Reliability and Validity of Single Axial Slice vs. Multiple Slice Quantitative Measurement of the Volume of Effusion-Synovitis on 3T Knee MRI in Knees with Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, Participants
2.2. Radiography Acquisition and Kellgren–Lawrence Grade Assessment
2.3. MRI Acquisition and Semi-Quantitative Scoring Assessment of Effusion-Synovitis
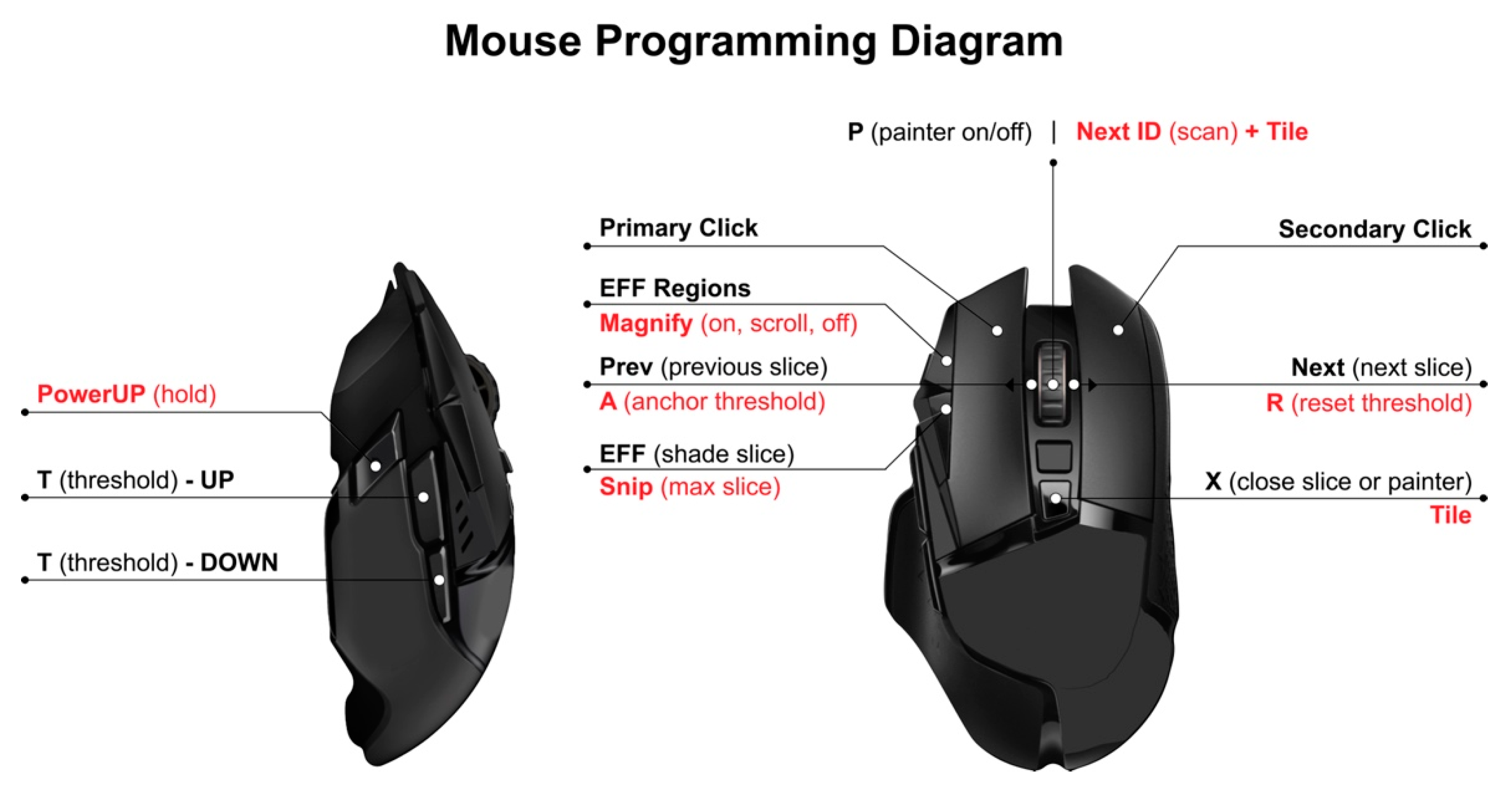
2.4. Semi-Automated Quantitative Assessment of Effusion-Synovitis
2.5. Statistical Analysis
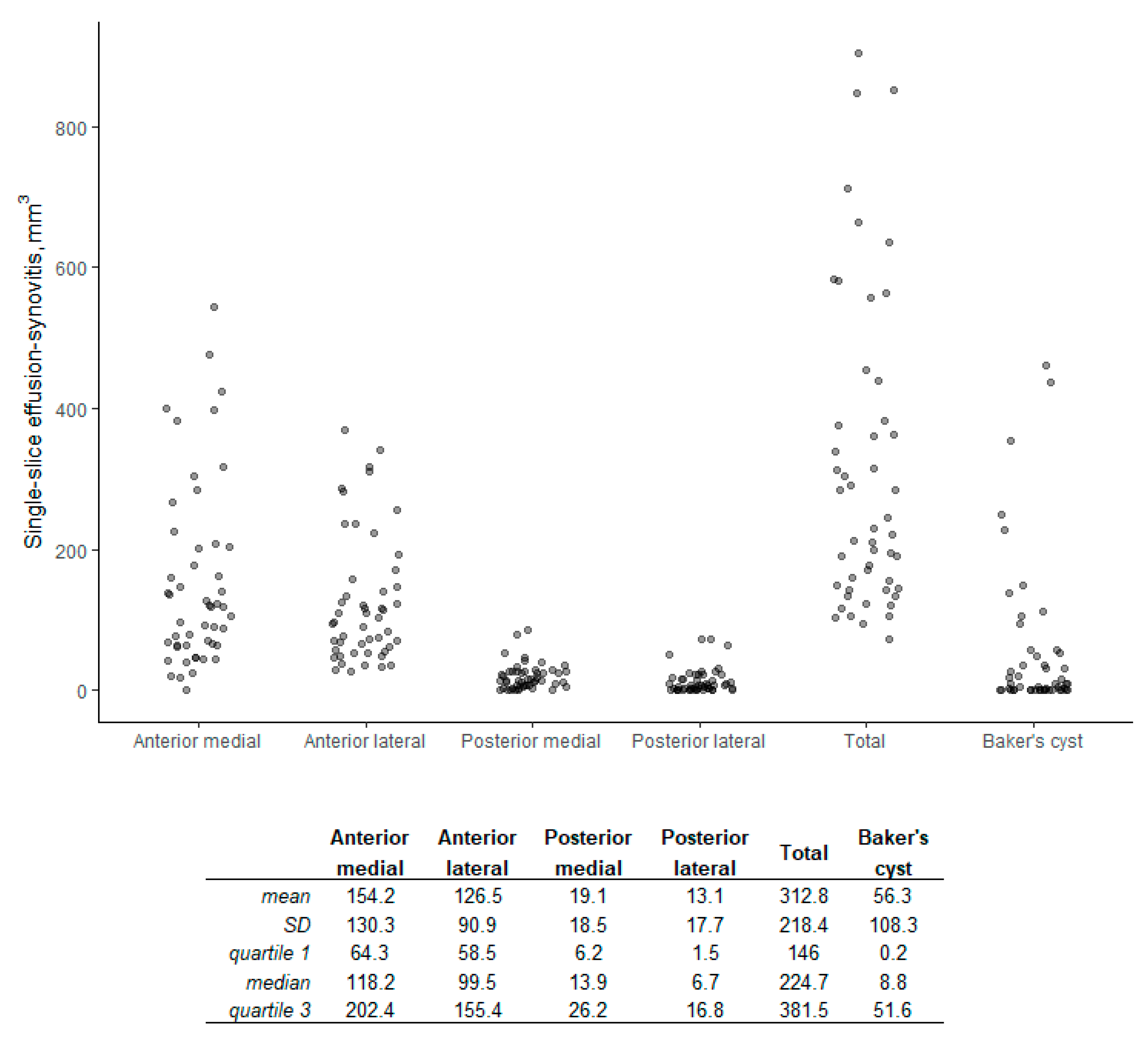
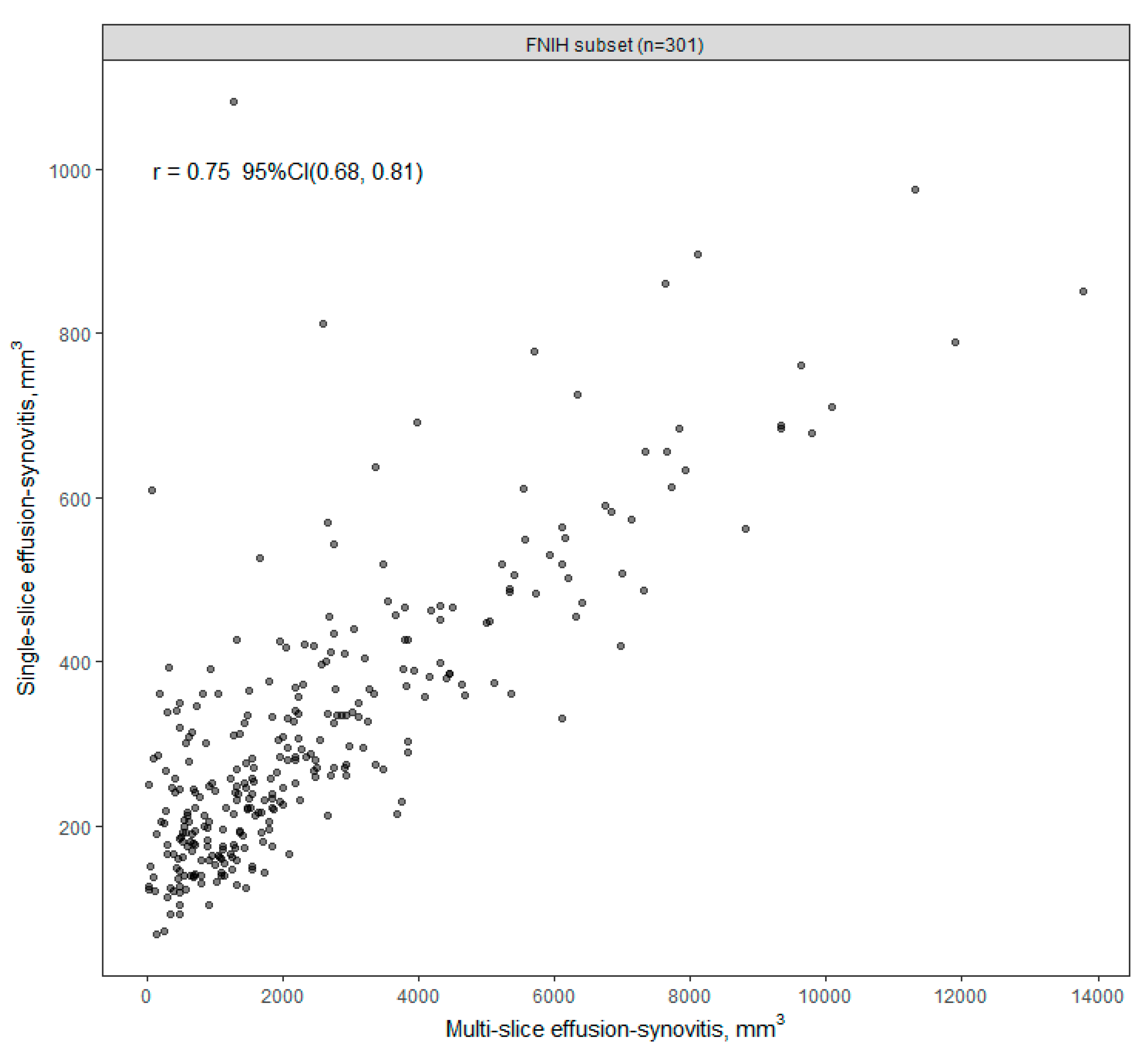
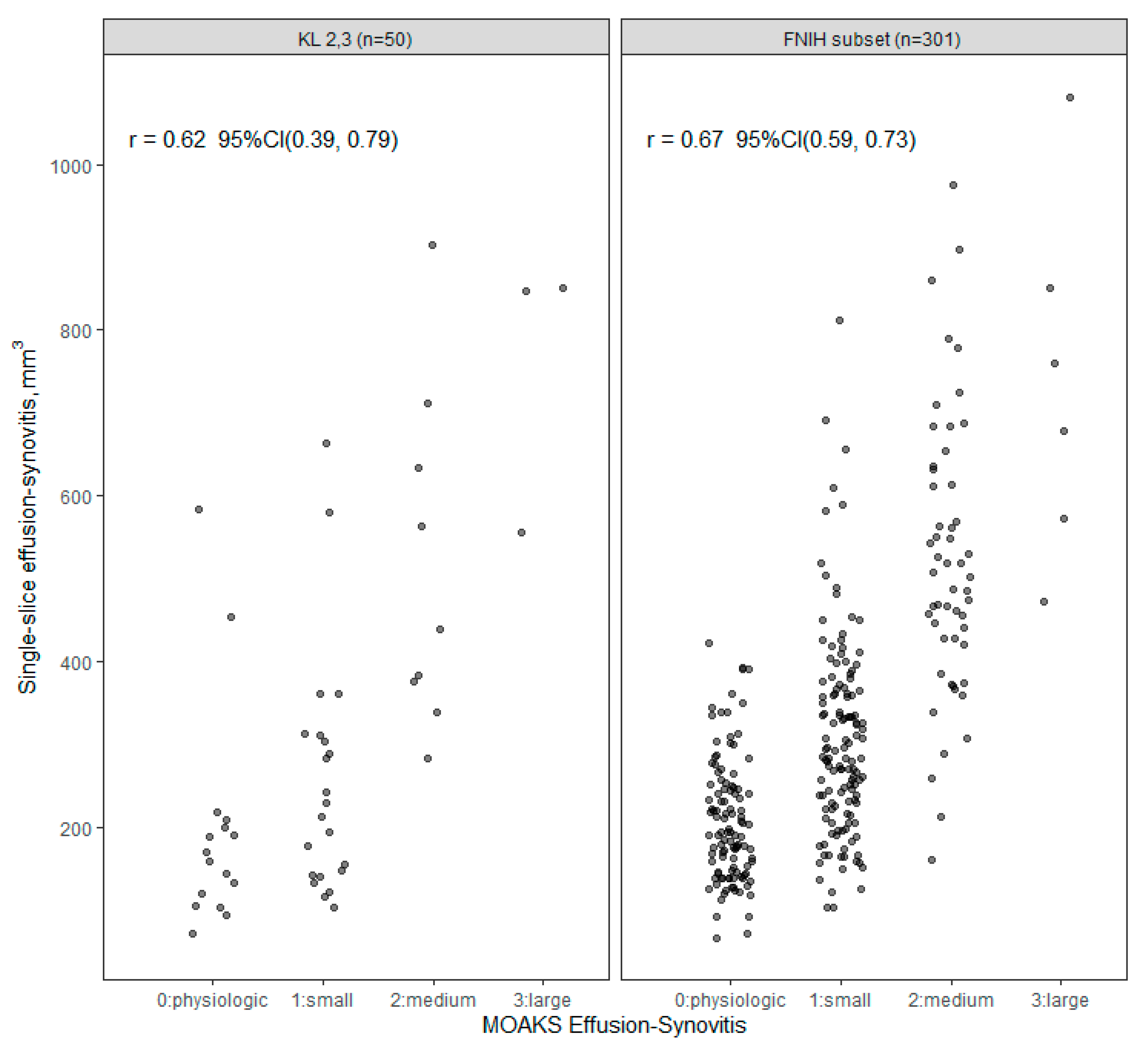
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- March, L.; Cross, M.; Lo, C.; Arden, N.K.; Gates, L.; Leyland, K.; Hawker, G.; King, L.; Leyland, K. Osteoarthritis: A Serious Disease: Submitted to the US Food and Drug Administration. 2016. Available online: https://www.oarsi.org/sites/default/files/docs/2016/oarsi_white_paper_oa_serious_disease_121416_1.pdf (accessed on 6 August 2022).
- Murray, C.J.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthr. Cartil. 2019, 27, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2019. Available online: https://population.un.org/wpp/ (accessed on 6 August 2022).
- Murtagh, E.; Collaboration, N.R.F. Worldwide trends in children’s and adolescents’ body mass index, underweight, overweight and obesity, in comparison with adults, from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies with 128.9 million participants. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Oo, W.M.; Little, C.; Duong, V.; Hunter, D.J. The development of disease-modifying therapies for osteoarthritis (DMOADs): The evidence to date. Drug Des. Dev. Ther. 2021, 15, 2921. [Google Scholar] [CrossRef]
