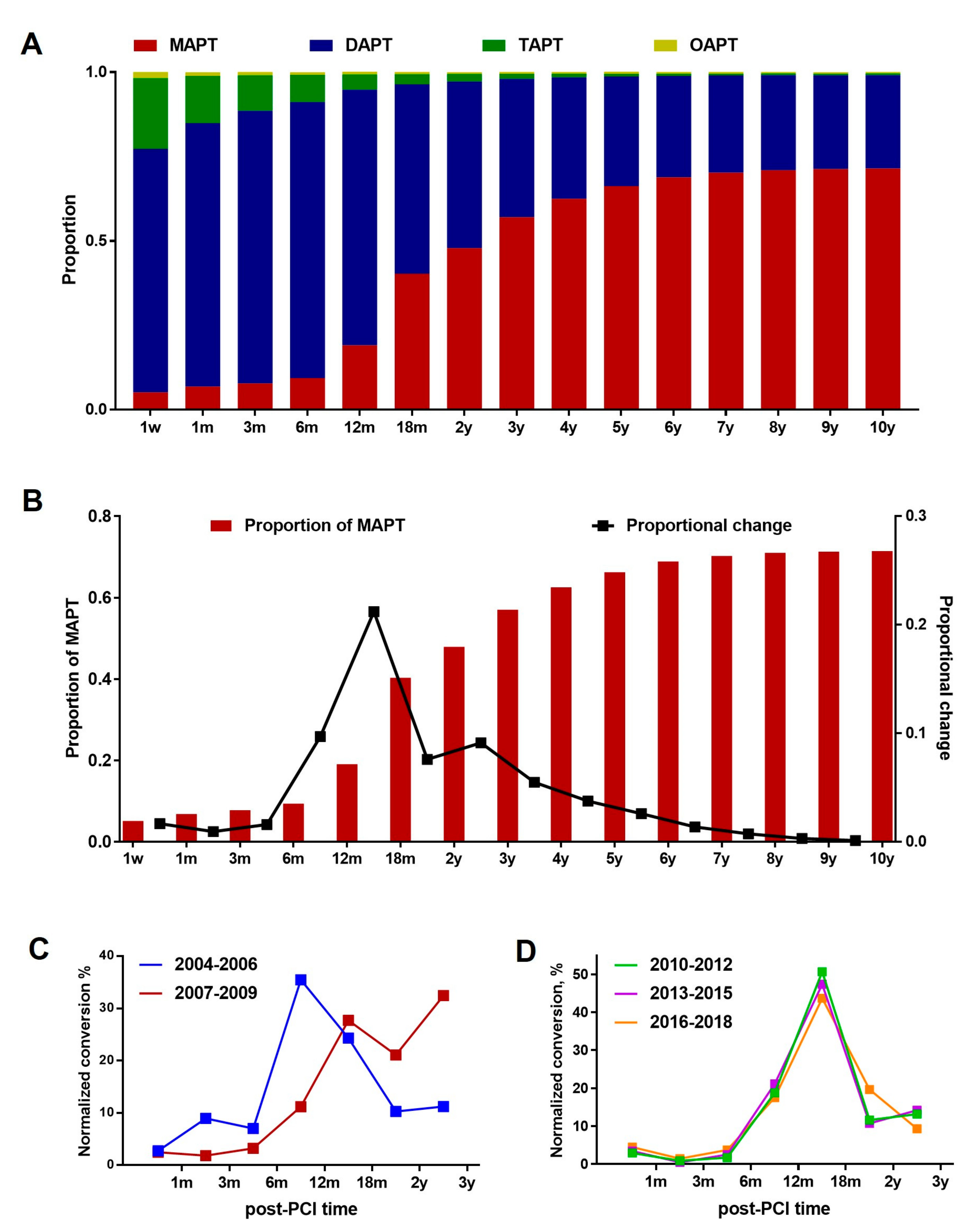
3.2. Overall APT Prescription Patterns: MAPT vs. DAPT vs. TAPT
3.3. DAPT-to-MAPT Conversion
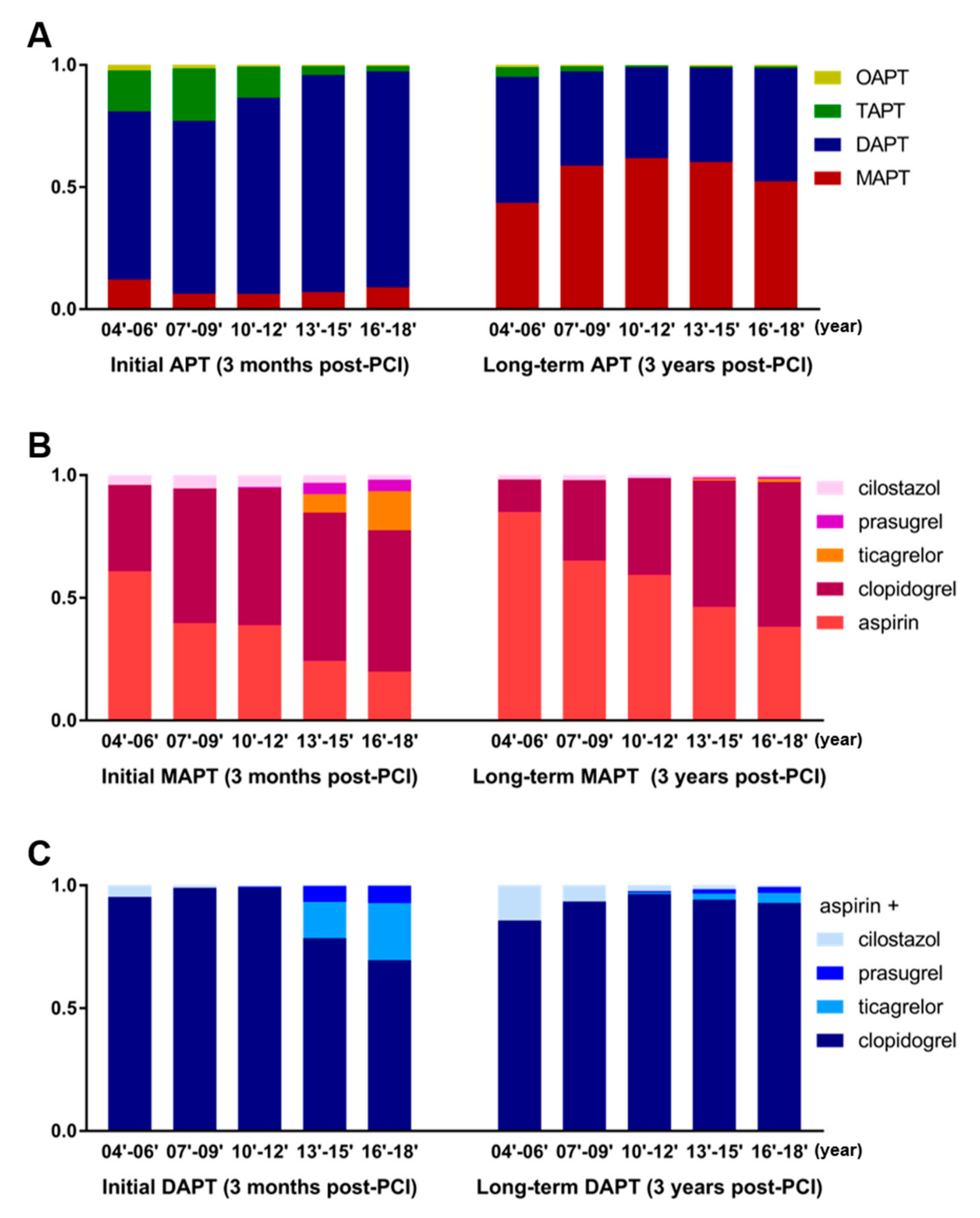
3.4. Preferred Antiplatelet Agents for MAPT and Preferred Combination for DAPT
3.5. Changes in the APT Strategy during the Past 15 Years
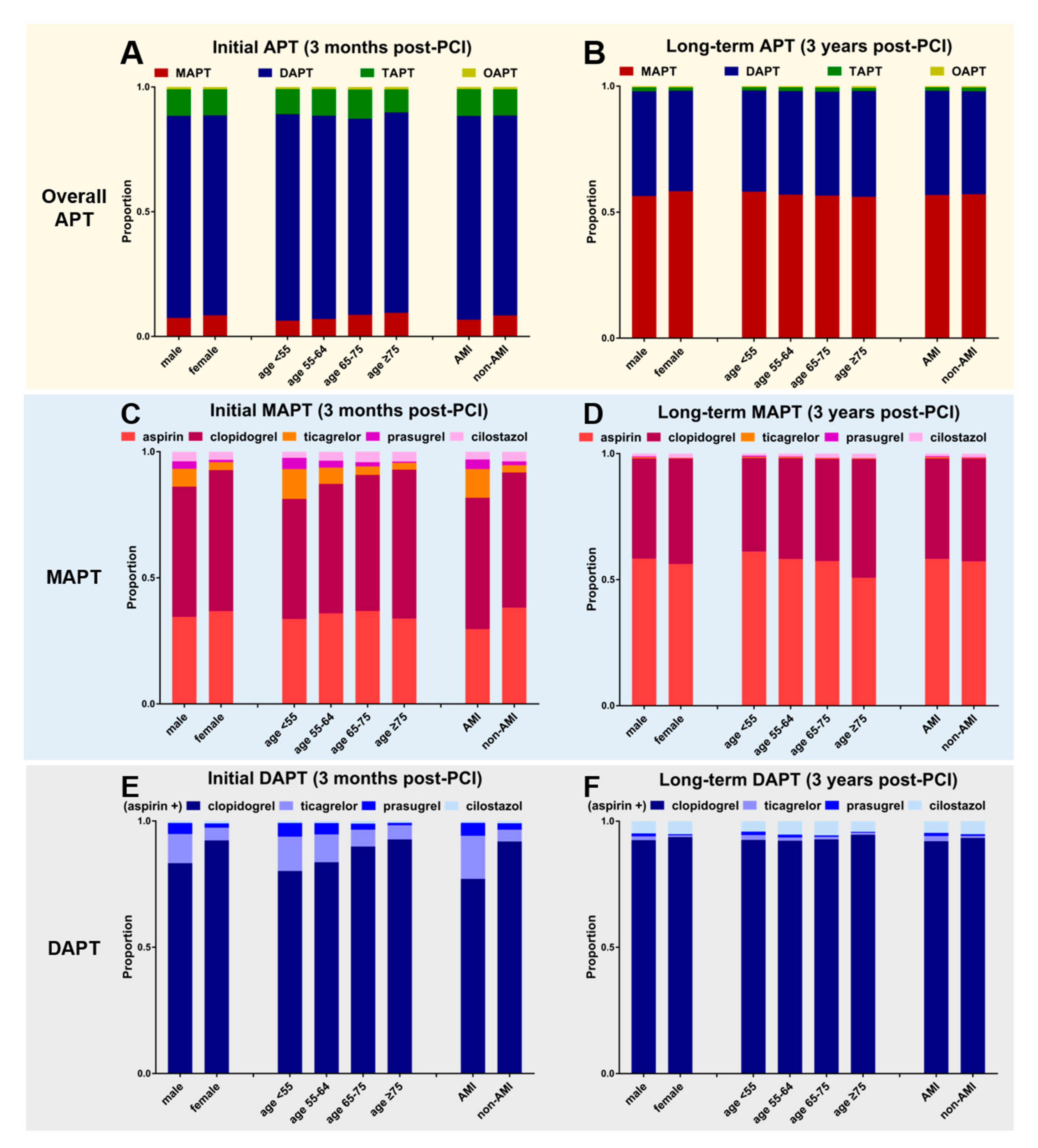
3.6. Drug Preference and APT Strategy According to the Patients’ Clinical Characteristics
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, S.; Bourantas, C.; Serruys, P.W. New concepts in the design of drug-eluting coronary stents. Nat. Rev. Cardiol. 2013, 10, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jeong, M.H.; Ahn, Y.; Kim, J.H.; Hong, Y.J.; Sim, D.S.; Kim, M.C.; Kim, H.S.; Park, S.J.; Gwon, H.C.; et al. Results of a 10-Year Experience in Korea Using Drug-Eluting Stents During Percutaneous Coronary Intervention for Acute Myocardial Infarction (from the Korea Acute Myocardial Infarction Registry). Am. J. Cardiol. 2018, 122, 365–373. [Google Scholar] [CrossRef]
- Park, S.J.; Kang, S.J.; Virmani, R.; Nakano, M.; Ueda, Y. In-stent neoatherosclerosis: A final common pathway of late stent failure. J. Am. Coll. Cardiol. 2012, 59, 2051–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, T.; Polimeni, A.; Indolfi, C.; Räber, L.; Adriaenssens, T.; Münzel, T. Predictors of stent thrombosis and their implications for clinical practice. Nat. Rev. Cardiol. 2019, 16, 243–256. [Google Scholar] [CrossRef]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016, 134, e123–e155. [Google Scholar]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Juni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar]
- Koo, B.K.; Kang, J.; Park, K.W.; Rhee, T.M.; Yang, H.M.; Won, K.B.; Rha, S.W.; Bae, J.W.; Lee, N.H.; Hur, S.H.; et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): An investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 2021, 397, 2487–2496. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Yasmina, A.; de Boer, A.; Deneer, V.H.; Souverein, P.C.; Klungel, O.H. Patterns of antiplatelet drug use after a first myocardial infarction during a 10-year period. Br. J. Clin. Pharmacol. 