Usefulness of the Duke Activity Status Index to Assess Exercise Capacity and Predict Risk Stratification in Patients with Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Methods
2.1. Study Setting
2.2. Participants and Recruitment
2.3. Procedure
2.4. Assessment of Exercise Capacity
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassoun, P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm. Circ. 2021, 11, 2045894020977300. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.M.T.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Malenfant, S.; Lebret, M.; Breton-Gagnon, É.; Potus, F.; Paulin, R.; Bonnet, S.; Provencher, S. Exercise intolerance in pulmonary arterial hypertension: Insight into central and peripheral pathophysiological mechanisms. Eur. Respir. Rev. 2021, 30, 200284. [Google Scholar] [CrossRef]
- Matura, L.A.; McDonough, A.; Carroll, D.L. Cluster analysis of symptoms in pulmonary arterial hypertension: A pilot study. Eur. J. Cardiovasc. Nurs. 2012, 11, 51–61. [Google Scholar] [CrossRef]
- Matura, L.A.; Shou, H.; Fritz, J.S.; Smith, K.A.; Vaidya, A.; Pinder, D.; Archer-Chicko, C.; Dubow, D.; Palevsky, H.I.; Sommers, M.S.; et al. Physical Activity and Symptoms in Pulmonary Arterial Hypertension. Chest 2016, 150, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Okumus, G.; Aslan, G.K.; Arseven, O.; Ongen, G.; Issever, H.; Kiyan, E. The role of an activity monitor in the objective evaluation of patients with pulmonary hypertension. Clin. Respir. J. 2018, 12, 119–125. [Google Scholar] [CrossRef]
- Arena, R.; Lavie, C.J.; Milani, R.V.; Myers, J.; Guazzi, M. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: An evidence-based review. J. Heart Lung Transplant. 2010, 29, 159–173. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partington, S.; Atwood, J.E. Exercise Capacity and Mortality among Men Referred for Exercise Testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef]
- Wensel, R.; Francis, D.P.; Meyer, F.J.; Opitz, C.F.; Bruch, L.; Halank, M.; Winkler, J.; Seyfarth, H.-J.; Gläser, S.; Blumberg, F.; et al. Incremental prognostic value of cardiopulmonary exercise testing and resting haemodynamics in pulmonary arterial hypertension. Int. J. Cardiol. 2013, 167, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Deboeck, G.; Scoditti, C.; Huez, S.; Vachiéry, J.-L.; Lamotte, M.; Sharples, L.; Melot, C.; Naeije, R. Exercise testing to predict outcome in idiopathic versus associated pulmonary arterial hypertension. Eur. Respir. J. 2012, 40, 1410–1419. [Google Scholar] [CrossRef]