- Cooper, C.; Javaid, M.K.; Arden, N. Epidemiology of osteoarthritis. In Atlas of Osteoarthritis; Springer: Berlin/Heidelberg, Germany, 2014; pp. 21–36. [Google Scholar]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee osteoarthritis: A review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Deveza, L.A.; Nelson, A.E.; Loeser, R.F. Phenotypes of osteoarthritis-current state and future implications. Clin. Exp. Rheumatol. 2019, 37, 64. [Google Scholar]
- Kampen, W.U.; Fischer, M. Local Treatment of Inflammatory Joint Diseases: Benefits and Risks; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Attur, M.; Samuels, J.; Krasnokutsky, S.; Abramson, S.B. Targeting the synovial tissue for treating osteoarthritis (OA): Where is the evidence? Best Pract. Res. Clin. Rheumatol. 2010, 24, 71–79. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Roemer, F.; Javaid, M.K.; Guermazi, A.; Thomas, M.; Kiran, A.; Keen, R.; King, L.; Arden, N. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthr. Cartil. 2010, 18, 1269–1274. [Google Scholar] [CrossRef]
- Hayashi, D.; Guermazi, A.; Kwoh, C.K. Clinical and translational potential of MRI evaluation in knee osteoarthritis. Curr. Rheumatol. Rep. 2014, 16, 391. [Google Scholar] [CrossRef][Green Version]
- Guermazi, A.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Niu, J.; Zhang, Y.; Marra, M.D.; Katur, A.; Lynch, J.A.; El-Khoury, G.Y. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: The MOST study. Ann. Rheum. Dis. 2011, 70, 805–811. [Google Scholar] [CrossRef]
- Maximova, N.; Gregori, M.; Zennaro, F.; Sonzogni, A.; Simeone, R.; Zanon, D. Hepatic gadolinium deposition and reversibility after contrast agent–enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology 2016, 281, 418–426. [Google Scholar] [CrossRef]
