Association between Sex-Related ALOX5 Gene Polymorphisms and Lung Atopy Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Data Collection and Genomic DNA Extraction
2.3. SNPs Genotyping and Serum 5-LO and LTB4 ELISA Quantification
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. ALOX5 Mutations Frequencies in Men and Women
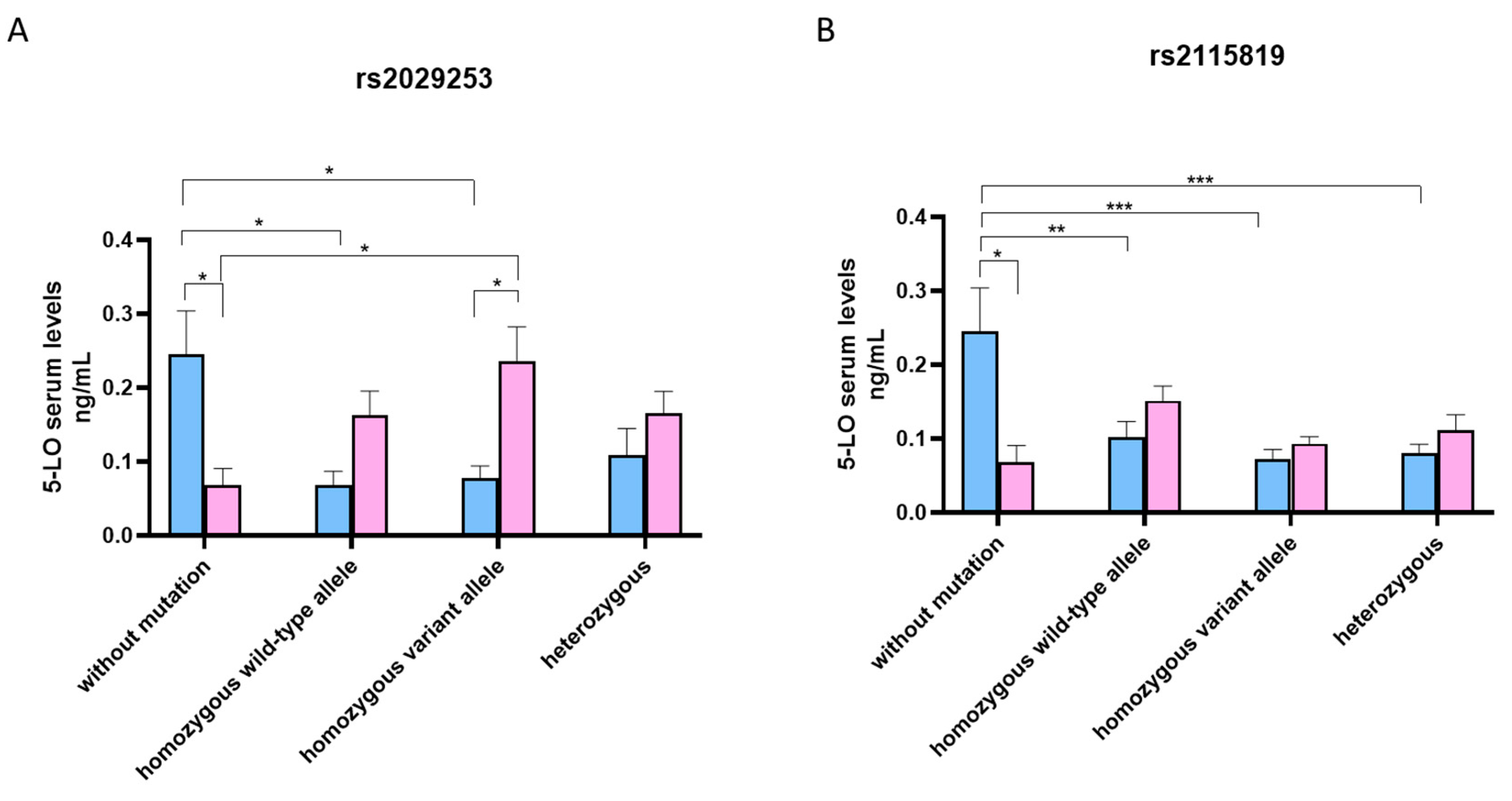
3.3. 5-LO Serum Levels According to Genotype ALOX5 rs2029253 and rs2115819 Polymorphisms
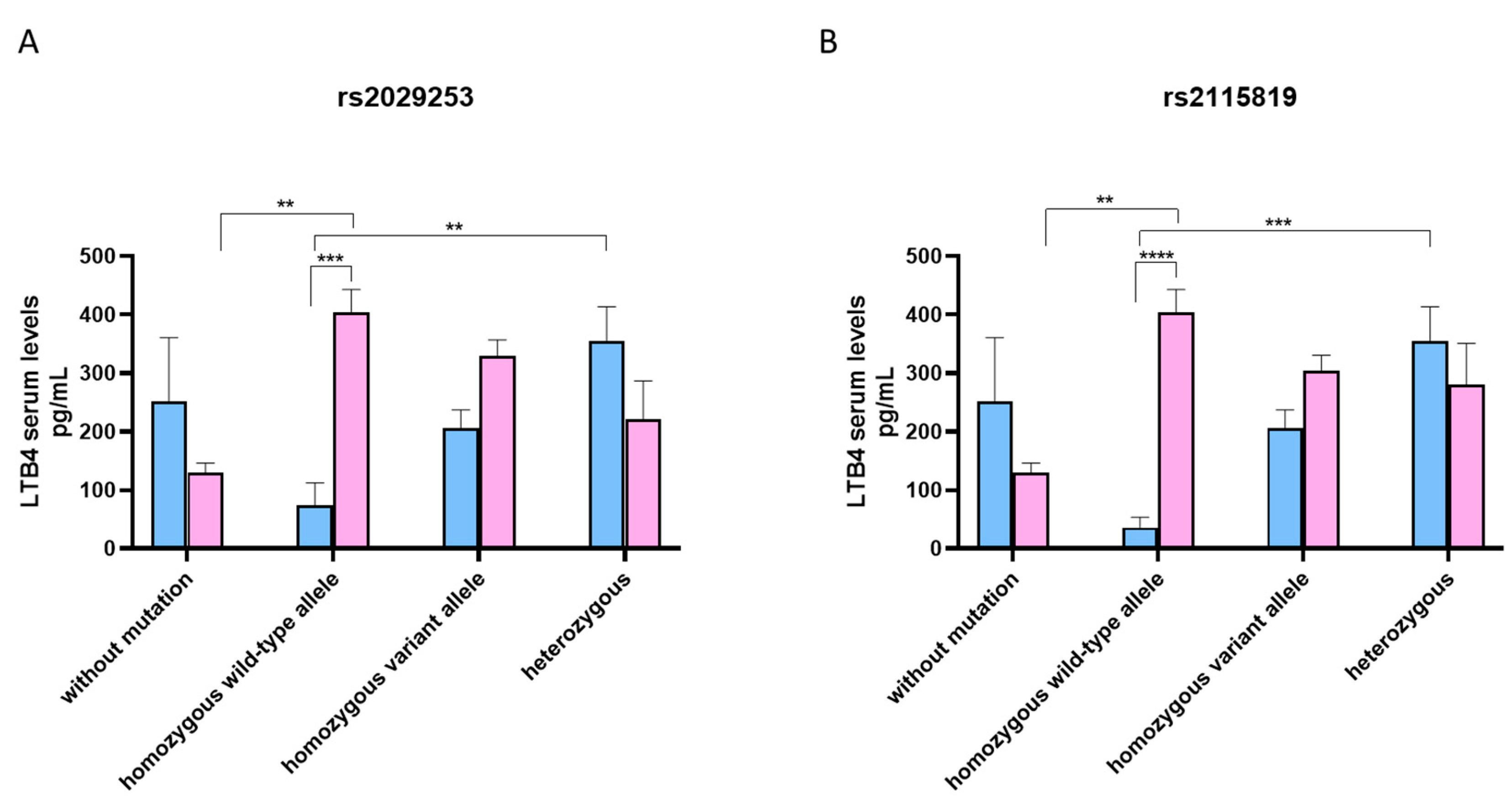
3.4. LTB4 Serum Levels According to Genotype ALOX5 rs2029253 and rs2115819 Polymorphisms
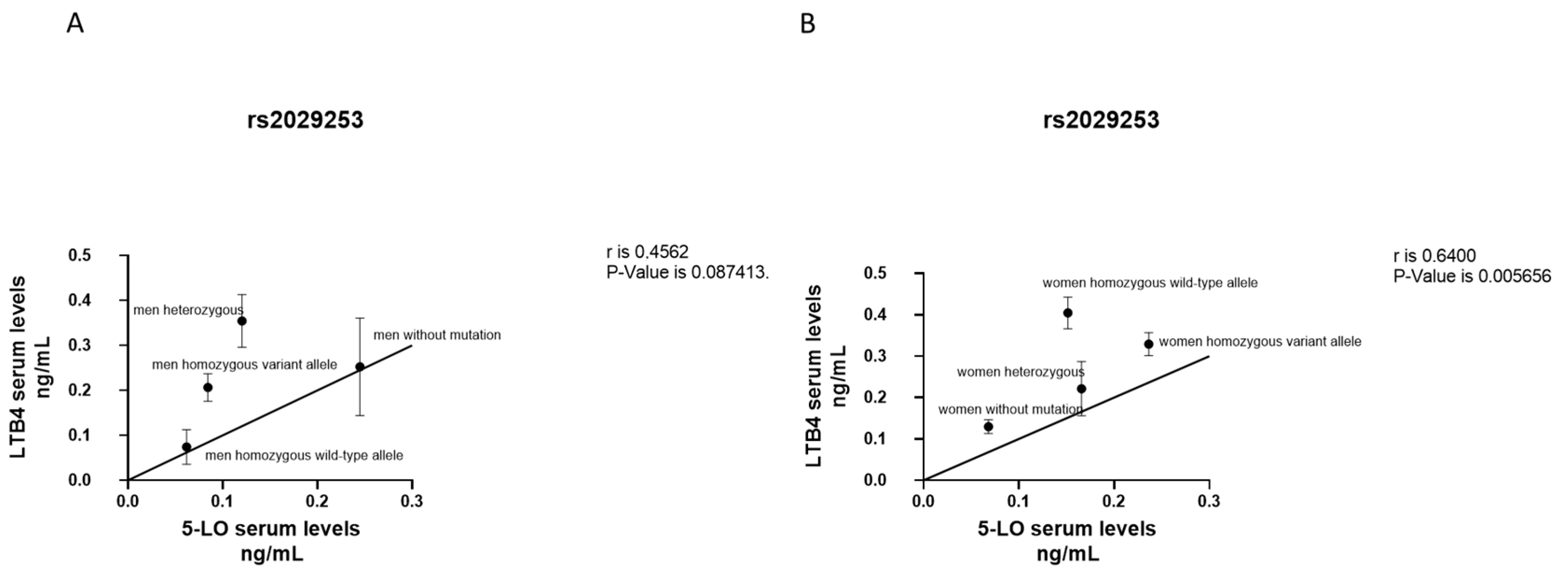
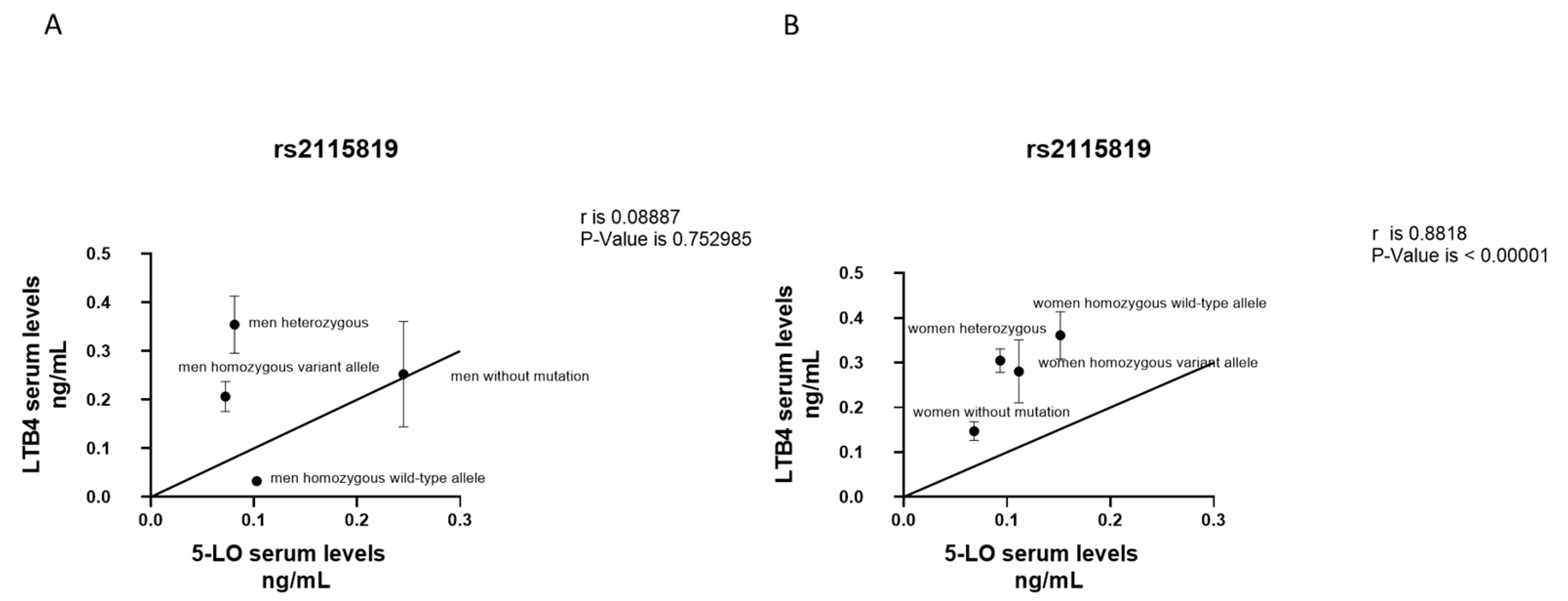
3.5. Correlation between LTB4 and 5-LO Serum Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, I.P.; Sayers, I. Pharmacogenetics and Asthma: False Hope or New Dawn? Eur. Respir. J. 2007, 29, 1239–1245. [Google Scholar] [CrossRef]
- Palmer, L.J.; Silverman, E.S.; Weiss, S.T.; Drazen, J.M. Pharmacogenetics of Asthma. Am. J. Respir. Crit. Care Med. 2002, 165, 861–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, G.Y.; Broide, D.H. Genes and Pathways Regulating Decline in Lung Function and Airway Remodeling in Asthma. Allergy Asthma Immunol. Res. 2019, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Mougey, E.; Lang, J.E.; Allayee, H.; Teague, W.G.; Dozor, A.J.; Wise, R.A.; Lima, J.J. ALOX5 Polymorphism Associates with Increased Leukotriene Production and Reduced Lung Function and Asthma Control in Children with Poorly Controlled Asthma. Clin. Exp. Allergy 2013, 43, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duroudier, N.P.; Tulah, A.S.; Sayers, I. Leukotriene Pathway Genetics and Pharmacogenetics in Allergy. Allergy 2009, 64, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Bizzintino, J.A.; Khoo, S.-K.; Zhang, G.; Martin, A.C.; Rueter, K.; Geelhoed, G.C.; Goldblatt, J.; Laing, I.A.; Le Souëf, P.N.; Hayden, C.M. Leukotriene Pathway Polymorphisms Are Associated with Altered Cysteinyl Leukotriene Production in Children with Acute Asthma. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 9–15. [Google Scholar] [CrossRef]