2017, 83, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimminiello, C.; Dondi, L.; Pedrini, A.; Ronconi, G.; Calabria, S.; Piccinni, C.; Polo Friz, H.; Martini, N.; Maggioni, A.P. Patterns of treatment with antiplatelet therapy after an acute coronary syndrome: Data from a large database in a community setting. Eur. J. Prev. Cardiol. 2019, 26, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Oh, Y.; Park, S.; Cho, S.; Park, H. Stratified sampling design based on data mining. Healthc. Inform. Res. 2013, 19, 186–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.K. Cardiovascular Research Using the Korean National Health Information Database. Korean Circ. J. 2020, 50, 754–772. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Mehran, R.; Baber, U.; Steg, P.G.; Ariti, C.; Weisz, G.; Witzenbichler, B.; Henry, T.D.; Kini, A.S.; Stuckey, T.; Cohen, D.J.; et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013, 382, 1714–1722. [Google Scholar] [CrossRef]
- Mulukutla, S.R.; Marroquin, O.C.; Vlachos, H.A.; Selzer, F.; Toma, C.; Kip, K.E.; Abbott, J.D.; Holper, E.; Lee, J.S.; Khandhar, S.; et al. Benefit of long-term dual anti-platelet therapy in patients treated with drug-eluting stents: From the NHLBI dynamic registry. Am. J. Cardiol. 2013, 111, 486–492. [Google Scholar] [CrossRef] [Green Version]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.L.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [Green Version]
- Helft, G.; Steg, P.G.; Le Feuvre, C.; Georges, J.L.; Carrie, D.; Dreyfus, X.; Furber, A.; Leclercq, F.; Eltchaninoff, H.; Falquier, J.F.; et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: The OPTIDUAL randomized trial. Eur. Heart J. 2016, 37, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Collet, J.P.; Silvain, J.; Barthelemy, O.; Range, G.; Cayla, G.; Van Belle, E.; Cuisset, T.; Elhadad, S.; Schiele, F.; Lhoest, N.; et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): A randomised trial. Lancet 2014, 384, 1577–1585. [Google Scholar] [CrossRef]
