Osteomalacia in Adults: A Practical Insight for Clinicians
Abstract
:1. Introduction
2. Rickets and Osteomalacia
3. Phosphate as a Key Element of Bone Mineralization
3.1. General Aspects of Phosphate Homeostasis
3.2. Hormonal Control of Inorganic Phosphate
3.3. Fibroblast Growth Factor 23 (FGF-23)
4. Clinical Diagnosis of Osteomalacia
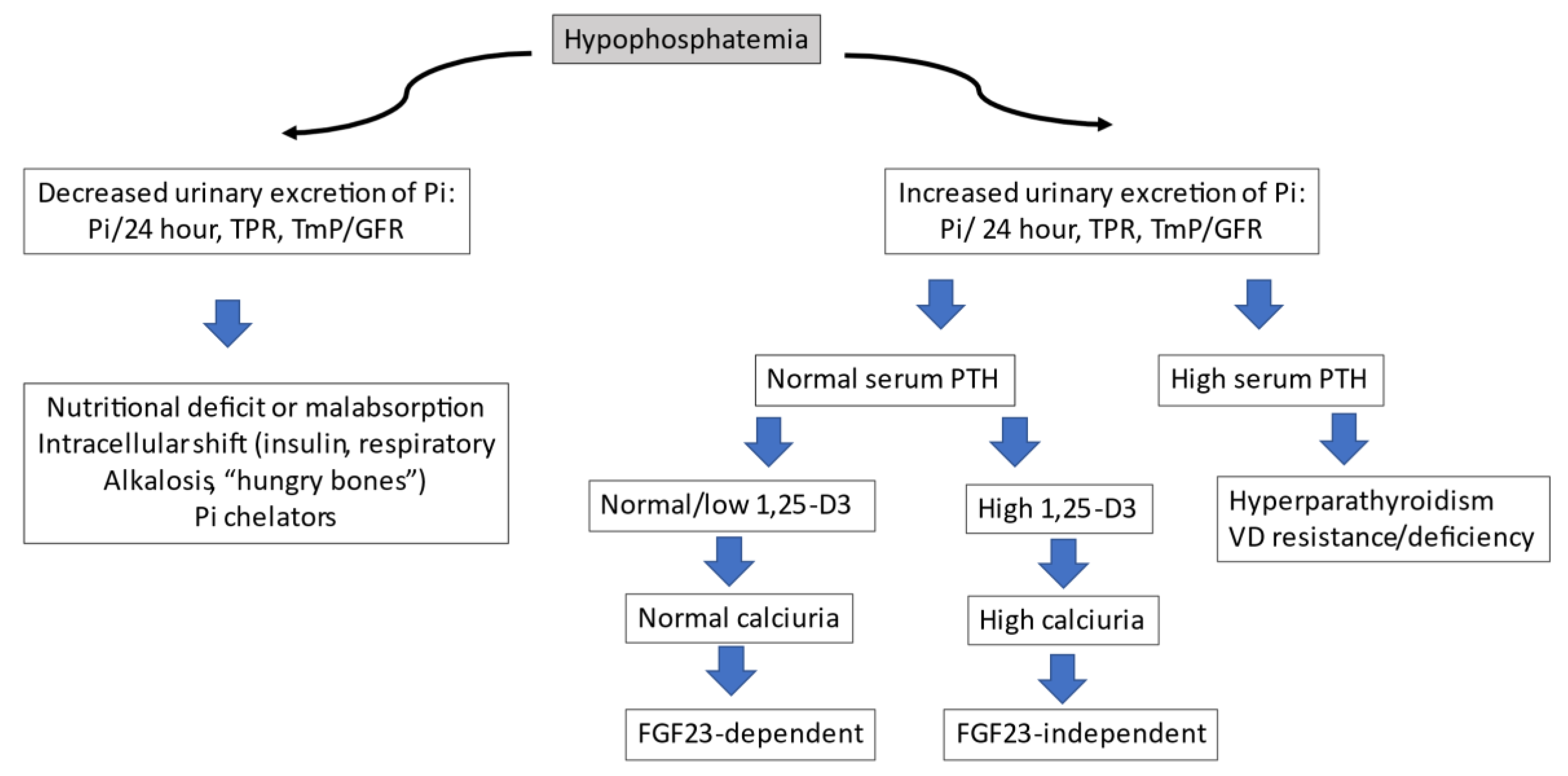
5. Etiology of Osteomalacia
5.1. Nutritional Osteomalacia
- Asymptomatic calcium deprivation phase: only initial biochemical signs are observed, mainly calcidiol below 15 ng/mL, PTH above 50 pg/mL, and a moderately decreased urinary Ca/Cr ratio;
- Pre-OM phase: the previous biochemical signs are more pronounced, with secondary hyperparathyroidism already existing and total alkaline phosphatase beginning to rise. We can observe a decreased serum calcium or phosphate and if we had the option of performing a bone histomorphometry, hyperosteoidosis would already be observed in the static study and delayed mineralization time in the dynamic study of double labeling with tetracycline. In this phase, there are usually no signs on imaging tests, although OM is already beginning to manifest clinically: patients report, to a variable degree, musculoskeletal pain, recurrent falls, hypotonia and muscle weakness, fatigue, and bone fragility, while presenting fractures that are generally wrongly attributed to osteoporosis;
- OM phase: in this phase, signs are observed in the imaging tests, which are added to the previous clinical picture.