- Errante, Y.; Cirimele, V.; Mallio, C.A.; Di Lazzaro, V.; Zobel, B.B.; Quattrocchi, C.C. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig. Radiol. 2014, 49, 685–690. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Thoenen, J.; MacKay, J.W.; Sandford, H.J.; Gold, G.E.; Kogan, F. Imaging of Synovial Inflammation in Osteoarthritis, From the AJR Special Series on Inflammation. AJR Am. J. Roentgenol. 2022, 218, 405. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Demehri, S.; Wirth, W.; Kijowski, R. Imaging in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 913–934. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kwoh, C.K.; Hannon, M.J.; Hunter, D.J.; Eckstein, F.; Fujii, T.; Boudreau, R.M.; Guermazi, A. What comes first? Multitissue involvement leading to radiographic osteoarthritis: Magnetic resonance imaging–based trajectory analysis over four years in the Osteoarthritis Initiative. Arthritis Rheumatol. 2015, 67, 2085–2096. [Google Scholar] [CrossRef]
- Atukorala, I.; Kwoh, C.K.; Guermazi, A.; Roemer, F.; Boudreau, R.; Hannon, M.; Hunter, D. Synovitis in knee osteoarthritis: A precursor of disease? Ann. Rheum. Dis. 2016, 75, 390–395. [Google Scholar] [CrossRef]
- Ramezanpour, S.; Kanthawang, T.; Lynch, J.; McCulloch, C.E.; Nevitt, M.C.; Link, T.M.; Joseph, G.B. Impact of Sustained Synovitis on Knee Joint Structural Degeneration: 4-Year MRI Data from the Osteoarthritis Initiative. J. Magn. Reson. Imaging 2022, 57, 153–164. [Google Scholar] [CrossRef]
- Wang, Y.; Teichtahl, A.J.; Pelletier, J.-P.; Abram, F.; Wluka, A.E.; Hussain, S.M.; Martel-Pelletier, J.; Cicuttini, F.M. Knee effusion volume assessed by magnetic resonance imaging and progression of knee osteoarthritis: Data from the Osteoarthritis Initiative. Rheumatology 2019, 58, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, X.; Han, W.; Cao, Y.; Halliday, A.; Blizzard, L.; Pan, F.; Antony, B.; Cicuttini, F.; Jones, G. Cross-sectional and longitudinal associations between knee joint effusion synovitis and knee pain in older adults. J. Rheumatol. 2016, 43, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Guermazi, A.; Felson, D.T.; Niu, J.; Nevitt, M.C.; Crema, M.D.