- Lee, C.W.; Ahn, J.M.; Park, D.W.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Park, S.W.; Han, S.; Lee, S.G.; Seong, I.W.; et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: A randomized, controlled trial. Circulation 2014, 129, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmerini, T.; Benedetto, U.; Bacchi-Reggiani, L.; Della Riva, D.; Biondi-Zoccai, G.; Feres, F.; Abizaid, A.; Hong, M.K.; Kim, B.K.; Jang, Y.; et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: A pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015, 385, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Udell, J.A.; Bonaca, M.P.; Collet, J.P.; Lincoff, A.M.; Kereiakes, D.J.; Costa, F.; Lee, C.W.; Mauri, L.; Valgimigli, M.; Park, S.J.; et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: A collaborative meta-analysis of randomized trials. Eur. Heart J. 2016, 37, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.H.; Xu, P.; Wang, B.; Lu, Y.; Wu, Q.Y.; Zhou, M.L.; Wu, J.R.; Cai, J.J.; Sun, X.; Yuan, H. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: Systematic review and network meta-analysis. BMJ 2019, 365, l2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butala, N.M.; Faridi, K.F.; Tamez, H.; Strom, J.B.; Song, Y.; Shen, C.; Secemsky, E.A.; Mauri, L.; Kereiakes, D.J.; Curtis, J.P.; et al. Estimation of DAPT Study Treatment Effects in Contemporary Clinical Practice: Findings From the EXTEND-DAPT Study. Circulation 2022, 145, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kazi, D.S.; Leong, T.K.; Chang, T.I.; Solomon, M.D.; Hlatky, M.A.; Go, A.S. Association of spontaneous bleeding and myocardial infarction with long-term mortality after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2015, 65, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Baber, U.; Dangas, G.; Chandrasekhar, J.; Sartori, S.; Steg, P.G.; Cohen, D.J.; Giustino, G.; Ariti, C.; Witzenbichler, B.; Henry, T.D. Time-dependent associations between actionable bleeding, coronary thrombotic events, and mortality following percutaneous coronary intervention: Results from the PARIS registry. JACC: Cardiovasc. Interv. 2016, 9, 1349–1357. [Google Scholar] [CrossRef]
