Surrogates of Muscle Mass on Cardiac MRI Correlate with Exercise Capacity in Patients with Fontan Circulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. CMR Data Analysis
2.4. Muscle Measurements
2.5. Cardiopulmonary Exercise Testing
2.6. Statistical Analyses
3. Results
3.1. Anterior and Paraspinal Muscle Index to BSA in the Fontan Population
3.2. Intra-Observer and Inter-Observer Reliability:
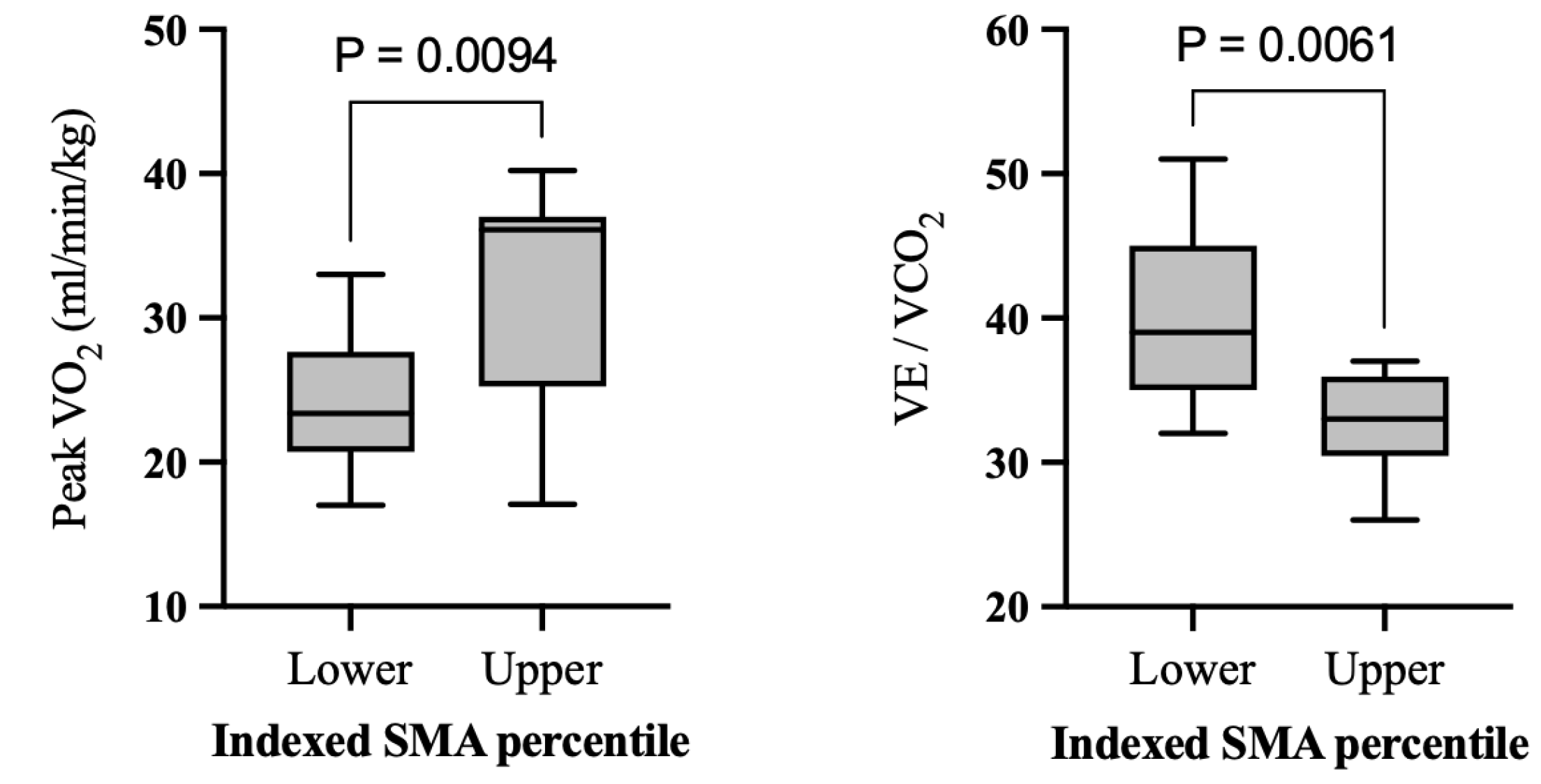
3.3. Comparison between High- and Low-Muscle Groups
3.4. Correlation of Muscle Mass with Exercise Capacity and Other Clinical Parameters
4. Discussion
4.1. Indexed Skeletal Muscle Area and Cardiorespiratory Function
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Budts, W.; Ravekes, W.J.; Danford, D.A.; Kutty, S. Diastolic Heart Failure in Patients With the Fontan Circulation: A Review. JAMA Cardiol. 2020, 5, 590. [Google Scholar] [CrossRef]
- Stout, K.K.; Broberg, C.S.; Book, W.M.; Cecchin, F.; Chen, J.M.; Dimopoulos, K.; Everitt, M.D.; Gatzoulis, M.; Harris, L.; Hsu, D.T.; et al. Chronic Heart Failure in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 133, 770–801. [Google Scholar] [CrossRef] [Green Version]
- Mertens, L.; Hagler, D.J.; Sauer, U.; Somerville, J.; Gewillig, M. Protein-losing enteropathy after the Fontan operation: An international multicenter study. J. Thorac. Cardiovasc. Surg. 1998, 115, 1063–1073. [Google Scholar] [CrossRef] [Green Version]
- Ostrow, A.M.; Freeze, H.; Rychik, J. Protein-Losing Enteropathy After Fontan Operation: Investigations Into Possible Pathophysiologic Mechanisms. Ann. Thorac. Surg. 2006, 82, 695–700. [Google Scholar] [CrossRef]
- Cordina, R.; O’Meagher, S.; Gould, H.; Rae, C.; Kemp, G.; Pasco, J.A.; Celermajer, D.S. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart 2013, 99, 1530–1534. [Google Scholar] [CrossRef]
- Ohuchi, H.; Negishi, J.; Miike, H.; Toyoshima, Y.; Morimoto, H.; Fukuyama, M.; Iwasa, T.; Sakaguchi, H.; Miyazaki, A.; Shiraishi, I.; et al. Positive pediatric exercise capacity trajectory predicts better adult Fontan physiology rationale for early establishment of exercise habits. Int. J. Cardiol. 2019, 274, 80–87. [Google Scholar] [CrossRef]