5.2. X-Linked Hypophosphatemia
5.3. Oncogenic Osteomalacia
5.4. Drug-Induced Osteomalacia
5.4.1. Methotrexate
5.4.2. Intravenous Iron Salts
5.4.3. Drug-Induced Tubulopathies/Fanconi Syndrome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, M.T.; Marcucci, G.; Anders, H.J.; Beltrami, G.; Cauley, J.A.; Ebeling, P.R.; Kumar, R.; Linglart, A.; Sangiorgi, L.; Towler, D.A.; et al. Skeletal and extraskeletal disorders of biomineralization. Nat. Rev. Endocrinol. 2022, 18, 473–489. [Google Scholar] [CrossRef]
- Burr, D.B.; Akkus, O. Bone Morphology and Organization. In Basic and Applied Bone Biology; Burr, D.B., Allend, M.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 3–26. [Google Scholar]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. Horm. Res. Paediatr. 2016, 85, 83–106. [Google Scholar] [CrossRef] [Green Version]
- Uday, S.; Högler, W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised concepts, public health, and prevention strategies. Curr. Osteoporos. Rep. 2017, 15, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shore, R.M.; Chesney, R.W. Rickets: Part I. Pediatr. Radiol. 2013, 43, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Pike, J.; Glorieux, F. (Eds.) Vitamin D, 2nd ed.; Elsevier: London, UK, 2004. [Google Scholar]
- Figueres, L.; Beck-Cormier, S.; Beck, L.; Marks, J. The complexities of organ crosstalk in phosphate homeostasis: Time to put phosphate sensing back in the lime light. Int. J. Mol. Sci. 2021, 22, 5701. [Google Scholar] [CrossRef]
- Manghat, P.; Sodi, R.; Swaminathan, R. Phosphate homeostasis and disorders. Ann. Clin. Biochem. 2014, 51, 631–656. [Google Scholar] [CrossRef] [Green Version]
- Lotscher, M.; Scarpetta, Y.; Levi, M.; Halaihel, N.; Wang, H.; Zajicek, H.K.; Biber, J.; Murer, H.; Kaissling, B. Rapid downregulation of rat renal Na/P(i) cotransporter in response to parathyroid hormone involves microtubule rearrangement. J. Clin. Invest. 1999, 104, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006, 70, 1548–1559. [Google Scholar] [CrossRef] [Green Version]
- Prie, D.; Ureña Torres, P.; Friedlander, G. Latest findings in phosphate homeostasis. Kidney Int. 2009, 75, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R.A., Jr.; Meyer, M.H.; Gray, R.W. Parabiosis suggests a humoral factor is involved in X-linkedhypophosphatemia in mice. J. Bone Miner. Res. 1989, 4, 493–500. [Google Scholar] [CrossRef]
- Cai, Q.; Hodgson, S.F.; Kao, P.C.; Lennon, V.A.; Klee, G.G.; Zinsmiester, A.R.; Kumar, R. Brief report: Inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N. Engl. J. Med. 1994, 330, 1645–1649. [Google Scholar] [CrossRef]
- ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000, 26, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Ohta, H.; Konishi, M. Endocrine FGFs: Evolution, physiology, pathophysiology, and pharmacotherapy. Front. Endocrinol. 2015, 6, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, Y.; Tamada, T.; Kasai, N.; Urakawa, I.; Aono, Y.; Hasegawa, H.; Fujita, T.; Kuroki, R.; Yamashita, T.; Fukumoto, S.; et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 2008, 23, 1509–1518. [Google Scholar] [CrossRef]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef] [Green Version]
- Gohil, A.; Imel, E.A. FGF23 and associated disorders of phosphate wasting. Pediatr. Endocrinol. Rev. 2019, 17, 17–34. [Google Scholar]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia—Proposal by an expert panel supported by the Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research, and the Japan Endocrine Society. J. Bone Miner. Metab. 2015, 33, 467–473. [Google Scholar] [CrossRef]
- Uday, S.; Högler, W. Spot the silent sufferers: A call for clinical diagnostic criteria for solar and nutritional osteomalacia. J. Steroid Biochem. Mol. Biol. 2019, 188, 141–146. [Google Scholar] [CrossRef]
- Sundaram, M.; Schils, J. Hyperparathyroidism, renal osteodystrophy, osteomalacia and rickets. In Imaging of the Musculoskeletal System, 1st ed; Pope, T.L., Ed.; Saunders: Philadelphia, PA, USA, 2008; pp. 1509–1523. [Google Scholar]
- Bredella, M.A.; Van de Berg, B.C. Metabolic-Endocrine. In Musculoskeletal Diseases 2021–2024; Hodler, J., Kubik-Huc, R.A., von Schulthess, G.K., Eds.; IDKD Springer Series; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Collins, M.T.; Chebli, C.; Jones, J.; Kushner, H.; Consugar, M.; Rinaldo, P.; Wientroub, S.; Bianco, P.; Robey, P.G. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J. Bone Miner. Res. 2001, 16, 806–813. [Google Scholar] [CrossRef]
- Pettifor, J.M. Raquitismo y osteomalacia nutricionales e inducidos por fármacos. In Primer on the Metabolic Bone Disorders and Disorders of Mineral Metabolism. ASBMR, 6th ed.; Favus, M., Ed.; Spanish Edition; Medical Trends S.L.: Barcelona, Spain, 2007; Volume 60, pp. 402–411. [Google Scholar]
- Bhattacharyya, N.; Wiench, M.; Dumitrescu, C.; Connolly, B.M.; Bugge, T.H.; Patel, H.V.; Gafni, R.I.; Cherman, N.; Cho, M.; Hager, G.L.; et al. Mechanism of FGF23 processing in fibrous dysplasia. J. Bone Miner. Res. 2012, 27, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S. Vitamin D: A critical regulator of intestinal physiology. J. Bone Miner. Res. Plus 2021, 5, e10554. [Google Scholar] [CrossRef]
- Bouillon, R.; Antonio, L.; Olarte, O.R. Calcifediol (25OH Vitamin D3) deficiency: A risk factor from early to old age. Nutrients 2022, 14, 1168. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of Vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 1, 1109–1151. [Google Scholar] [CrossRef] [Green Version]
- Minisola, S.; Colangelo, L.; Pepe, J.; Diacinti, D.; Cipriani, C.; Rao, S.D. Osteomalacia and vitamin D status: A clinical update 2020. J. Bone Miner. Res. Plus 2020, 21, e10447. [Google Scholar] [CrossRef] [PubMed]
- Kopic, S.; Geibel, J.P. Gastric acid, calcium absorption, and their impact on bone health. Physiol. Rev. 2013, 93, 189–268. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, M. Assessing vitamin D metabolism-four decades of experience. Clin. Chem. Lab. Med. 2023, 61, 880–894. [Google Scholar] [CrossRef]