- Gragnano, F.; Mehran, R.; Branca, M.; Franzone, A.; Baber, U.; Jang, Y.; Kimura, T.; Hahn, J.Y.; Zhao, Q.; Windecker, S.; et al. P2Y(12) Inhibitor Monotherapy or Dual Antiplatelet Therapy After Complex Percutaneous Coronary Interventions. J. Am. Coll. Cardiol. 2023, 81, 537–552. [Google Scholar] [CrossRef]
- Yeh, R.W.; Secemsky, E.A.; Kereiakes, D.J.; Normand, S.L.; Gershlick, A.H.; Cohen, D.J.; Spertus, J.A.; Steg, P.G.; Cutlip, D.E.; Rinaldi, M.J.; et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA 2016, 315, 1735–1749. [Google Scholar] [CrossRef] [Green Version]
- D’Ascenzo, F.; De Filippo, O.; Gallone, G.; Mittone, G.; Deriu, M.A.; Iannaccone, M.; Ariza-Sole, A.; Liebetrau, C.; Manzano-Fernandez, S.; Quadri, G.; et al. Machine learning-based prediction of adverse events following an acute coronary syndrome (PRAISE): A modelling study of pooled datasets. Lancet 2021, 397, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Kumar, S.; Fusaro, M.; Amoroso, N.; Attubato, M.J.; Feit, F.; Bhatt, D.L.; Slater, J. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: A mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012, 125, 2873–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iakovou, I.; Schmidt, T.; Bonizzoni, E.; Ge, L.; Sangiorgi, G.M.; Stankovic, G.; Airoldi, F.; Chieffo, A.; Montorfano, M.; Carlino, M.; et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 2005, 293, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Moses, J.W.; Ellis, S.G.; Schofer, J.; Dawkins, K.D.; Morice, M.C.; Colombo, A.; Schampaert, E.; Grube, E.; Kirtane, A.J.; et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 2007, 356, 998–1008. [Google Scholar] [CrossRef]
- Alfonso, F.; Fernandez, C. Second-generation drug-eluting stents. Moving the field forward. J. Am. Coll. Cardiol. 2011, 58, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Escaned, J.; Collet, C.; Ryan, N.; De Maria, G.L.; Walsh, S.; Sabate, M.; Davies, J.; Lesiak, M.; Moreno, R.; Cruz-Gonzalez, I.; et al. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur. Heart J. 2017, 38, 3124–3134. [Google Scholar] [CrossRef] [Green Version]