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Zhang, Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: The MOST study. Ann. Rheum. Dis. 2011, 70, 1804–1809. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Zhang, Y.; Niu, J.; Lynch, J.A.; Crema, M.D.; Marra, M.D.; Nevitt, M.C.; Felson, D.T.; Hughes, L.B.; El-Khoury, G.Y. Tibiofemoral joint osteoarthritis: Risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology 2009, 252, 772. [Google Scholar] [CrossRef]
- Guermazi, A.; Kwoh, C.; Hannon, M.; Boudreau, B.; Hayashi, D.; Hunter, D.; Eckstein, F.; John, M.; Wang, Z.; Roemer, F. Hoffa-synovitis and effusion-synovitis are associated with knees undergoing total knee replacement: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2012, 20, S235–S236. [Google Scholar] [CrossRef][Green Version]
- Dell’Isola, A.; Allan, R.; Smith, S.; Marreiros, S.; Steultjens, M. Identification of clinical phenotypes in knee osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2016, 17, 425. [Google Scholar] [CrossRef]
- Hafezi-Nejad, N.; Demehri, S.; Guermazi, A.; Carrino, J. Osteoarthritis year in review 2017: Updates on imaging advancements. Osteoarthr. Cartil. 2018, 26, 341–349. [Google Scholar] [CrossRef]
- Perry, T.A.; Gait, A.; O’Neill, T.W.; Parkes, M.J.; Hodgson, R.; Callaghan, M.J.; Arden, N.K.; Felson, D.T.; Cootes, T.F. Measurement of synovial tissue volume in knee osteoarthritis using a semiautomated MRI-based quantitative approach. Magn. Reson. Med. 2019, 81, 3056–3064. [Google Scholar] [CrossRef]
- Fotinos-Hoyer, A.K.; Guermazi, A.; Jara, H.; Eckstein, F.; Ozonoff, A.; Khard, H.; Norbash, A.; Bohndorf, K.; Roemer, F.W. Assessment of synovitis in the osteoarthritic knee: Comparison between manual segmentation, semiautomated segmentation, and semiquantitative assessment using contrast-enhanced fat-suppressed T1-weighted MRI. Magn. Reson. Med. 2010, 64, 604–609. [Google Scholar] [CrossRef]