- Thompson, M.D.; Capra, V.; Clunes, M.T.; Rovati, G.E.; Stankova, J.; Maj, M.C.; Duffy, D.A. Cysteinyl Leukotrienes Pathway Genes, Atopic Asthma and Drug Response: From Population Isolates to Large Genome-Wide Association Studies. Front. Pharmacol. 2016, 7, 299. [Google Scholar] [CrossRef] [Green Version]
- Funk, C.D.; Hoshiko, S.; Matsumoto, T.; Rdmark, O.; Samuelsson, B. Characterization of the Human 5-Lipoxygenase Gene. Proc. Natl. Acad. Sci. USA 1989, 86, 2587–2591. [Google Scholar] [CrossRef] [Green Version]
- Peters-Golden, M.; Henderson, W.R., Jr. Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef]
- Folco, G.; Murphy, R.C. Eicosanoid Transcellular Biosynthesis: From Cell-Cell Interactions to In Vivo Tissue Responses. Pharm. Rev. 2006, 58, 375–388. [Google Scholar] [CrossRef]
- Singh, R.K.; Tandon, R.; Dastidar, S.G.; Ray, A. A Review on Leukotrienes and Their Receptors with Reference to Asthma. J. Asthma 2013, 50, 922–931. [Google Scholar] [CrossRef]
- Gelfand, E.W. Importance of the Leukotriene B4-BLT1 and LTB4-BLT2 Pathways in Asthma. Semin. Immunol. 2017, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T.; Peters-Golden, M.; Panettieri, R.A.; Henderson, W.R. Roles of Cysteinyl Leukotrienes in Airway Inflammation, Smooth Muscle Function, and Remodeling. J. Allergy Clin. Immunol. 2003, 111, S18–S36. [Google Scholar] [CrossRef] [PubMed]
- Schaible, A.M.; Filosa, R.; Temml, V.; Krauth, V.; Matteis, M.; Peduto, A.; Bruno, F.; Luderer, S.; Roviezzo, F.; Di Mola, A.; et al. Elucidation of the Molecular Mechanism and the Efficacy In Vivo of a Novel 1,4-Benzoquinone That Inhibits 5-Lipoxygenase: 5-Lipoxygenase Inhibition by a 1,4-Benzoquinone. Br. J. Pharmacol. 