- Sempos, C.T.; Durazo-Arvizu, R.A.; Fischer, P.R.; Munns, C.F.; Pettifor, J.M.; Thacher, T.D. Serum 25-hydroxyvitamin D requirements to prevent nutritional rickets in Nigerian children on a low-calcium diet. A multivariable reanalysis. Am. J. Clin. Nutr. 2021, 114, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Imel, E.A. Rickets, vitamin D, and Ca/P metabolism. Horm. Res. Paediatr. 2022, 95, 579–592. [Google Scholar] [CrossRef]
- Miyauchi, M.; Hirai, C.; Nakajima, H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J. Nutr. Sci. Vitaminol. 2013, 59, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck-Nielsen, S.S.; Brock-Jacobsen, B.; Gram, J.; Brixen, K.; Jensen, T.K. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur. J. Endocrinol. 2009, 160, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Endo, I.; Fukumoto, S.; Ozono, K.; Namba, N.; Inoue, D.; Okazaki, R.; Yamauchi, M.; Yamauchi, M.; Sugimoto, T.; Minagawa, M.; et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: Prevalence, biochemical data and treatment. Endocr. J. 2015, 62, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafaelsen, S.; Johansson, S.; Ræder, H.; Bjerknes, R. Hereditary hypophosphatemia in Norway: A retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur. J. Endocrinol. 2016, 174, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Zhang, Y.; Ruan, D.; Huang, J.; Zhu, Y.; Lin, X.; Xiao, X.P.; Cheng, Q.; Geng, Z.B.; Liao, L.S.; et al. Function of PHEX mutations p.Glu145* and p.Trp749Arg in families with X-linked hypophosphatemic rickets by the negative regulation mechanism on FGF23 promoter transcription. Cell Death Dis. 2022, 13, 518. [Google Scholar] [CrossRef]
- Ruppel, M.D.; Jan de Beur, S.M. Disorders of phosphate homeostasis. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. ASBMR, 9th ed.; Bilezikian, J.P., Ed.; Wiley: Hoboken, NJ, USA, 2019; Volume 88, pp. 674–683. [Google Scholar]
- Pettifor, J.M. What’s new in hypophosphataemic rickets? Eur. J. Pediatr. 2008, 167, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Seefried, L.; Smyth, M.; Keen, R.; Harvengt, P. Burden of disease associated with X-linked hypophosphataemia in adults: A systematic literature review. Osteoporos. Int. 2021, 32, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Bubbear, O.; Clarke, S.; Keen, R.; Roy, M.; Anilkumar, A.; Schini, M.; Walsh, J.S.; Javaid, M.K.; Ireland, A. Physical function and physical activity in adults with X-linked hypophosphatemia G. Osteoporos. Int. 2022, 33, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Forestier-Zhang, L.; Watts, L.; Turner, A.; Teare, H.; Kaye, J.; Barrett, J.; Cooper, C.; Eastell, R.; Wordsworth, P.; Javaid, M.K.; et al. Health-related quality of life and a cost-utility simulation of adults in the UK with osteogenesis imperfecta, X-linked hypophosphatemia and fbrous dysplasia. Orphanet. J. Rare Dis. 2016, 11, 160. [Google Scholar] [CrossRef] [Green Version]
- Orlando, G.; Pinedo-Villanueva, R.; Reeves, N.D.; Javaid, M.K.; Ireland, A. Physical function in UK adults with osteogenesis imperfecta: A cross-sectional analysis of the RUDY study. Osteoporos. Int. 2021, 32, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, W.; Wang, Y.; Jin, J.; Zhao, Y.; Zhu, J.; Huang, F. Comparative analysis of clinical and imaging features of osteomalacia and spondyloarthritis. Front. Med. 2021, 8, 680598. [Google Scholar] [CrossRef]
- Lyles, K.W.; Harrelson, J.M.; Drezner, M.K. The efficacy of vitamin D2 and oral phosphorus therapy in X-linked hypophosphatemic rickets and osteomalacia. J. Clin. Endocrinol. Metab. 1982, 54, 307–315. [Google Scholar] [CrossRef]
- Latta, K.; Hisano, S.; Chan, J.C. Therapeutics of X-linked hypophosphatemic rickets. Pediatr. Nephrol. 1993, 7, 744–748. [Google Scholar] [CrossRef]
- Imel, E.A.; Econs, M.J. Approach to the hypophosphatemic patient. J. Clin. Endocrinol. Metab. 2012, 97, 696–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aono, Y.; Yamazaki, Y.; Yasutake, J.; Kawata, T.; Hasegawa, H.; Urakawa, I.; Fujita, T.; Wada, M.; Yamashita, T.; Fukumoto, S.; et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. 2009, 24, 1879–1888. [Google Scholar] [CrossRef]
- Lee, S.K.; Gosselin, N.H.; Taylor, J.; Roberts, M.S.; McKeever, K.; Shi, J. Population pharmacokinetics and pharmacodynamics of Burosumab in adult and pediatric patients with X-linked Hypophosphatemia. J. Clin. Pharmacol. 2022, 62, 87–98. [Google Scholar] [CrossRef]
- Linglart, A.; Imel, E.A.; Whyte, M.P.; Portale, A.A.; Högler, W.; Boot, A.M.; Padidela, R.; Van’t Hoff, W.; Gottesman, G.S.; Chen, A.; et al. Sustained efficacy and safety of Burosumab, a monoclonal antibody to FGF23, in children with X-Linked Hypophosphatemia. J. Clin. Endocrinol. Metab. 2022, 107, 813–824. [Google Scholar] [CrossRef]
- Kritmetapak, K.; Kumar, R. Phosphatonins: From discovery to therapeutics. Endocr. Pract. 2022, 29, 69–79. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Whyte, M.P.; Imel, E.A.; Boot, A.M.; Högler, W.; Linglart, A.; Padidela, R.; Van’t Hoff, W.; Mao, M.; Chen, C.Y.; et al. Burosumab therapy in children with X-Linked Hypophosphatemia. N. Engl. J. Med. 2018, 378, 1987–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imel, E.A.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Ward, L.M.; Nilsson, O.; Simmons, J.H.; Padidela, R.; Namba, N.; Cheong, H.I.; et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: A randomised, active-controlled, open-label, phase 3 trial. Lancet 2019, 393, 2416–2427. [Google Scholar] [CrossRef]
- The European Medicines Agency. European Public Assessment Report. Burosumab (Crysvita®). Available online: https://www.ema.europa.eu/en/documents/assessment-report/crysvita-epar-publicassessment-report_en.pdf (accessed on 2 December 2022).