- Li, W.; Abram, F.; Pelletier, J.-P.; Raynauld, J.-P.; Dorais, M.; d’Anjou, M.-A.; Martel-Pelletier, J. Fully automated system for the quantification of human osteoarthritic knee joint effusion volume using magnetic resonance imaging. Arthritis Res. Ther. 2010, 12, R173. [Google Scholar] [CrossRef]
- Felfeliyan, B.; Cornell, D.; Hareendranathan, A.; Jaremko, J.; Ronsky, J. Automatic Effusion Measurement with Limited Labeled Data. Osteoarthr. Imaging 2022, 2, 100038. [Google Scholar] [CrossRef]
- Kellgren, J.H. Epidemiology of chronic rheumatism. Atlas Stand. Radiogr. Arthritis 1963, 2, 22–23. [Google Scholar]
- Collins, J.E.; Losina, E.; Nevitt, M.C.; Roemer, F.W.; Guermazi, A.; Lynch, J.A.; Katz, J.N.; Kent Kwoh, C.; Kraus, V.B.; Hunter, D.J. Semiquantitative imaging biomarkers of knee osteoarthritis progression: Data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthritis Rheumatol. 2016, 68, 2422–2431. [Google Scholar] [CrossRef]
- Smith, S.E.; Hosseinzadeh, S.; Maetani, T.; Shilpa, P.; Collins, J.E.; Kwoh, C.K.; Duryea, J. Association of quantitative measures of effusion-synovitis and hoffa-synovitis with radiographic and pain progression: Data from the FNIH OA biomarkers consortium. Osteoarthr. Cartil. Open 2021, 3, 100138. [Google Scholar] [CrossRef]
- Schaefer, L.; Nikac, V.; Lynch, J.; Duryea, J. Quantitative measurement of cartilage volume is possible using two-dimensional magnetic resonance imaging data sets. Osteoarthr. Cartil. 2018, 26, 920–923. [Google Scholar] [CrossRef]
- Osteoarthritis Initiative. Central Reading of Knee X-rays for Kellgren and Lawrence Grade and Individual Radiographic Features of Tibiofemoral Knee OA. 2016, pp. 1–30. Available online: http://oai.epi-ucsf.org/datarelease/SASDocs/kXR_SQ_BU_descrip.pdf (accessed on 30 December 2022).