- Badagliacca, R.; Papa, S.; Poscia, R.; Valli, G.; Pezzuto, B.; Manzi, G.; Torre, R.; Gianfrilli, D.; Sciomer, S.; Palange, P.; et al. The added value of cardiopulmonary exercise testing in the follow-up of pulmonary arterial hypertension. J. Heart Lung Transplant. 2019, 38, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Huscher, D.; Vonk-Noordegraaf, A.; Ewert, R.; Lange, T.J.; Klose, H.; Dumitrescu, D.; Halank, M.; Held, M.; Gall, H.; et al. The 6MWT as a prognostic tool in pulmonary arterial hypertension: Results from the COMPERA registry. Clin. Res. Cardiol. 2018, 107, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Paolillo, S.; Costanzo, P.; D’Amore, C.; Cecere, M.; Losco, T.; Musella, F.; Gargiulo, P.; Marciano, C.; Perrone-Filardi, P. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J. Am. Coll. Cardiol. 2012, 60, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; Boineau, R.E.; Higginbotham, M.B.; Lee, K.L.; Mark, D.B.; Califf, R.M.; Cobb, F.R.; Pryor, D.B. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am. J. Cardiol. 1989, 64, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.-G.; Bolshinsky, V.; Ismail, H.; Ho, K.-M.; Heriot, A.; Riedel, B. Comparison of Duke Activity Status Index with cardiopulmonary exercise testing in cancer patients. J. Anesth. 2018, 32, 576–584. [Google Scholar] [CrossRef]
- Parissis, J.T.; Nikolaou, M.; Birmpa, D.; Farmakis, D.; Paraskevaidis, I.; Bistola, V.; Katsoulas, T.; Filippatos, G.; Kremastinos, D.T. Clinical and prognostic value of Duke’s Activity Status Index along with plasma B-type natriuretic peptide levels in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2009, 103, 73–75. [Google Scholar] [CrossRef]
- Reed, J.L.; Cotie, L.M.; Cole, C.A.; Harris, J.; Moran, B.; Scott, K.; Terada, T.; Buckley, J.P.; Pipe, A.L. Submaximal Exercise Testing in Cardiovascular Rehabilitation Settings (BEST Study). Front. Physiol. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Struthers, R.; Erasmus, P.; Holmes, K.; Warman, P.; Collingwood, A.; Sneyd, J.R. Assessing fitness for surgery: A comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. Br. J. Anaesth. 2008, 101, 774–780. [Google Scholar] [CrossRef]
- Wijeysundera, D.N.; Pearse, R.M.; Shulman, M.A.; Abbott, T.E.F.; Torres, E.; Ambosta, A.; Croal, B.L.; Granton, J.T.; Thorpe, K.E.; Grocott, M.P.W.; et al. Assessment of functional capacity before major non-cardiac surgery: An international, prospective cohort study. Lancet 2018, 391, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Hammadah, M.; Fan, Y.; Hazen, S.L.; Tang, W.H.W. Prognostic value of estimating functional capacity with the use of the duke activity status index in stable patients with chronic heart failure. J. Card. Fail. 2015, 21, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, 2215–2245. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef] [PubMed]