2014, 171, 2399–2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaible, A.M.; Filosa, R.; Krauth, V.; Temml, V.; Pace, S.; Garscha, U.; Liening, S.; Weinigel, C.; Rummler, S.; Schieferdecker, S.; et al. The 5-Lipoxygenase Inhibitor RF-22c Potently Suppresses Leukotriene Biosynthesis in Cellulo and Blocks Bronchoconstriction and Inflammation In Vivo. Biochem. Pharmacol. 2016, 112, 60–71. [Google Scholar] [CrossRef]
- Liparulo, A.; Esposito, R.; Santonocito, D.; Muñoz-Ramírez, A.; Spaziano, G.; Bruno, F.; Xiao, J.; Puglia, C.; Filosa, R.; Berrino, L.; et al. Formulation and Characterization of Solid Lipid Nanoparticles Loading RF22-c, a Potent and Selective 5-LO Inhibitor, in a Monocrotaline-Induced Model of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Çobanoğlu, B.; Toskala, E.; Ural, A.; Cingi, C. Role of Leukotriene Antagonists and Antihistamines in the Treatment of Allergic Rhinitis. Curr. Allergy Asthma Rep. 2013, 13, 203–208. [Google Scholar] [CrossRef]
- Montuschi, P. Exhaled Leukotrienes and Prostaglandins in COPD. Thorax 2003, 58, 585–588. [Google Scholar] [CrossRef] [Green Version]
- Bleecker, E.; Ortega, V.; Meyers, D. Asthma Pharmacogenetics and the Development of Genetic Profiles for Personalized Medicine. PGPM 2015, 8, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Horn, T.; Reddy Kakularam, K.; Anton, M.; Richter, C.; Reddanna, P.; Kuhn, H. Functional Characterization of Genetic Enzyme Variations in Human Lipoxygenases. Redox Biol. 2013, 1, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Kalayci, O.; Birben, E.; Sackesen, C.; Keskin, O.; Tahan, F.; Wechsler, M.E.; Civelek, E.; Soyer, O.U.; Adalioglu, G.; Tuncer, A.; et al. ALOX5 Promoter Genotype, Asthma Severity and LTC4 Production by Eosinophils: ALOX5 Genotype Variants in Asthma. Allergy 2005, 61, 97–103. [Google Scholar] [CrossRef]