- Osteoarthritis Initiative. Overview and Description of Central Image Assessments. 2016; pp. 1–17. Available online: https://nda.nih.gov/binaries/content/documents/ndacms/resources/oai/oai-image-assessment-overview/oai-image-assessment-overview/ndacms:resource (accessed on 30 December 2022).
- Hunter, D.J.; Guermazi, A.; Lo, G.H.; Grainger, A.J.; Conaghan, P.G.; Boudreau, R.M.; Roemer, F.W. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef]
- Loeuille, D.; Sauliere, N.; Champigneulle, J.; Rat, A.; Blum, A.; Chary-Valckenaere, I. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: Associations with clinical, macroscopic and microscopic features. Osteoarthr. Cartil. 2011, 19, 1433–1439. [Google Scholar] [CrossRef]
- De Vet, H.C.; Terwee, C.B.; Mokkink, L.B.; Knol, D.L. Measurement in Medicine: A Practical Guide; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Streiner, D.L.; Norman, G.R.; Cairney, J. Health Measurement Scales: A practical Guide to Their Development and Use, 4th ed.; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Leeden, R.v.d.; Meijer, E.; Busing, F.M.T.A. Resampling Multilevel Models. In Handbook of Multilevel Analysis; Leeuw, J.d., Meijer, E., Eds.; Springer: New York, NY, USA, 2008; pp. 401–433. [Google Scholar]
- Loy, A.; Steele, S.; Korobova, J.; lmeresampler: Bootstrap Methods for Nested Linear Mixed-Effects Models. R Package Version 0.2.2. Available online: https://cran.r-project.org/package=lmeresampler (accessed on 9 December 2022).
- Harrell Jr, F.E. Biostatistics for Biomedical Research. 1987. Available online: https://hbiostat.org/bbr/obsvar.html (accessed on 9 December 2022).
- Liu, Z. Analysis of Observer Variability. Available online: https://biostat.app.vumc.org/wiki/Main/AnalysisOfObserverVariability (accessed on 9 December 2022).
- Harrell, F.E., Jr. _rms: Regression Modeling Strategies_. R Package Version 6.3-0. 2022. Available online: https://CRAN.R-project.org/package=rms (accessed on 30 December 2022).