- Wijeysundera, D.N.; Beattie, W.S.; Hillis, G.S.; Abbott, T.E.F.; Shulman, M.A.; Ackland, G.L.; Mazer, C.D.; Myles, P.S.; Pearse, R.M.; Cuthbertson, B.H.; et al. Integration of the Duke Activity Status Index into preoperative risk evaluation: A multicentre prospective cohort study. Br. J. Anaesth. 2020, 124, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Mustafaoglu, R.; Demir, R.; Aslan, G.K.; Sinan, U.Y.; Zeren, M.; Kucukoglu, M.S. Does Duke Activity Status Index help predicting functional exercise capacity and long-term prognosis in patients with pulmonary hypertension? Respir. Med. 2021, 181, 106375. [Google Scholar] [CrossRef]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Zhang, J.; Hua, D.; Deng, D.; Meng, D.; Zhang, Y.; Dai, J.; Tu, W. The relationship between functional capacity (FC) and cardiac autonomic nervous dysfunction to surgery stress in senile patients. Arch. Gerontol. Geriatr. 2011, 53, 95–99. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Qiu, X.; Luo, W.; Luo, X.; Liu, H.; Geng, Q.; Ma, H.; Xue, L.; Guo, L. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity on Peak Oxygen Uptake and Myocardial Fibrosis in Patients With Myocardial Infarction: Protocol for a Randomized Controlled Trial. Front. Cardiovasc. Med. 2022, 9, 860071. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Shariat, A.; Cleland, J.A.; Danaee, M.; Alizadeh, R.; Sangelaji, B.; Kargarfard, M.; Ansari, N.N.; Sepehr, F.H.; Tamrin, S.B.M. Borg CR-10 scale as a new approach to monitoring office exercise training. Work 2018, 60, 549–554. [Google Scholar] [CrossRef] [PubMed]
- George, M.J.; Kasbekar, S.A.; Bhagawati, D.; Hall, M.; Buscombe, J.R. The value of the Duke Activity Status Index (DASI) in predicting ischaemia in myocardial perfusion scintigraphy—A prospective study. Nucl. Med. Rev. Cent. East. Eur. 2010, 13, 59–63. [Google Scholar] [PubMed]
- Meijer, R.; van Hooff, M.; Papen-Botterhuis, N.E.; Molenaar, C.J.L.; Regis, M.; Timmers, T.; van de Poll-Franse, L.V.; Savelberg, H.H.C.M.; Schep, G. Estimating VO2peak in 18-90 Year-Old Adults: Development and Validation of the FitMáx©-Questionnaire. Int. J. Gen. Med. 2022, 15, 3727–3737. [Google Scholar] [CrossRef] [PubMed]
- Coute, R.A.; Ehrenfeld, J.M.; Gupta, D.K.; Terekhov, M.A.; Wanderer, J.P. Electronically self-assessed functional capacity and exercise testing: A comparison of the Duke Activity Status Index and Patient-Reported Outcomes Measurement Information System tools. Am. Heart J. 2017, 188, 82–86. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Olson, M.; McGorray, S.; Pakstis, D.L.; Zell, K.; Rickens, C.R.; Kelsey, S.F.; Bittner, V.; Sharaf, B.L.; Sopko, G. Physical activity and functional capacity measurement in women: A report from the NHLBI-sponsored WISE study. J. Womens Health Gend. Based Med. 2000, 9, 769–777. [Google Scholar] [CrossRef]