- Schlag, K.; Steinhilber, D.; Karas, M.; Sorg, B.L. Analysis of Proximal ALOX5 Promoter Binding Proteins by Quantitative Proteomics. FEBS J. 2020, 287, 4481–4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- In, K.H.; Asano, K.; Beier, D.; Grobholz, J.; Finn, P.W.; Silverman, E.K.; Silverman, E.S.; Collins, T.; Fischer, A.R.; Keith, T.P.; et al. Naturally Occurring Mutations in the Human 5-Lipoxygenase Gene Promoter That Modify Transcription Factor Binding and Reporter Gene Transcription. J. Clin. Investig. 1997, 99, 1130–1137. [Google Scholar] [CrossRef]
- Drazen, J.M.; Yandava, C.N.; Dubé, L.; Szczerback, N.; Hippensteel, R.; Pillari, A.; Israel, E.; Schork, N.; Silverman, E.S.; Katz, D.A.; et al. Pharmacogenetic Association between ALOX5 Promoter Genotype and the Response to Anti-Asthma Treatment. Nat. Genet. 1999, 22, 168–170. [Google Scholar] [CrossRef]
- Sayers, I.; Barton, S.; Rorke, S.; Sawyer, J.; Peng, Q.; Beghé, B.; Ye, S.; Keith, T.; Clough, J.B.; Holloway, J.W.; et al. Promoter Polymorphism in the 5-Lipoxygenase (ALOX5) and 5-Lipoxygenase-Activating Protein (ALOX5AP) Genes and Asthma Susceptibility in a Caucasian Population: ALOX5 and ALOX5AP Promoter Polymorphism. Clin. Exp. Allergy 2003, 33, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Bae, J.-S.; Suh, C.-H.; Nahm, D.-H.; Holloway, J.W.; Park, H.-S. Polymorphism of Tandem Repeat in Promoter of 5-Lipoxygenase in ASA-Intolerant Asthma: A Positive Association with Airway Hyperresponsiveness. Allergy 2005, 60, 760–765. [Google Scholar] [CrossRef]
- Crosslin, D.R.; Shah, S.H.; Nelson, S.C.; Haynes, C.S.; Connelly, J.J.; Gadson, S.; Goldschmidt-Clermont, P.J.; Vance, J.M.; Rose, J.; Granger, C.B.; et al. Genetic Effects in the Leukotriene Biosynthesis Pathway and Association with Atherosclerosis. Hum. Genet. 2009, 125, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, E.V.; Doehring, A.; Kirchhof, A.; Lötsch, J. Functional Variants of the Human 5-Lipoxygenase Gene and Their Genetic Diagnosis. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 255–262. [Google Scholar] [CrossRef]