- Hunter, D.; Zhang, W.; Conaghan, P.G.; Hirko, K.; Menashe, L.; Li, L.; Reichmann, W.; Losina, E. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthr. Cartil. 2011, 19, 557–588. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; McReynolds, A.; Pedersen, S.J.; Weber, U.; Paschke, J.; Wichuk, S.; Jaremko, J.L.; Lambert, R.G. The OMERACT knee inflammation MRI scoring system: Validation of quantitative methodologies and tri-compartmental overlays in osteoarthritis. Semin. Arthritis Rheum. 2021, 51, 925–928. [Google Scholar] [CrossRef]
- Wang, X.; Cicuttini, F.; Jin, X.; Wluka, A.; Han, W.; Zhu, Z.; Blizzard, L.; Antony, B.; Winzenberg, T.; Jones, G. Knee effusion-synovitis volume measurement and effects of vitamin D supplementation in patients with knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1304–1312. [Google Scholar] [CrossRef]

| Variable | KL 2–3 (n = 50) | FNIH Subset (n = 301) |
|---|---|---|
| Age, years [mean, (SD)] | 61.6 (9.2) | 62.0 (9.2) |
| Female sex | 33 (66%) | 189 (63%) |
| Race, ethnicity | ||
| White NH | 44 (88%) | 233 (78%) |
| Black NH | 6 (12%) | 56 (19%) |
| Other | 0 (0%) | 11 (4%) |
| BMI, kg/m2 [mean, (SD)] | 29.6 (4.9) | 30.2 (4.6) |
| BMI categories | ||
| Normal | 9 (18%) | 40 (13%) |
| Overweight | 16 (32%) | 116 (39%) |
| Obese | 25 (50%) | 144 (48%) |
| Kellgren–Lawrence grade | ||
| 1 | 0 (0%) | 44 (15%) |
| 2 | 31 (62%) | 163 (54%) |
| 3 | 19 (38%) | 94 (31%) |
| Joint space narrowing, medial | ||
| 0 | 25 (50%) | 97 (32%) |
| 1 | 10 (20%) | 110 (37%) |
| 2 | 15 (30%) | 94 (31%) |
| Joint space narrowing, lateral | ||
| 0 | 45 (90%) | 296 (98%) |
| 1 | 1 (2%) | 5 (2%) |
| 2 | 4 (8%) | 0 (0%) |
| WOMAC pain | 2.8 (3.4) | 2.2 (3.0) |
| Frequent knee pain | 20 (41%) | 90 (30%) |
| Mean Absolute Difference, mm3 | ||||
|---|---|---|---|---|
| Variable | Intra-Reader | (95% CI) | Inter-Reader | (95% CI) |
| Total volume, mm3 | 36 | (28, 44) | 61 | (48, 75) |
| Anterior medial | 21 | (16, 28) | 32 | (26, 39) |
| Anterior lateral | 29 | (23, 37) | 37 | (30, 44) |
| Posterior medial | 12 | (9, 16) | 17 | (12, 22) |
| Posterior lateral | 10 | (7, 13) | 13 | (9, 18) |
| Baker’s cyst | 18 | (10, 27) | 26 | (14, 39) |
| Case Type | Model/Hypothesis | Likelihood Ratio Χ2 | d.f. | Adequacy Index * | p |
|---|---|---|---|---|---|
| Radiographic and Pain Progression case | |||||
| X: KL grade + BMI + sex + age | 10.2 | 5 | 0.51 | ||
| XA: X + MOAKS ES | 11.5 | 8 | 0.57 | ||
| XB: X + Multi-slice ES | 11.0 | 6 | 0.54 | ||
| XC: X + Single-slice ES | 15.5 | 6 | 0.77 | ||
| XAB: X + MOAKS ES + Multi-slice ES | 12.0 | 9 | 0.60 | ||
| XAC: X + MOAKS ES + Single-slice ES | 19.3 | 9 | 0.95 | ||
| XBC: X + Multi-slice ES + Single-slice ES | 17.9 | 7 | 0.89 | ||
| XABC: X + MOAKS ES + Multi-slice ES + Single-slice ES | 20.2 | 10 | 1.00 | ||
| Hypothesis (model comparison) | |||||
| Ho: No additional information provided by MOAKS ES beyond quantitative methods (Model XABC vs. XBC) | 2.3 | 3 | 0.51 | ||
| Ho: No additional information provided by quantitative methods beyond MOAKS ES (Model XABC vs. XA) | 8.6 | 2 | 0.01 | ||
| Ho: No additional information provided by multi-slice ES volume beyond single-slice ES volume (Model XBC vs. XC) | 2.3 | 1 | 0.13 | ||
| Ho: No additional information provided by single-slice ES volume beyond multi-slice ES volume (Model XBC vs. XB) | 6.9 | 1 | <0.01 | ||
| Radiographic Progression case | |||||
| X: KL grade + BMI + sex + age | 25.5 | 5 | 0.60 | ||
| XA: X + MOAKS ES | 31.5 | 8 | 0.75 | ||
| XB: X + Multi-slice ES | 32.8 | 6 | 0.78 | ||