- Faridi, K.F.; Garratt, K.N.; Kennedy, K.F.; Maddox, T.M.; Secemsky, E.A.; Butala, N.M.; Yeh, R.W. Physician and Hospital Utilization of P2Y12 Inhibitors in ST-Segment-Elevation Myocardial Infarction in the United States: A Study From the National Cardiovascular Data Registry’s Research to Practice Initiative. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006275. [Google Scholar] [CrossRef]
- Samuelsen, P.-J.; Eggen, A.E.; Steigen, T.; Wilsgaard, T.; Kristensen, A.; Skogsholm, A.; Holme, E.; van den Heuvel, C.; Nordrehaug, J.E.; Bendz, B. Incidence and risk factors for major bleeding among patients undergoing percutaneous coronary intervention: Findings from the Norwegian Coronary Stent Trial (NORSTENT). PLoS ONE 2021, 16, e0247358. [Google Scholar] [CrossRef]
- Genereux, P.; Giustino, G.; Witzenbichler, B.; Weisz, G.; Stuckey, T.D.; Rinaldi, M.J.; Neumann, F.J.; Metzger, D.C.; Henry, T.D.; Cox, D.A.; et al. Incidence, Predictors, and Impact of Post-Discharge Bleeding After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2015, 66, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Baber, U.; Mastoris, I.; Dangas, G.; Sartori, S.; Steg, P.G.; Cohen, D.J.; Giustino, G.; Chandrasekhar, J.; Ariti, C.; et al. Sex-Based Differences in Cessation of Dual-Antiplatelet Therapy Following Percutaneous Coronary Intervention With Stents. JACC Cardiovasc. Interv. 2016, 9, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-H. “East asian paradox”: Challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr. Cardiol. Rep. 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Jeong, Y.H.; Goto, S.; Anderson, J.L.; Huo, Y.; Mega, J.L.; Taubert, K.; Smith, S.C., Jr. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat. Rev. Cardiol. 2014, 11, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, H.-S. The evolving concept of dual antiplatelet therapy after percutaneous coronary intervention: Focus on unique feature of East Asian and “Asian Paradox”. Korean Circ. J. 2018, 48, 537. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Y.; Wang, Z.; Wang, S.; Zhang, H.; Wang, Y.; Lu, C.; Xuan, H.; Wang, C.; Li, D.; et al. Efficacy and safety of low dose ticagrelor in patients with acute coronary syndrome: A systematic review and meta-analysis. Postgrad. Med. J. 2020, 96, 693–702. [Google Scholar] [CrossRef] [PubMed]