- Ficha Técnica de Burosumab (Crysvita®). Available online: https://cima.aemps.es/cima/pdfs/es/ft/1171262001/FT_1171262001.pdf (accessed on 2 December 2022).
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Letchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahir, K.; Roberts, M.S.; Krolczyk, S.; Simmons, J.H. X-Linked Hypophosphatemia: A new era in management. J. Endocr. Soc. 2020, 4, bvaa151. [Google Scholar] [CrossRef]
- McCance, R.A. Osteomalacia with Looser’s nodes (Milkman’s syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years. Q. J. Med. 1947, 16, 33–46. [Google Scholar]
- Prader, A.; Illig, R.; Uehlinger, E.; Stalder, G. Rickets following bone tumor. Helv. Paediatr. Acta 1959, 14, 554–565. [Google Scholar]
- Chiam, P.; Tan, H.C.; Bee, Y.M.; Chadran, M. Oncogenic osteomalacia—Hypophosphataemic spectrum from “benignancy” to “malignancy”. Bone 2013, 53, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.; Palermo, A.; Vanderhulst, J.; De Beur, S.M.J.; Fukumoto, S.; Minisola, S.; Xia, W.; Body, J.J.; Zillikens, M.C. Tumor-induced osteomalacia: A systematic clinical review of 895 cases. Calcif. Tissue Int. 2022, 111, 367–379. [Google Scholar] [CrossRef]
- Gupta, A.; Kandasamy, D.; Sharma, R.; Damle, N.; Goyal, A.; Goyal, A.; Agarwal, S.; Dharmashaktu, Y. Imaging characteristics of phosphaturic mesenchymal tumors. Acta Radiol. 2023, 2841851231160086. [Google Scholar] [CrossRef]
- Minisola, S.; Peacock, M.; Fukumoto, S.; Cipriani, C.; Pepe, J.; Tella, S.H.; Collins, M.T. Tumour-induced osteomalacia. Nat. Rev. Dis. Prim. 2017, 3, 17044. [Google Scholar] [CrossRef]
- Brandi, M.L.; Clunie, G.P.R.; Houillier, P.; Jan de Beur, S.M.; Minisola, S.; Oheim, R.; Seefried, L. Challenges in the management of tumor-induced osteomalacia (TIO). Bone 2021, 152, 116064. [Google Scholar] [CrossRef]
- Feng, J.; Jiang, Y.; Wang, O.; Li, M.; Xing, X.; Huo, L.; Li, F.; Yu, W.; Zhong, D.R.; Jin, J.; et al. The diagnostic dilemma of tumor induced osteomalacia: A retrospective analysis of 144 cases. Endocr. J. 2017, 64, 675–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, N.; Kubota, T.; Kitanaka, S.; Fujiwara, I.; Adachi, M.; Takeuchi, Y.; Yamagami, H.; Kimura, T.; Shinoda, T.; Minagawa, M.; et al. Clinical performance of a novel chemiluminescent enzyme immunoassay for FGF23. J. Bone Miner. Metab. 2021, 39, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Minisola, S.; Fukumoto, S.; Xia, W.; Corsi, A.; Colangelo, L.; Scillitani, A.; Pepe, J.; Cipriani, C.; Thakker, R.V. Tumor-induced osteomalacia: A comprehensive review. Endocr. Rev. 2022, 44, bnac026. [Google Scholar] [CrossRef]
- Florenzano, P.; Gafni, R.I.; Collins, M.T. Tumor-induced osteomalacia. Bone Rep. 2017, 20, 90–97. [Google Scholar] [CrossRef]
- Hartley, I.R.; Gafni, R.I.; Roszko, K.L.; Brown, S.M.; de Castro, L.F.; Saikali, A.; Ferreira, C.R.; Gahl, W.A.; Pacak, K.; Blau, J.E.; et al. Determination of FGF23 levels for the diagnosis of FGF23-mediated hypophosphatemia. J. Bone Miner. Res. 2022, 37, 2174–2185. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Koga, M.; Kinoshita, Y.; Hidaka, N.; Hoshino, Y.; Takashi, Y.; Arai, M.; Kobayashi, H.; Katsura, M.; Nakamoto, Y.; et al. Utility of multimodality aproach including systemic FGF23 venous sampling in localizing phosphaturic mesenchymal tumors. J. Endocr. Soc. 2022, 7, bvac181. [Google Scholar] [CrossRef]
- Underwood, J.; Sturchio, G.; Arnold, S. Patient release and instructions for Lutetium Dotatate Radiopharmaceutical Therapy. Health Phys. 2021, 121, 160–165. [Google Scholar] [CrossRef]