- Dulfer, K.; Bossers, S.S.M.; Utens, E.M.W.J.; Duppen, N.; Kuipers, I.M.; Kapusta, L.; van Iperen, G.; Schokking, M.; ten Harkel, A.D.J.; Takken, T.; et al. Does functional health status predict health-related quality of life in children after Fontan operation? Cardiol. Young 2016, 26, 459–468. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Williams, R.V.; Mital, S.; Clark, B.J.; Russell, J.L.; Klein, G.; Eisenmann, J.C. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch. Dis. Child. 2007, 92, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Ohuchi, H.; Ohashi, H.; Takasugi, H.; Yamada, O.; Yagihara, T.; Echigo, S. Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after fontan operation. Pediatr. Cardiol. 2004, 25, 513–521. [Google Scholar] [CrossRef]
- Zajac, A.; Tomkiewicz, L.; Podolec, P.; Tracz, W.; Malec, E. Cardiorespiratory response to exercise in children after modified fontan operation. Scand. Cardiovasc. J. SCJ 2002, 36, 80–85. [Google Scholar] [CrossRef]
- Diller, G.-P.; Dimopoulos, K.; Okonko, D.; Li, W.; Babu-Narayan, S.V.; Broberg, C.S.; Johansson, B.; Bouzas, B.; Mullen, M.J.; Poole-Wilson, P.A.; et al. Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation 2005, 112, 828–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diller, G.-P.; Dimopoulos, K.; Okonko, D.; Uebing, A.; Broberg, C.S.; Babu-Narayan, S.; Bayne, S.; Poole-Wilson, P.A.; Sutton, R.; Francis, D.P.; et al. Heart rate response during exercise predicts survival in adults with congenital heart disease. J. Am. Coll. Cardiol. 2006, 48, 1250–1256. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, K.; Okonko, D.O.; Diller, G.-P.; Broberg, C.S.; Salukhe, T.V.; Babu-Narayan, S.V.; Li, W.; Uebing, A.; Bayne, S.; Wensel, R.; et al. Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 2006, 113, 2796–2802. [Google Scholar] [CrossRef] [Green Version]
- Albouaini, K.; Egred, M.; Alahmar, A.; Wright, D.J. Cardiopulmonary exercise testing and its application. Postgrad. Med. J. 2007, 83, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Tran, D.; D’Ambrosio, P.; Verrall, C.E.; Attard, C.; Briody, J.; D’Souza, M.; Fiatarone Singh, M.; Ayer, J.; d’Udekem, Y.; Twigg, S.; et al. Body Composition in Young Adults Living With a Fontan Circulation: The Myopenic Profile. J. Am. Heart Assoc. 2020, 9, e015639. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.-Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef]