- Phillips, L.; Wang, J.W.; Pfeffer, B.; Gianos, E.; Fisher, D.; Shaw, L.J.; Mieres, J.H. Clinical role of the Duke Activity Status Index in the selection of the optimal type of stress myocardial perfusion imaging study in patients with known or suspected ischemic heart disease. J. Nucl. Cardiol. 2011, 18, 1015–1020. [Google Scholar] [CrossRef]
- Carter, R.; Holiday, D.B.; Grothues, C.; Nwasuruba, C.; Stocks, J.; Tiep, B. Criterion validity of the Duke Activity Status Index for assessing functional capacity in patients with chronic obstructive pulmonary disease. J. Cardpulm. Rehabil. 2002, 22, 298–308. [Google Scholar] [CrossRef]
- Shaw, L.J.; Olson, M.B.; Kip, K.; Kelsey, S.F.; Johnson, B.D.; Mark, D.B.; Reis, S.E.; Mankad, S.; Rogers, W.J.; Pohost, G.M.; et al. The value of estimated functional capacity in estimating outcome: Results from the NHBLI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 3), S36–S43. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mansoor, H.; Li, Q.; Guo, Y.; Handberg, E.M.; Bairey Merz, C.N.; Pepine, C.J. Long-term mortality and estimated functional capacity among women with symptoms of ischemic heart disease: From the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Am. Heart J. 2018, 206, 123–126. [Google Scholar] [CrossRef]
- Potter, E.; Yang, H.; Wright, L.; Wang, B.; Marwick, T.H. Measurement of Functional Capacity to Discriminate Clinical from Subclinical Heart Failure in Patients ≥65 Years of Age. Am. J. Cardiol. 2020, 127, 84–91. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Patients | IPAH | CHD–PAH | Others | p-Value |
|---|---|---|---|---|---|
| (n = 89) | (n = 20) | (n = 62) | (n = 7) | ||
| Age—years | 34.53 ± 8.97 | 36.25 ± 9.36 | 33.47 ± 8.58 | 36.29 ± 3.04 | 0.075 |
| BMI—kg/m2 | 20.29 ± 3.35 | 22.30 ± 2.76 | 19.46 ± 3.30 | 21.37 ± 3.08 | 0.003 |
| Smoking—yes/no | 14 (15.7%)/75 (84.3%) | 3 (15.0%)/17 (85.0%) | 9 (14.5%)/53 (85.5%) | 2 (28.6%)/5 (71.4) | 0.623 |
| Gender—female/male | 74 (83.1%)/15 (16.9%) | 16 (80.0%)/4 (20.0%) | 52 (83.9%)/10 (16.1%) | 6 (85.7%)/1 (14.3%) | 0.909 |
| Marital status | 0.293 | ||||
| Married | 58 (65.2%) | 17 (85.0%) | 35 (56.5%) | 6 (85.7%) | |
| Single | 25 (28.1%) | 2 (10.0%) | 22 (35.5%) | 1 (14.3%) | |
| Divorce | 6 (6.7%) | 1 (5.0%) | 5(8.0%) | 0 | |
| Educational level | 0.373 | ||||
| Primary school | 7 (7.8%) | 3 (15.0%) | 3 (4.8%) | 1 (14.3%) | |
| Middle school | 24 (27.0%) | 4 (20.0%) | 18 (29.0%) | 2 (28.6%) | |
| High school | 24 (27.0%) | 4 (20.0%) | 20 (32.3%) | 0 | |
| College or above | 34 (38.2%) | 9 (45.0%) | 21 (33.9%) | 4 (57.1%) | |
| Course of disease (year) | 5.73 ± 4.57 | 4.55 ± 3.85 | 5.95 ± 4.82 | 7.29 ± 4.82 | 0.112 |
| WHO-FC | 0.762 | ||||
| I | 21 (23.6%) | 3 (15.0%) | 16 (25.8%) | 2 (28.6%) | |
| II | 62 (69.7%) | 15 (75.0%) | 43 (69.4%) | 4 (57.1%) | |
| III | 6 (6.7%) | 2 (10.0%) | 3 (4.8%) | 1 (14.3%) | |