| Variables | N | % |
|---|---|---|
| Total patients | 79,654 | |
| Age, mean | 63.06 ± 11.53 | |
| <55 years | 19,315 | 24.2% |
| 55–64 years | 23,372 | 29.3% |
| 65–74 years | 23,094 | 29.0% |
| ≥75 years | 13,873 | 17.4% |
| Sex | ||
| Male | 54,566 | 68.5% |
| Female | 25,088 | 31.5% |
| Hospital type | ||
| Tertiary hospital | 39,794 | 50.0% |
| Secondary general hospital | 39,768 | 49.9% |
| Primary clinic | 92 | 0.1% |
| Clinical diagnosis at the index PCI | ||
| Acute myocardial infarction | 30,823 | 38.7% |
| Non-acute myocardial infarction | 48,831 | 61.3% |
| Comorbidities | ||
| Diabetes mellitus | 26,140 | 32.8% |
| Hypertension | 43,489 | 54.6% |
| Dyslipidemia | 31,833 | 40.0% |
| Peripheral vascular disease | 12,109 | 15.2% |
| Stroke (ischemic or hemorrhagic) | 5590 | 7.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, J.-S.; Lee, J.; Kim, Y.-H.; Kim, J.-S.; Lim, S.-Y.; Kim, S.H.; Ahn, J.-C.; Song, W.-H. Fifteen-Year Nationwide Trend in Antiplatelet Treatment among Drug-Eluting Stent Recipients in Korea: Many Patients Receive Very Prolonged Dual-Antiplatelet Treatment, and Newer Drugs Are Replacing the Older Ones. J. Clin. Med. 2023, 12, 2675. https://doi.org/10.3390/jcm12072675
Kim S, Lee J-S, Lee J, Kim Y-H, Kim J-S, Lim S-Y, Kim SH, Ahn J-C, Song W-H. Fifteen-Year Nationwide Trend in Antiplatelet Treatment among Drug-Eluting Stent Recipients in Korea: Many Patients Receive Very Prolonged Dual-Antiplatelet Treatment, and Newer Drugs Are Replacing the Older Ones. Journal of Clinical Medicine. 2023; 12(7):2675. https://doi.org/10.3390/jcm12072675
Chicago/Turabian StyleKim, Sunwon, Jong-Seok Lee, Jungkuk Lee, Yong-Hyun Kim, Jin-Seok Kim, Sang-Yup Lim, Seong Hwan Kim, Jeong-Cheon Ahn, and Woo-Hyuk Song. 2023. "Fifteen-Year Nationwide Trend in Antiplatelet Treatment among Drug-Eluting Stent Recipients in Korea: Many Patients Receive Very Prolonged Dual-Antiplatelet Treatment, and Newer Drugs Are Replacing the Older Ones" Journal of Clinical Medicine 12, no. 7: 2675. https://doi.org/10.3390/jcm12072675