- Tantisira, K.G.; Lima, J.; Sylvia, J.; Klanderman, B.; Weiss, S.T. 5-Lipoxygenase Pharmacogenetics in Asthma: Overlap with Cys-Leukotriene Receptor Antagonist Loci. Pharm. Genom. 2009, 19, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.J.; Zhang, S.; Grant, A.; Shao, L.; Tantisira, K.G.; Allayee, H.; Wang, J.; Sylvester, J.; Holbrook, J.; Wise, R.; et al. Influence of Leukotriene Pathway Polymorphisms on Response to Montelukast in Asthma. Am. J. Respir. Crit. Care Med. 2006, 173, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Storms, W. Update on Montelukast and Its Role in the Treatment of Asthma, Allergic Rhinitis and Exercise-Induced Bronchoconstriction. Expert. Opin. Pharmacother. 2007, 8, 2173–2187. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J.; Phillips, B.R.; Martinez, F.D.; Chinchilli, V.M.; Lemanske, R.F.; Strunk, R.C.; Zeiger, R.S.; Larsen, G.; Spahn, J.D.; Bacharier, L.B.; et al. Characterization of Within-Subject Responses to Fluticasone and Montelukast in Childhood Asthma. J. Allergy Clin. Immunol. 2005, 115, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Israel, E.; Chervinsky, P.S.; Friedman, B.; van Bavel, J.; Skalky, C.S.; Ghannam, A.F.; Bird, S.R.; Edelman, J.M. Effects of Montelukast and Beclomethasone on Airway Function and Asthma Control. J. Allergy Clin. Immunol. 2002, 110, 847–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, R.; Spaziano, G.; Giannattasio, D.; Ferrigno, F.; Liparulo, A.; Rossi, A.; Roviezzo, F.; Sessa, M.; Falciani, M.; Berrino, L.; et al. Montelukast Improves Symptoms and Lung Function in Asthmatic Women Compared With Men. Front. Pharmacol. 2019, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Roviezzo, F.; Sorrentino, R.; Riemma, M.A.; Cerqua, I.; Bilancia, R.; Spaziano, G.; Troisi, F.; Pace, S.; Pinto, A.; et al. Leukotriene-Mediated Sex Dimorphism in Murine Asthma-like Features during Allergen Sensitization. Pharmacol. Res. 2019, 139, 182–190. [Google Scholar] [CrossRef]