- Deng, H.-Y.; Hou, L.; Zha, P.; Huang, K.-L.; Peng, L. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: A comprehensive systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Schmitz, K.H.; Berger, N.A. Sarcopenia in aging, obesity, and cancer. Transl. Cancer Res. 2020, 9, 5760–5771. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pellicori, P.; Zhang, J.; Weston, J.; Clark, A.L. Identification of Frailty in Chronic Heart Failure. JACC Heart Fail. 2019, 7, 291–302. [Google Scholar] [CrossRef]
- von Haehling, S.; Ebner, N.; dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.M.; Goldberg, D.J.; Zemel, B.S.; Brodsky, J.L.; Dodds, K.; Hayden-Rush, C.; Whitehead, K.K.; Goldmuntz, E.; Rychik, J.; Leonard, M.B. Deficits in bone density and structure in children and young adults following Fontan palliation. Bone 2015, 77, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avitabile, C.M.; Leonard, M.B.; Zemel, B.S.; Brodsky, J.L.; Lee, D.; Dodds, K.; Hayden-Rush, C.; Whitehead, K.K.; Goldmuntz, E.; Paridon, S.M.; et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart 2014, 100, 1702–1707. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, K.W.; Bosmans, J.W.A.M.; Uittenbogaart, M.; Alsoumali, A.; Poeze, M.; Sosef, M.N.; Derikx, J.P.M. Loss of Skeletal Muscle Mass During Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann. Surg. Oncol. 2015, 22, 4445–4452. [Google Scholar] [CrossRef] [Green Version]
- Possner, M.; Alsaied, T.; Siddiqui, S.; Morales, D.; Trout, A.T.; Veldtman, G. Abdominal Skeletal Muscle Index as a Potential Novel Biomarker in Adult Fontan Patients. CJC Open 2020, 2, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Alsaied, T.; Allen, K.Y.; Anderson, J.B.; Anixt, J.S.; Brown, D.W.; Cetta, F.; Cordina, R.; D’udekem, Y.; Didier, M.; Ginde, S.; et al. The Fontan outcomes network: First steps towards building a lifespan registry for individuals with Fontan circulation in the United States. Cardiol. Young 2020, 30, 1070–1075. [Google Scholar] [CrossRef]