- Imanishi, Y.; Ito, N.; Rhee, Y.; Takeuchi, Y.; Shin, C.S.; Takahashi, Y.; Onuma, H.; Onuma, H.; Kojima, M.; Kanematsu, M.; et al. Interim analysis of a phase 2 open-label trial assessing Burosumab efficacy and safety in patients with Tumor-Induced Osteomalacia. J. Bone Miner. Res. 2021, 36, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Zucchi, F.; Alfieri, C.; Caporali, R.; Varenna, M. Long-term use of burosumab for the treatment of tumor-induced osteomalacia. Osteoporos. Int. 2023, 34, 201–206. [Google Scholar] [CrossRef]
- Jan de Beur, S.M.; Minisola, S.; Xia, W.B.; Abrahamsen, B.; Body, J.J.; Brandi, M.L.; Clifton-Bligh, R.; Collins, M.; Florenzano, P.; Houillier, P.; et al. Global guidance for the recognition, diagnosis, and management of tumor-induced osteomalacia. J. Intern. Med. 2023, 293, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, N.; Koga, M.; Kimura, S.; Hoshino, Y.; Kato, H.; Kinoshita, Y.; Makita, N.; Nangaku, M.; Horiguchi, K.; Furukawa, Y.; et al. Clinical challenges in diagnosis, tumor localization and treatment of Tumor-Induced Osteomalacia: Outcome of a retrospective surveillance. J. Bone Miner. Res. 2022, 37, 1479–1488. [Google Scholar] [CrossRef]
- Botrus, G.; Raman, P.; Oliver, T.; Bekaii-Saab, T. Infigratinib (BGJ398): An investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 309–316. [Google Scholar] [CrossRef]
- Hartley, I.R.; Roszko, K.L.; Li, X.; Pozo, K.; Streit, J.; Del Rivero, J.; Magone, M.T.; Smith, M.R.; Vold, R.; Dambkowski, C.L.; et al. Infigratinib reduces Fibroblast Growth Factor 23 (FGF23) and increases blood phosphate in Tumor-Induced Osteomalacia. JBMR Plus 2022, 6, e10661. [Google Scholar] [CrossRef]
- Hall, A.M.; Bass, P.; Unwin, R.J. Drug-induced renal Fanconi syndrome. QJM Int. J. Med. 2014, 107, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knights, M.J.; Finlay, E. The effects of sodium valproate on the renal function of children with epilepsy. Pediatr. Nephrol. 2014, 29, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nishida, R.; Maeda, K.; Sugawara, A.; Kuwahara, T. Chinese herb nephropathy in Japan presents adult-onset Fanconi syndrome: Could different components of aristolochic acids cause a different type of Chinese herb nephropathy? Clin. Nephrol. 2000, 53, 301–306. [Google Scholar]
- Wako, Y.; Hiratsuka, H.; Kurotaki, T.; Tsuchitani, M.; Umemura, T. Relationship between osteoid formation and iron deposition induced by chronic cadmium exposure in ovariectomized rats. J. Appl. Toxicol. 2021, 41, 1304–1315. [Google Scholar] [CrossRef]
- Verzicco, I.; Regolisti, G.; Quaini, F.; Bocchi, P.; Brusasco, I.; Ferrari, M.; Passeri, G.; Cannone, V.; Coghi, P.; Fiaccadori, E.; et al. Electrolyte disorders induced by antineoplastic drugs. Front. Oncol. 2020, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Zechner, C.; Adams-Huet, B.; Gregory, B.; Neyra, J.A.; Rule, J.A.; Li, X.; Rakela, J.; Moe, O.W.; Lee, W.M.; Acute Liver Failure Study Group. Hypophosphatemia in acute liver failure of a broad range of etiologies is associated with phosphaturia without kidney damage or phosphatonin elevation. Transl. Res. 2021, 238, 1–11. [Google Scholar] [CrossRef]
- Matsubara, N.; Uemura, H.; Nagamori, S.; Suzuki, H.; Uemura, H.; Kimura, G. A Phase II, randomized, open-label, multi-arm study of TAS-115 for castration-resistant prostate cancer patients with bone metastases. Clin. Genitourin. Cancer 2021, 19, 491–500. [Google Scholar] [CrossRef]
- Arora, E.; Singh, H.; Gupta, Y.K. Impact of antiepileptic drugs on bone health: Need for monitoring, treatment, and prevention strategies. J. Fam. Med. Prim. Care 2016, 5, 248–253. [Google Scholar]
- Regidor, B.; Swift, R.; Eades, B.; Emamy-Sadr, M.; Tarhini, F.; Spektor, T.M.; Berenson, J.R. Frequent occurrence of hypophosphatemia among multiple myeloma patients treated with elotuzumab: A single clinic retrospective study. Ann. Hematol. 2021, 100, 1079–1085. [Google Scholar] [CrossRef]