| XC: X + Single-slice ES | 40.0 | 6 | 0.95 | ||
| XAB: X + MOAKS ES + Multi-slice ES | 34.1 | 9 | 0.81 | ||
| XAC: X + MOAKS ES + Single-slice ES | 42.1 | 9 | 1.00 | ||
| XBC: X + Multi-slice ES + Single-slice ES | 40.2 | 7 | 0.95 | ||
| XABC: X + MOAKS ES + Multi-slice ES + Single-slice ES | 42.1 | 10 | 1.00 | ||
| Hypothesis (model comparison) | |||||
| Ho: No additional information provided by MOAKS ES beyond quantitative methods (Model XABC vs. XBC) | 1.9 | 3 | 0.59 | ||
| Ho: No additional information provided by quantitative methods beyond MOAKS ES (Model XABC vs. XA) | 10.6 | 2 | <0.01 | ||
| Ho: No additional information provided by multi-slice ES volume beyond single-slice ES volume (Model XBC vs. XC) | 0.2 | 1 | 0.68 | ||
| Ho: No additional information provided by single-slice ES volume beyond multi-slice ES volume (Model XBC vs. XB) | 7.4 | 1 | <0.01 | ||
| Pain Progression case | |||||
| X: KL grade + BMI + sex + age | 7.7 | 5 | 0.37 | ||
| XA: X + MOAKS ES | 9.4 | 8 | 0.44 | ||
| XB: X + Multi-slice ES | 8.9 | 6 | 0.42 | ||
| XC: X + Single-slice ES | 8.8 | 6 | 0.42 | ||
| XAB: X + MOAKS ES + Multi-slice ES | 9.6 | 9 | 0.46 | ||
| XAC: X + MOAKS ES + Single-slice ES | 15.7 | 9 | 0.75 | ||
| XBC: X + Multi-slice ES + Single-slice ES | 18.8 | 7 | 0.89 | ||
| XABC: X + MOAKS ES + Multi-slice ES + Single-slice ES | 21.1 | 10 | 1.00 | ||
| Hypothesis (model comparison) | |||||
| Ho: No additional information provided by MOAKS ES beyond quantitative methods (Model XABC vs. XBC) | 2.3 | 3 | 0.52 | ||
| Ho: No additional information provided by quantitative methods beyond MOAKS ES (Model XABC vs. XA) | 11.7 | 2 | <0.01 | ||
| Ho: No additional information provided by multi-slice ES volume beyond single-slice ES volume (Model XBC vs. XC) | 10.0 | 1 | <0.01 | ||
| Ho: No additional information provided by single-slice ES volume beyond multi-slice ES volume (Model XBC vs. XB) | 9.9 | 1 | <0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilles, G.; Vohra, A.; Robles, D.; Taljanovic, M.S.; Ashbeck, E.L.; Caruso, C.; Duryea, J.; Bedrick, E.J.; Guermazi, A.; Kwoh, C.K. Reliability and Validity of Single Axial Slice vs. Multiple Slice Quantitative Measurement of the Volume of Effusion-Synovitis on 3T Knee MRI in Knees with Osteoarthritis. J. Clin. Med. 2023, 12, 2691. https://doi.org/10.3390/jcm12072691
Gilles G, Vohra A, Robles D, Taljanovic MS, Ashbeck EL, Caruso C, Duryea J, Bedrick EJ, Guermazi A, Kwoh CK. Reliability and Validity of Single Axial Slice vs. Multiple Slice Quantitative Measurement of the Volume of Effusion-Synovitis on 3T Knee MRI in Knees with Osteoarthritis. Journal of Clinical Medicine. 2023; 12(7):2691. https://doi.org/10.3390/jcm12072691
Chicago/Turabian StyleGilles, Greg, Arjun Vohra, Dagoberto Robles, Mihra S. Taljanovic, Erin L. Ashbeck, Chelsea Caruso, Jeffrey Duryea, Edward J. Bedrick, Ali Guermazi, and C. Kent Kwoh. 2023. "Reliability and Validity of Single Axial Slice vs. Multiple Slice Quantitative Measurement of the Volume of Effusion-Synovitis on 3T Knee MRI in Knees with Osteoarthritis" Journal of Clinical Medicine 12, no. 7: 2691. https://doi.org/10.3390/jcm12072691
APA StyleGilles, G., Vohra, A., Robles, D., Taljanovic, M. S., Ashbeck, E. L., Caruso, C., Duryea, J., Bedrick, E. J., Guermazi, A., & Kwoh, C. K. (2023). Reliability and Validity of Single Axial Slice vs. Multiple Slice Quantitative Measurement of the Volume of Effusion-Synovitis on 3T Knee MRI in Knees with Osteoarthritis. Journal of Clinical Medicine, 12(7), 2691. https://doi.org/10.3390/jcm12072691