- Gunsaulus, M.; Wang, L.; Haack, L.; Christopher, A.; Feingold, B.; Squires, J.; Horslen, S.; Hoskoppal, A.; Rose-Felker, K.; West, S.; et al. Cardiac MRI-Derived Inferior Vena Cava Cross-Sectional Area Correlates with Measures of Fontan-Associated Liver Disease. Pediatr. Cardiol. 2022. Available online: https://link.springer.com/10.1007/s00246-022-03054-0 (accessed on 20 December 2022). [CrossRef]
- Mathur, S.; Rodrigues, N.; Mendes, P.; Rozenberg, D.; Singer, L.G. Computed Tomography–Derived Thoracic Muscle Size as an Indicator of Sarcopenia in People With Advanced Lung Disease. Cardiopulm. Phys. Ther. J. 2017, 28, 99–105. [Google Scholar] [CrossRef]
- Mosteller, R. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Myers, J.; Kaminsky, L.A.; Lima, R.; Christle, J.W.; Ashley, E.; Arena, R. A Reference Equation for Normal Standards for VO2 Max: Analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry). Prog. Cardiovasc. Dis. 2017, 60, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [Green Version]
- McDonald, M.-L.N.; Diaz, A.A.; Ross, J.C.; San Jose Estepar, R.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ufuk, F.; Demirci, M.; Sagtas, E.; Akbudak, I.H.; Ugurlu, E.; Sari, T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur. J. Radiol. 2020, 131, 109271. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Negishi, J.; Noritake, K.; Hayama, Y.; Sakaguchi, H.; Miyazaki, A.; Kagisaki, K.; Yamada, O. Prognostic Value of Exercise Variables in 335 Patients after the Fontan Operation: A 23-year Single-center Experience of Cardiopulmonary Exercise Testing: Prognostic Variables in Fontan. Congenit. Heart Dis. 2015, 10, 105–116. [Google Scholar] [CrossRef]
- Weinreb, S.J.; Dodds, K.M.; Burstein, D.S.; Huang, J.; Rand, E.B.; Mancilla, E.; Heimall, J.R.; McBride, M.G.; Paridon, S.M.; Goldberg, D.J.; et al. End-Organ Function and Exercise Performance in Patients With Fontan Circulation: What Characterizes the High Performers? J. Am. Heart Assoc. 2020, 9, e016850. [Google Scholar] [CrossRef]
- Cordina, R.L.; O’Meagher, S.; Karmali, A.; Rae, C.L.; Liess, C.; Kemp, G.J.; Puranik, R.; Singh, N.; Celermajer, D.S. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int. J. Cardiol. 2013, 168, 780–788. [Google Scholar] [CrossRef]