- Sessa, M.; Mascolo, A.; D’Agostino, B.; Casciotta, A.; D’Agostino, V.; Michele, F.D.; Polverino, M.; Spaziano, G.; Andersen, M.P.; Kragholm, K.; et al. Relationship Between Gender and the Effectiveness of Montelukast: An Italian/Danish Register-Based Retrospective Cohort Study. Front. Pharmacol. 2018, 9, 844. [Google Scholar] [CrossRef] [Green Version]
- Telleria, J.J.; Blanco-Quiros, A.; Varillas, D.; Armentia, A.; Fernandez-Carvajal, I.; Jesus Alonso, M.; Diez, I. ALOX5 Promoter Genotype and Response to Montelukast in Moderate Persistent Asthma. Respir. Med. 2008, 102, 857–861. [Google Scholar] [CrossRef]
- Klotsman, M.; York, T.P.; Pillai, S.G.; Vargas-Irwin, C.; Sharma, S.S.; van den Oord, E.J.C.G.; Anderson, W.H. Pharmacogenetics of the 5-Lipoxygenase Biosynthetic Pathway and Variable Clinical Response to Montelukast. Pharm. Genom. 2007, 17, 189–196. [Google Scholar] [CrossRef]
- Koshy, L.; Anju, A.L.; Harikrishnan, S.; Kutty, V.R.; Jissa, V.T.; Kurikesu, I.; Jayachandran, P.; Jayakumaran Nair, A.; Gangaprasad, A.; Nair, G.M.; et al. Evaluating Genomic DNA Extraction Methods from Human Whole Blood Using Endpoint and Real-Time PCR Assays. Mol. Biol. Rep. 2017, 44, 97–108. [Google Scholar] [CrossRef]
- Passalacqua, G.; Ciprandi, G. Allergy and the Lung. Clin. Exp. Immunol. 2008, 153 (Suppl. S1), 12–16. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The Development of Allergic Inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Mirra, D.; Cione, E.; Spaziano, G.; Esposito, R.; Sorgenti, M.; Granato, E.; Cerqua, I.; Muraca, L.; Iovino, P.; Gallelli, L.; et al. Circulating MicroRNAs Expression Profile in Lung Inflammation: A Preliminary Study. JCM 2022, 11, 5446. [Google Scholar] [CrossRef] [PubMed]