- Berg, S.L.; Cairo, M.S.; Russell, H.; Ayello, J.; Ingle, A.M.; Lau, H.; Chen, N.; Adamson, P.C.; Blaney, S.M. Safety, pharmacokinetics, and immunomodulatory effects of lenalidomide in children and adolescents with relapsed/refractory solid tumors or myelodysplastic syndrome: A Children’s Oncology Group Phase I Consortium report. J. Clin. Oncol. 2011, 29, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Higgins, M.J.; Tolaney, S.M.; Come, S.E.; Smith, M.R.; Fornier, M.; Mahmood, U.; Baselga, J.; Yeap, B.Y.; Chabner, B.A.; et al. A phase II trial of Cabozantinib in Hormone Receptor-Positive Breast Cancer with Bone Metastases. Oncologist 2020, 25, 652–660. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Mehra, R.; Tan, D.S.; Felip, E.; Chow, L.Q.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Klute, K.A.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; Nazemzadeh, R.; Yost, K.J.; Duvivier, H.L.; Ahn, E.R.; Cannon, T.L.; Alese, O.B.; et al. Cobimetinib plus Vemurafenib in patients with Colorectal Cancer with BRAF mutations: Results from the targeted agent and profiling utilization registry (TAPUR) study. JCO Precis. Oncol. 2022, 6, e2200191. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Blumenthal, G.M.; Luo, L.; He, K.; Fran, I.; Lemery, S.; Pazdur, R. Benefit-Risk Summary of Crizotinib for the treatment of patients with ROS1 Alteration-Positive, Metastatic Non-Small Cell Lung Cancer. Oncologist 2016, 21, 974–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanchoo, R.; Jhaveri, K.D.; Deray, G.; Launay-Vacher, V. Renal effects of BRAF inhibitors: A systematic review by the Cancer and the Kidney International Network. Clin. Kidney J. 2016, 9, 245–251. [Google Scholar] [CrossRef]
- Jaeckle, K.A.; Anderson, S.K.; Twohy, E.L.; Dixon, J.G.; Giannini, C.; Jenkins, R.; Egorin, M.J.; Sarkaria, J.N.; Brown, P.D.; Flynn, P.J.; et al. Phase I-II trial of imatinib mesylate (Gleevec; STI571) in treatment of recurrent oligodendroglioma and mixed oligoastrocytoma. North central cancer treatment group study N0272 (ALLIANCE/NCCTG). J. Neurooncol. 2019, 143, 573–581. [Google Scholar] [CrossRef]
- Al-Kali, A.; Tibes, R.; Atherton, P.; Palmer, J.; Alkhateeb, H.B.; Patnaik, M.; Begna, K.; Gangat, N.; Hashmi, S.; He, R.; et al. A Phase II Study of combination Daunorubicin, Cytarabine (Ara-c) and Nilotinib (TAsigna) (DATA) in patients newly diagnosed with Acute Myeloid Leukemia with KIT Expression. Am. J. Hematol. 2023, 98, 472–480. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Takata, N.; Takeda, Y.; Kido, H.; Iida, N.; Kitahara, M.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Safety and efficacy of sorafenib followed by regorafenib or lenvatinib in patients with hepatocellular carcinoma. Hepatol. Res. 2021, 51, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Bolejack, V.; Ganjoo, K.N.; George, S.; Agulnik, M.; Rushing, D.; Loggers, E.T.; Livingston, M.B.; Wright, J.; Chawla, S.P.; et al. A phase II trial of regorafenib in patients with advanced Ewing sarcoma and related tumors of soft tissue and bone: SARC024 trial results. Cancer Med. 2022, 12, 1532–1539. [Google Scholar] [CrossRef]
- Schelman, W.R.; Mohammed, T.A.; Traynor, A.M.; Kolesar, J.M.; Marnocha, R.M.; Eickhoff, J.; Keppen, M.; Alberti, D.B.; Wilding, G.; Takebe, N.; et al. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Investig. New Drugs 2014, 32, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Valencia, D.N.; Fershko, A. Partial Fanconi syndrome induced by Ifosfamide. Cureus 2019, 11, e3947. [Google Scholar] [CrossRef] [Green Version]
- Jebali, M.; Elaidi, R.; Brizard, M.; Fouque, J.; Takouchop, C.; Sabatier, B.; Oudard, S.; Medioni, J. Biological toxicities as surrogate markers of efficacy in patients treated with mTOR inhibitors for metastatic renal cell carcinoma. BMC Cancer 2017, 17, 27. [Google Scholar] [CrossRef] [Green Version]