- Duppen, N.; Etnel, J.R.; Spaans, L.; Takken, T.; van den Berg-Emons, R.J.; Boersma, E.; Schokking, M.; Dulfer, K.; Utens, E.M.; Helbing, W.; et al. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am. Heart J. 2015, 170, 606–614. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, M.L.; Desai, S.; Lane, M.; McBride, M.; Paridon, S.; Goldmuntz, E. Relationship Between Habitual Exercise and Performance on Cardiopulmonary Exercise Testing Differs Between Children With Single and Biventricular Circulations. Pediatr. Cardiol. 2017, 38, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhang, X.; Ma, W.; Song, H.; Gong, Z.; Wang, Q.; Che, L.; Xu, W.; Jiang, J.; Xu, J.; et al. VE/VCO2 slope and its prognostic value in patients with chronic heart failure. Exp. Ther. Med. 2015, 9, 1407–1412. [Google Scholar] [CrossRef] [Green Version]
- Arena, R.; Myers, J.; Aslam, S.S.; Varughese, E.B.; Peberdy, M.A. Peak VO2 and VE/VCO2 slope in patients with heart failure: A prognostic comparison. Am. Heart J. 2004, 147, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Cicoira, M.; Zanolla, L.; Franceschini, L.; Rossi, A.; Golia, G.; Zamboni, M.; Tosoni, P.; Zardini, P. Skeletal muscle mass independently predicts peak oxygen consumption and ventilatory response during exercise in noncachectic patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 2080–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, S.M.; Alexander, M.E.; Graham, D.A.; Khairy, P.; Clair, M.; Rodriguez, E.; Pearson, D.D.; Landzberg, M.J.; Rhodes, J. Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery. Congenit. Heart Dis. 2011, 6, 294–303. [Google Scholar] [CrossRef] [PubMed]
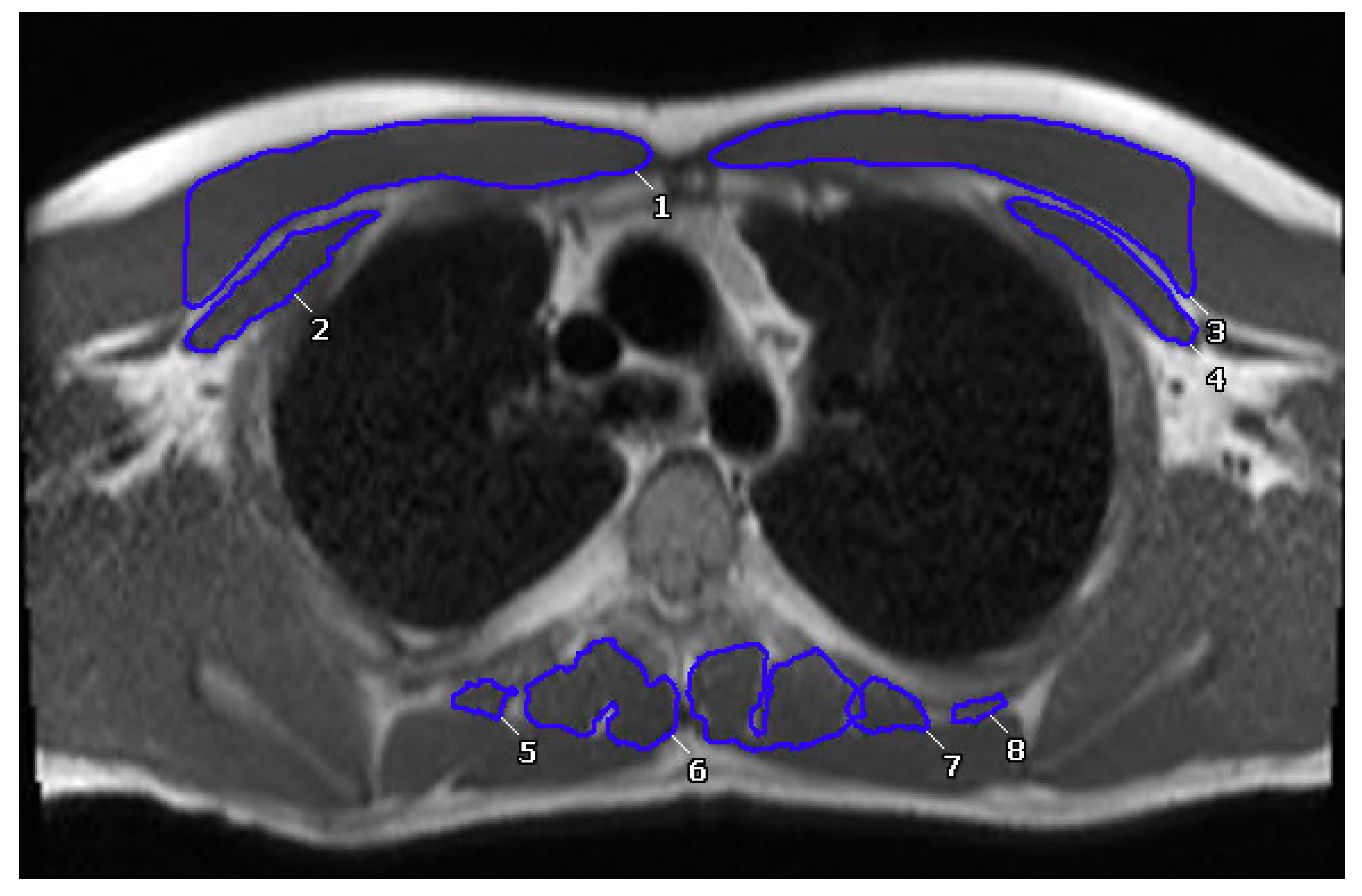


| Variables | N | Results |
|---|---|---|
| Age (y), mean ± SD | 75 | 19.1 ± 8.6 |
| Male sex, n (%) | 75 | 49 (65%) |
| Muscle characteristics (N = 75) | ||
| BSA-indexed anterior muscle (cm2/m2), mean ± SD | 20.8 ± 5.7 | |
| BSA-indexed paraspinal muscle (cm2/m2), mean ± SD | 6.5 ± 1.7 | |
| BSA-indexed total skeletal muscle (cm2/m2), mean ± SD | 27.3 ± 6.6 | |
| Fontan type (N = 75) | ||
| Atriopulmonary and other, n (%) | 1 (1.3%) | |
| Lateral tunnel, n (%) | 23 (31%) | |
| Extracardiac, n (%) | 49 (65%) | |
| Unkown, n (%) | 2 (2.6%) | |
| Ventricular Morphology (N = 75) | ||
| Left, n (%) | 35 (47%) | |
| Right, n (%) | 28 (37%) | |
| Both, n (%) | 12 (16%) |
| Variables | N | Results |
|---|---|---|
| Exercise parameters | ||