| Right heart catheterization | |||||
| PAP (mean)—mmHg | 57.28 ± 21.67 | 49.50 ± 20.03 | 59.70 ± 22.19 | 53.83 ± 19.5 | 0.383 |
| 3wPAWP—mmHg | 8.97 ± 3.15 | 8.67 ± 3.11 | 9.02 ± 3.24 | 9.17 ± 3.24 | 0.937 |
| CI—L/min/m2 | 3.46 ± 1.09 | 3.25 ± 1.08 | 3.46 ± 1.08 | 3.84 ± 1.28 | 0.403 |
| RAP—mmHg | 5.03 ± 3.19 | 7.17 ± 5.02 | 4.51 ± 2.47 | 4.67 ± 1.86 | 0.201 |
| Echocardiography | |||||
| LVEF % | 68.78 ± 5.74 | 69.10 ± 5.19 | 68.80 ± 5.90 | 67.71 ± 6.53 | 0.988 |
| TAPSE mm | 18.01 ± 3.84 | 19.84 ± 4.10 | 17.64 ± 3.51 | 16.03 ± 4.53 | 0.084 |
| DASI total score | 36.47 ± 13.58 | 33.09 ± 13.45 | 37.52 ± 13.39 | 36.84 ± 16.08 | 0.451 |
| PeakVO2—mL/min/kg | 13.70 ± 3.74 | 13.97 ± 2.26 | 13.71 ± 3.90 | 12.87 ± 7.74 | 0.890 |
| Peak VO2 < 11 mL/min/kg | 20 (22.4%) | 4 (20.0%) | 14 (22.5%) | 2 (28.6%) | 0.342 |
| 6MWD—meters | 478.10 ± 100.80 | 516.45 ± 89.54 | 467.97 ± 103.16 | 467.97 ± 100.40 | 0.161 |
| All the PAH (n = 89) | CHD–PAH (n = 63) | |||||||
|---|---|---|---|---|---|---|---|---|
| r | r2 | Standardization Coefficient | p | r | r2 | Standardization Coefficient | p | |
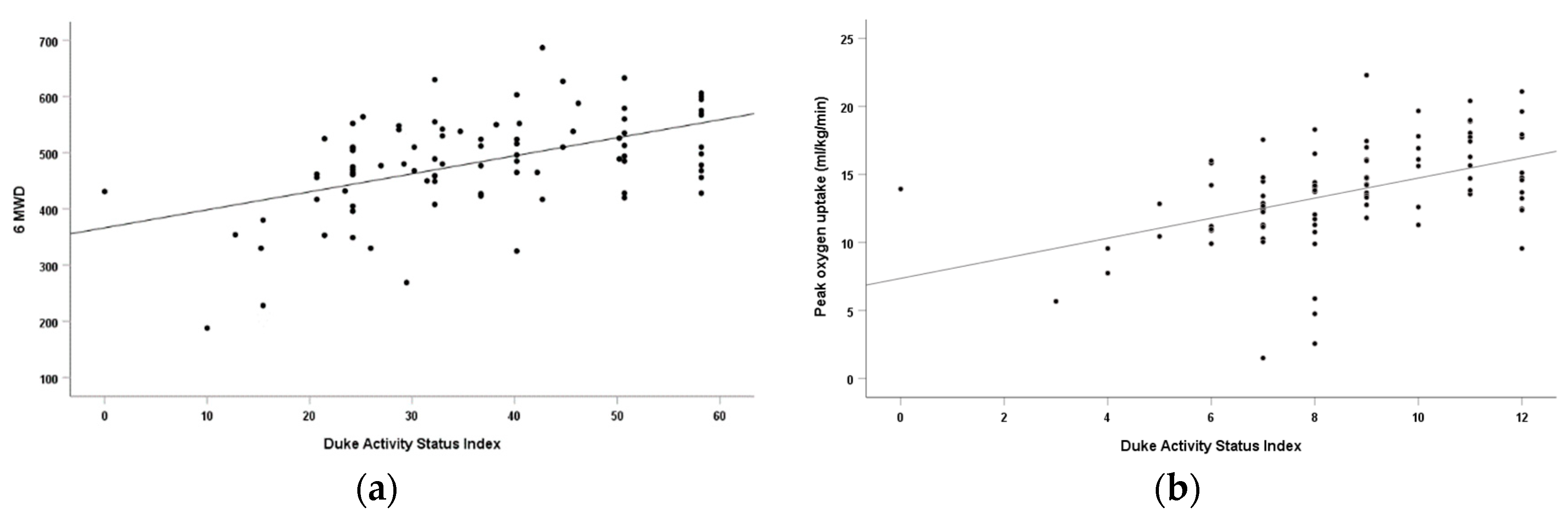
| DASI and PeakVO2 | 0.467 | 0.218 | 0.467 | <0.001 | 0.515 | 0.265 | 0.515 | <0.001 |
| DASI and 6MWD | 0.501 | 0.251 | 0.501 | <0.001 | 0.605 | 0.366 | 0.605 | <0.001 |
| WHO Function Class | High-Risk (DASI < 33.8) | Non-High-Risk (DASI > 33.8) | Sig. |
|---|---|---|---|
| I | 6 | 16 | χ2 = 7.267 p = 0.001 |
| II | 32 | 29 | |
| III | 5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Wang, Y.; Li, W.; Yang, L.; Liao, Y.; Xu, M.; Zhang, C.; Ma, H. Usefulness of the Duke Activity Status Index to Assess Exercise Capacity and Predict Risk Stratification in Patients with Pulmonary Arterial Hypertension. J. Clin. Med. 2023, 12, 2761. https://doi.org/10.3390/jcm12082761
Zhou H, Wang Y, Li W, Yang L, Liao Y, Xu M, Zhang C, Ma H. Usefulness of the Duke Activity Status Index to Assess Exercise Capacity and Predict Risk Stratification in Patients with Pulmonary Arterial Hypertension. Journal of Clinical Medicine. 2023; 12(8):2761. https://doi.org/10.3390/jcm12082761
Chicago/Turabian StyleZhou, Haofeng, Yu Wang, Weiya Li, Lifang Yang, Yingxue Liao, Mingyu Xu, Caojin Zhang, and Huan Ma. 2023. "Usefulness of the Duke Activity Status Index to Assess Exercise Capacity and Predict Risk Stratification in Patients with Pulmonary Arterial Hypertension" Journal of Clinical Medicine 12, no. 8: 2761. https://doi.org/10.3390/jcm12082761
APA StyleZhou, H., Wang, Y., Li, W., Yang, L., Liao, Y., Xu, M., Zhang, C., & Ma, H. (2023). Usefulness of the Duke Activity Status Index to Assess Exercise Capacity and Predict Risk Stratification in Patients with Pulmonary Arterial Hypertension. Journal of Clinical Medicine, 12(8), 2761. https://doi.org/10.3390/jcm12082761