- Naesens, M.; Kuypers, D.R.; Sarwal, M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009, 4, 481–508. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Li, T.; Dai, H.; Feng, C.; Xie, X.; Peng, F.; Lan, G.; Yu, S.; Wang, Y.; Fang, C.; et al. Drug-induced Fanconi syndrome in patients with kidney allograft transplantation. Front. Immunol. 2022, 13, 979983. [Google Scholar] [CrossRef]
- Portales-Castillo, I.; Mount, D.B.; Nigwekar, S.U.; Yu, E.W.; Rennke, H.G.; Gupta, S. Zoledronic acid-associated Fanconi syndrome in patients with cancer. Am. J. Kidney Dis. 2022, 80, 555–559. [Google Scholar] [CrossRef]
- Bagger, L.W.; Hansen, P.K.D.; Schwarz, P.; Nielsen, B.R. Severe hypophosphataemia following oral bisphosphonate treatment in a patient with osteoporosis. Drug Ther. Bull. 2021, 59, 107–111. [Google Scholar] [CrossRef]
- Nachankar, A.; Katyal, A.; Bansal, N.; Bishnoi, A. Hungry bone syndrome like presentation following single-dose denosumab for hypercalcaemia secondary to sarcoidosis with IgA nephropathy. BMJ Case Rep. 2022, 15, e250647. [Google Scholar] [CrossRef]
- Rashed, R.; Hyassat, D.; Batieha, A.; Aldabbas, M.; Aldarabah, F.; El-Khateeb, M.; Ajlouni, K. Prevalence and correlates of hypophosphatemia among type 2 diabetic patients attending the National Center for Diabetes, Endocrinology and Genetics (NCDEG). Ann. Med. Surg. 2022, 78, 103770. [Google Scholar] [CrossRef]
- Ragab, A.H.; Frech, R.S.; Vietti, T.J. Osteoporotic fractures secondary to methotrexate therapy of acute leukemia in remission. Cancer 1970, 25, 580–585. [Google Scholar] [CrossRef]
- Rolvien, T.; Jandl, N.M.; Stürznickel, J.; Beil, F.T.; Kötter, I.; Oheim, R.; Lohse, A.W.; Barvencik, F.; Amling, M. Clinical and radiological characterization of patients with immobilizing and progressive stress fractures in Methotrexate Osteopathy. Calcif. Tissue Int. 2021, 108, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Rolvien, T.; Creutzfeldt, A.M.; Lohse, A.W.; Amling, M. Stress fractures in systemic lupus erythematosus after long-term MTX use successfully treated by MTX discontinuation and individualized bone-specific therapy. Lupus 2019, 28, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Glaspy, J.A.; Wolf, M.; Strauss, W.E. Intravenous Iron-Induced Hypophosphatemia: An emerging syndrome. Adv. Ther. 2021, 38, 3531–3549. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ganz, T.; Trumbo, H.; Seid, M.H.; Goodnough, L.T.; Levine, M.A. Parenteral iron therapy and phosphorus homeostasis: A review. Am. J. Hematol. 2021, 96, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Detlie, T.E.; Lindstrom, J.C.; Jahnsen, M.E.; Finnes, E.; Zoller, H.; Moum, B.; Jahnsen, J. Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment. Pharmacol. Ther. 2019, 50, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.W. Fanconi síndrome and other tubule proximal disorders. In Comprehensive Clinical Nephrology, 6th ed.; Feehally, J., Floege, J., Tonnelli, M., Johnson, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 48, pp. 586–596. [Google Scholar]
- Casado, J.L. Renal and bone toxicity with the use of Tenofovir: Understanding at the end. AIDS Rev. 2016, 18, 59–68. [Google Scholar] [PubMed]
- Kohler, J.J.; Hosseini, S.H. Subcellular renal proximal tubular mitochondrial toxicity with tenofovir treatment. Methods Mol. Biol. 2011, 755, 267–277. [Google Scholar]
- Sano, T.; Kawaguchi, T.; Ide, T.; Amano, K.; Kuwahara, R.; Arinaga-Hino, T.; Torimura, T. Tenofovir Alafenamide rescues renal tubules in patients with Chronic Hepatitis B. Life 2021, 11, 263. [Google Scholar] [CrossRef]
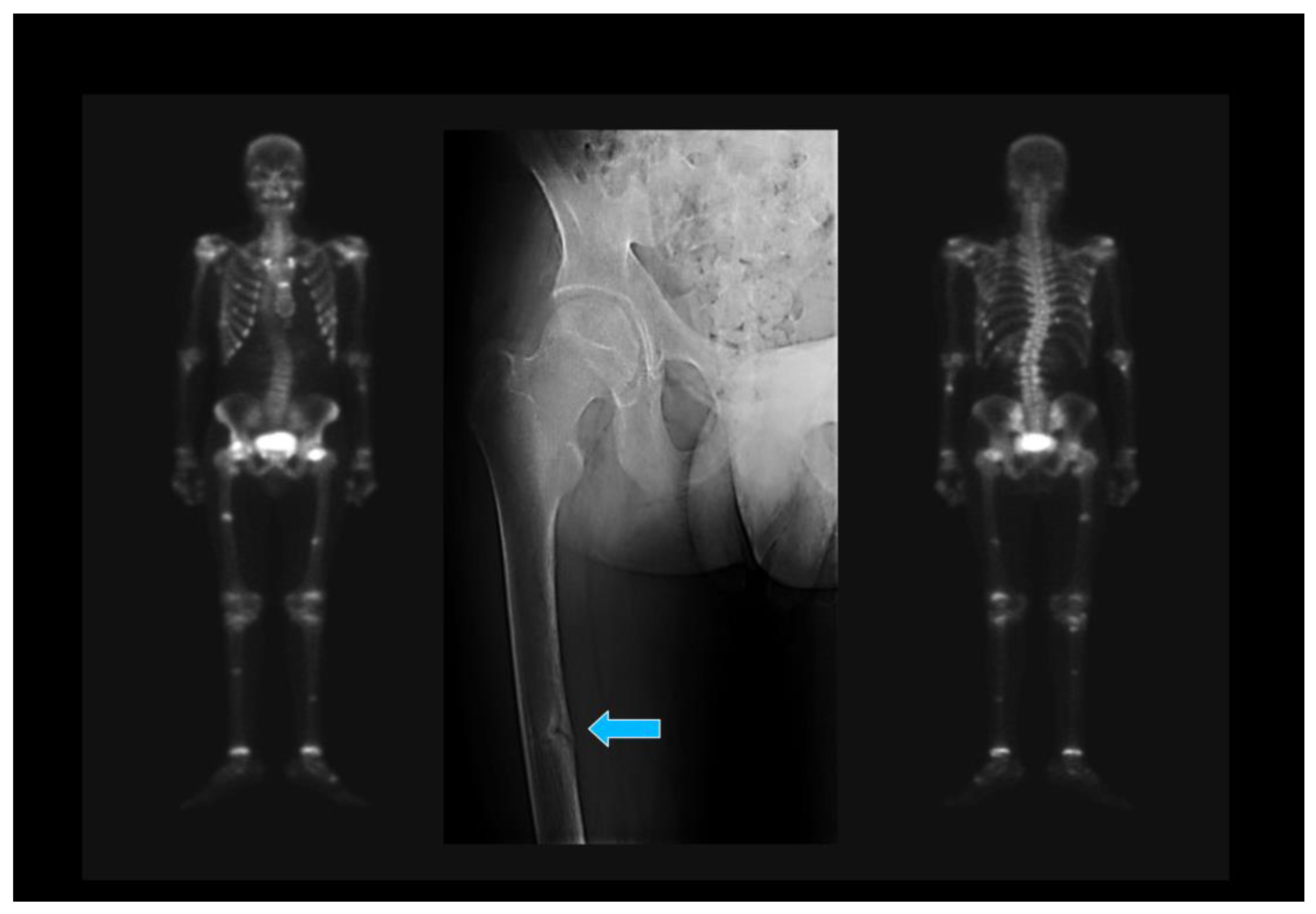

| 1. Japanese Criteria [20] |
| (a) Hypophosphatemia or hypocalcemia; (b) Elevated bone alkaline phosphatase; (c) Muscle weakness or bone pain; (d) BMD <80% in young adults; (e) Image: multiple uptake bone zones or Looser–Milkman fractures. |