| Peak VO2 (mL/kg/min) | 38 | 27.1 ± 6.6 |
| Peak O2 pulse (mL/beat), mean ± SD | 19 | 9.6 ± 2.7 |
| Peak HR (beats/min), mean ± SD | 51 | 165.7 ± 25.5 |
| % Predicted peak HR | 45 | 83.1 ± 12.2 |
| Peak RER | 43 | 1.2 ± 0.1 |
| VE/VCO2 | 42 | 36.5 ± 6.0 |
| CMR Ventricular Volumetry | ||
| EF (%), mean ± SD | 75 | 49.4 ± 9.6 |
| BSA-indexed SVEDV (mL/m2), mean ± SD | 75 | 100.6 ± 41.0 |
| BSA-indexed SVESV (mL/m2), mean ± SD | 75 | 50.7 ± 21.7 |
| BSA-indexed ventricle mass, mean ± SD | 41 | 43.8 ± 17.2 |
| Variables | Female | Male | p-Value Effect Size (95% CI) |
|---|---|---|---|
| Mean ± SD (N) [1st Quartile, Median, 3rd Quartile] | Mean ± SD (N) [1st Quartile, Median, 3rd Quartile] | ||
| Age (years) | 17.2 ± 7.0 (20) [13.3, 15, 21.5] | 17.8 ± 7.1 (19) [12, 17, 22] | 0.499 0.085 (−0.39, 0.56) |
| Sex (N, %) | Male: 8 (40%) Female: 12 (60%) | Male: 18 (95%) Female: 1 (5%) | 0.0003 * |
| Height (cm) | 158.5 ± 17.0 (20) [155.5, 162.9, 168] | 154.6 ± 26.1 (19) [130, 165, 174.4] | 0.704 0.28 (−0.20, 0.75) |
| Weight (kg) | 65.0 ± 20.0 (20) [54.4, 68.8, 77.8] | 54.7 ± 25.4 (19) [28, 59.1, 70.4] | 0.136 0.24 (−0.24, 0.72) |
| BMI (kg/m2) | 25.1 ± 5.3 (20) [21.1, 25.4, 28] | 21.3 ± 4.9 (19) [17.1, 20.8, 24.9] | 0.027 * 0.32 (−0.44, 0.51) |
| Anterior Wall Muscle Area (cm2) | 25.8 ± 7.0 (20) [23, 25.6, 31.4] | 41.4 ± 15.5 (19) [25.2, 42.8, 52.8] | 0.002 * 0.86 (0.36, 1.35) |
| Paraspinal Wall Muscle Area (cm2) | 8.7 ± 2.0 (20) [7.7, 9.3, 9.8] | 12.1 ± 3.8 (19) [9.6, 11.9, 14.3] | 0.002 * 0.77 (0.28, 1.26) |
| Peak VO2 (mL/kg/min) | 23.8 ± 4.7 (12) [20.7, 23.4, 27.6] | 32.2 ± 8.5 (9) [25.2, 36.1, 37] | 0.009 * 0.15 (−0.48, 0.78) |
| Peak O2 Pulse (mL/beat) | 8.1 ± 0.9 (5) [7.3, 8, 8.9] | 11.1 ± 3.6 (5) [8, 12.2, 13.7] | 0.144 0.83 (−0.10, 1.82) |
| Peak HR (beats/min) | 167.3 ± 21.4 (14) [154.5, 171, 181] | 173.7 ± 12.0 (10) [163.5, 173, 185.3] | 0.519 0.07 (−0.51, 0.65) |
| % Predicted Peak HR (%) | 82.8 ± 8.1 (13) [78.5, 84, 87.5] | 88.6 ± 5.2 (8) [83.5, 88, 94] | 0.103 0.02 (−0.61, 0.66) |
| Peak RER | 1.1 ± 0.04 (12) [1.1, 1.1, 1.2] | 1.2 ± 0.1 (9) [1.2, 1.2, 1.3] | 0.006 * 0.51 (−0.14, 1.16) |
| VE/VCO2 | 40.2 ± 6.2 (11) [35, 39, 45] | 32.9 ± 3.6 (9) [30.5, 33, 36] | 0.006 * −0.55 (−1.21, 0.12) |
| Variable | N | Indexed Anterior Muscle | Indexed Paraspinal Muscle | Indexed Total Muscle | |||
|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | ||
| Peak VO2 (mL/kg/min) | 38 | 0.3364 | 0.039 * | 0.4303 | 0.007 * | 0.4023 | 0.012 * |
| Peak O2 Pulse (mL/beat) | 19 | 0.1339 | 0.123 | 0.1515 | 0.536 | 0.3692 | 0.120 |
| Peak HR (beats/min) | 45 | 0.1256 | 0.411 | 0.0478 | 0.755 | 0.1210 | 0.429 |
| % Predicted Peak HR (%) | 39 | 0.1969 | 0.230 | −0.0297 | 0.858 | 0.1634 | 0.320 |
| Peak RER | 37 | 0.1297 | 0.444 | 0.2836 | 0.089 | 0.1823 | 0.280 |
| VE/VCO2 | 36 | −0.2013 | 0.239 | −0.4450 | 0.006 * | −0.2865 | 0.090 |
| EF (%) | 75 | −0.0899 | 0.443 | −0.0212 | 0.857 | −0.0827 | 0.481 |
| Ventricle Mass (g/m2) | 41 | 0.0743 | 0.644 | −0.1936 | 0.225 | 0.0181 | 0.911 |
| BSA-Indexed Ventricle Mass | 41 | 0.0623 | 0.699 | −0.1733 | 0.279 | 0.0023 | 0.988 |
| Variable | Low Muscle (N = 20) | High Muscle (N = 19) | p-Value Effect Size (95% CI) |
|---|---|---|---|
| Mean ± SD (N) [1st Quartile, Median, 3rd Quartile] | Mean ± SD (N) [1st Quartile, Median, 3rd Quartile] | ||
| Age (years) | 17.2 ± 7.0 (20) [13.3, 15, 21.5] | 17.8 ± 7.1 (19) [12, 17, 22] | 0.499 0.10 (−0.53, 0.73) |
| Sex (N, %) | Male: 8 (40%) Female: 12 (60%) | Male: 18 (95%) Female: 1 (5%) | 0.0003 * |
| Height (cm) | 158.5 ± 17.0 (20) [155.5, 162.9, 168] | 154.6 ± 26.1 (19) [130, 165, 174.4] | 0.704 −0.18 (−0.81, 0.45) |
| Weight (kg) | 65.0 ± 20.0 (20) [54.4, 68.8, 77.8] | 54.7 ± 25.4 (19) [28, 59.1, 70.4] | 0.136 −0.45 (−1.08, 0.19) |
| BMI (kg/m2) | 25.1 ± 5.3 (20) [21.1, 25.4, 28] | 21.3 ± 4.9 (19) [17.1, 20.8, 24.9] | 0.027 * −0.76 (−1.41, −0.10) |
| Anterior Wall Muscle Area (cm2) | 25.8 ± 7.0 (20) [23, 25.6, 31.4] | 41.4 ± 15.5 (19) [25.2, 42.8, 52.8] | 0.002 * 1.31 (0.61, 2.0) |
| Paraspinal Wall Muscle Area (cm2) | 8.7 ± 2.0 (20) [7.7, 9.3, 9.8] | 12.1 ± 3.8 (19) [9.6, 11.9, 14.3] | 0.002 * 1.14 (0.46, 1.82) |
| BSA-Indexed Anterior Muscle (cm2/m2) | 15.4 ± 2.2 (20) [13.6, 15.7, 16.2] | 27.5 ± 5.8 (19) [24.2, 26.2, 28.4] | <0.0001 * 2.77 (1.87, 3.65) |
| BSA-Indexed Paraspinal Muscle (cm2/m2) | 5.2 ± 0.7 (20) [5, 5.2, 5.7] | 8.2 ± 1.8 (19) [7, 7.8, 8.7] | <0.0001 * 2.18 (1.37, 2.97) |
| Exercise parameters: | |||
| Peak VO2 (mL/kg/min) | 23.8 ± 4.7 (12) [20.7, 23.4, 27.6] | 32.2 ± 8.5 (9) [25.2, 36.1, 37] | 0.009 * 1.22 (0.30, 2.13) |