| Defined OM: a–e. Probable OM: a+b and 2 out of c–e. BMD: Bone mineral density. |
| 2. Uday–Hogler Criteria [21] |
| (a) Elevated parathormone (PTH); (b) Elevated total alkaline phosphatase; (c) Calcium intake <300 mg/d or serum calcidiol <30 nmol/L; (d) Low urinary calcium. |
| The above criteria, applied in the absence of kidney or liver disease, suggest the diagnosis of OM. The additional presence of symptoms and Looser zones helps in advanced phases. |
| Name | Gen | Hereditary Transmission | Clinical Picture |
|---|---|---|---|
| XLH | PHEX | X-linked | Post rickety sequelae. Hypophosphatemia. Low calcitriol |
| ADHR | FGF23 | AD | Similar to XLH |
| ARHR1 | DMP1 | AR | Similar to XLH |
| ARHR2 | ENPP1 | AR | Similar to XLH with arterial calcifications in childhood (GACI syndrome) |
| ARHR3 | FAM20c | AR | Hypophosphatemia with osteosclerosis, facial dysmorphia, brain calcifications, and severe dental alterations |
| OGD | FGFR1 | Hypophosphatemic rickets with craniosynostosis, facial dysmorphism, and dwarfism | |
| McCune–Albright syndrome | Sporadic congenital disorders due to postzygotic mutations in genes that activate the signal or levels of FGF23 | Classic triad: precocious puberty, café au lait spots, and fibrous dysplasia. Associated rickets is rare and is due to FGF23 production by bone lesions. | |
| TIO | Severe acquired hypophosphatemia secondary to ectopic production of FGF23. There may be hypocalcemia caused by a decrease in FGF23 dependent on the synthesis of calcitriol. | ||
| HHRH | SLC34A3 | AR | Postrickets sequelae, hypophosphatemia, hypercalciuria, and lithiasis with nephrocalcinosis. |
| HRHPT | α-KLOTHO | AD | Macrocephaly, dysplasia of the nasal bones, hypercalcemia, and hyperparathyroidism. |
| Congenital and acquired tubulopathies | They can affect the elimination of phosphates in an isolated way or within Fanconi syndrome. |
| Place | Latitude | July | December |
|---|---|---|---|
| Sapporo (Japan) | 43.06 N | 4.6–7.4 | 76.4–497.4 |
| Asturias (Spain) | 44.46 N | 6 | 286 |
| Tsukuba (Japan) | 32.02 N | 3.5–5.9 | 22.4–106.0 |
| Cadiz (Spain) | 36.32 N | 4–5 | 64 |
| Naha (Japan) | 26.12 N | 2.9–8.8 | 7.5–78.0 |
| Tenerife (Spain) | 28.0 N | 6 | 43 |
| Drug Class | Drug |
|---|---|
| Analgesics | Acetaminophen [85] |
| Antiandrogens | Abiraterone acetate [86] |
| Anticonvulsants | Phenytoin [87], topiramate [87] |
| Antineoplasics | Elotuzumab [88] Lenalidomide [89], Cabozantinib [90], Ceritinib [91], Cobimetinib [92], Crizotinib [93], Dabrafenib [94], Imatinib [95], Nilotinib [96], Sorafenib [97], Regorafenib [98], Cis-platinum [99], Ifosfamide [100] |
| mTOR inhibitors | Sirolimus [101], Everolimus [101], Temsirolimus [101] |
| Calcineurin inhibitors | Cyclosporine [102], Tacrolimus [103] |
| Antiresorptives | Zoledronate [104], Etidronate [105], Alendronate [105], Denosumab [106] |
| Diuretics | Thiazides [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arboleya, L.; Braña, I.; Pardo, E.; Loredo, M.; Queiro, R. Osteomalacia in Adults: A Practical Insight for Clinicians. J. Clin. Med. 2023, 12, 2714. https://doi.org/10.3390/jcm12072714
Arboleya L, Braña I, Pardo E, Loredo M, Queiro R. Osteomalacia in Adults: A Practical Insight for Clinicians. Journal of Clinical Medicine. 2023; 12(7):2714. https://doi.org/10.3390/jcm12072714
Chicago/Turabian StyleArboleya, Luis, Ignacio Braña, Estefanía Pardo, Marta Loredo, and Rubén Queiro. 2023. "Osteomalacia in Adults: A Practical Insight for Clinicians" Journal of Clinical Medicine 12, no. 7: 2714. https://doi.org/10.3390/jcm12072714
APA StyleArboleya, L., Braña, I., Pardo, E., Loredo, M., & Queiro, R. (2023). Osteomalacia in Adults: A Practical Insight for Clinicians. Journal of Clinical Medicine, 12(7), 2714. https://doi.org/10.3390/jcm12072714