| Peak O2 Pulse (mL/beat) | 8.1 ± 0.9 (5) [7.3, 8, 8.9] | 11.1 ± 3.6 (5) [8, 12.2, 13.7] | 0.144 1.03 (−0.22, 2.23) |
| Peak HR (beats/min) | 167.3 ± 21.4 (14) [154.5, 171, 181] | 173.7 ± 12.0 (10) [163.5, 173, 185.3] | 0.519 0.34 (−0.45, 1.13) |
| % Predicted Peak HR (%) | 82.8 ± 8.1 (13) [78.5, 84, 87.5] | 88.6 ± 5.2 (8) [83.5, 88, 94] | 0.103 0.78 (−0.11, 1.66) |
| Peak RER | 1.1 ± 0.04 (12) [1.1, 1.1, 1.2] | 1.2 ± 0.1 (9) [1.2, 1.2, 1.3] | 0.006 * 1.47 (0.50, 2.40) |
| VE/VCO2 | 40.2 ± 6.2 (11) [35, 39, 45] | 32.9 ± 3.6 (9) [30.5, 33, 36] | 0.006 * −1.34 (−2.27, −0.37) |
| Variables | Indexed Paraspinal Muscle | Indexed Anterior Muscle | Indexed Total Muscle | |
|---|---|---|---|---|
| Parameter Estimate ± SD (p-Value) | Parameter Estimate ± SD (p-Value) | Parameter Estimate ± SD (p-Value) | ||
| Age-adjusted | Peak VO2 | 1.7 ± 0.6 (0.008) * | 0.4 ± 0.2 (0.043) * | 0.4 ± 0.2 (0.012) * |
| VE/VCO2 | −1.4 ± 0.6 (0.019) * | −0.1 ± 0.2 (0.522) | −0.1 ± 0.2 (0.522) | |
| Sex-adjusted | Peak VO2 | 1.5 ± 0.6 (0.012) * | 0.4 ± 0.2 (0.013) * | 0.4 ± 0.1 (0.005) * |
| VE/VCO2 | −1.6 ± 0.5 (0.004) * | −0.06 ± 0.1 (0.616) | −0.2 ± 0.1 (0.115) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, K.L.; Gordon, E.B.; Gunsaulus, M.E.; Christopher, A.; Olivieri, L.J.; Tadros, S.S.; Harris, T.; Saraf, A.P.; Kreutzer, J.; Feingold, B.; et al. Surrogates of Muscle Mass on Cardiac MRI Correlate with Exercise Capacity in Patients with Fontan Circulation. J. Clin. Med. 2023, 12, 2689. https://doi.org/10.3390/jcm12072689
Smith KL, Gordon EB, Gunsaulus ME, Christopher A, Olivieri LJ, Tadros SS, Harris T, Saraf AP, Kreutzer J, Feingold B, et al. Surrogates of Muscle Mass on Cardiac MRI Correlate with Exercise Capacity in Patients with Fontan Circulation. Journal of Clinical Medicine. 2023; 12(7):2689. https://doi.org/10.3390/jcm12072689
Chicago/Turabian StyleSmith, Kevin L., Emile B. Gordon, Megan E. Gunsaulus, Adam Christopher, Laura J. Olivieri, Sameh S. Tadros, Tyler Harris, Anita P. Saraf, Jacqueline Kreutzer, Brian Feingold, and et al. 2023. "Surrogates of Muscle Mass on Cardiac MRI Correlate with Exercise Capacity in Patients with Fontan Circulation" Journal of Clinical Medicine 12, no. 7: 2689. https://doi.org/10.3390/jcm12072689