- Mirra, D.; Spaziano, G.; Esposito, R.; Santonocito, D.; Filosa, R.; Roviezzo, F.; Malgieri, G.; D’Abrosca, G.; Iovino, P.; Gallelli, L.; et al. Formulation of Solid Lipid Nanoparticles Loaded with Nociceptin/Orphanin FQ (N/OFQ) and Characterization in a Murine Model of Airway Hyperresponsiveness. Pharmaceuticals 2022, 15, 1210. [Google Scholar] [CrossRef] [PubMed]
- Rouget, C.; Cui, Y.Y.; D’Agostino, B.; Faisy, C.; Naline, E.; Bardou, M.; Advenier, C. Nociceptin Inhibits Airway Microvascular Leakage Induced by HCl Intra-Oesophageal Instillation: Nociceptin and Gastro-Oesophageal Reflux. Br. J. Pharmacol. 2004, 141, 1077–1083. [Google Scholar] [CrossRef]
- D’Agostino, B.; Marrocco, G.; De Nardo, M.; Calò, G.; Guerrini, R.; Gallelli, L.; Advenier, C.; Rossi, F. Activation of the Nociceptin/Orphanin FQ Receptor Reduces Bronchoconstriction and Microvascular Leakage in a Rabbit Model of Gastroesophageal Reflux: N/OFQ Effects in the Airways in a GER Animal Model. Br. J. Pharmacol. 2005, 144, 813–820. [Google Scholar] [CrossRef]
- Gallelli, L.; D’Agostino, B.; Marrocco, G.; De Rosa, G.; Filippelli, W.; Rossi, F.; Advenier, C. Role of Tachykinins in the Bronchoconstriction Induced by HCl Intraesophageal Instillation in the Rabbit. Life Sci. 2003, 72, 1135–1142. [Google Scholar] [CrossRef]
- D’Agostino, B.; Advenier, C.; De Palma, R.; Gallelli, L.; Marrocco, G.; Abbate, G.F.; Rossi, F. The Involvement of Sensory Neuropeptides in Airway Hyper-Responsiveness in Rabbits Sensitized and Challenged to Parietaria Judaica: Sensory Neuropeptides in Airway Hyper-Responsiveness. Clin. Exp. Allergy 2002, 32, 472–479. [Google Scholar] [CrossRef]
- Cappetta, D.; De Angelis, A.; Spaziano, G.; Tartaglione, G.; Piegari, E.; Esposito, G.; Ciuffreda, L.P.; Liparulo, A.; Sgambato, M.; Russo, T.P.; et al. Lung Mesenchymal Stem Cells Ameliorate Elastase-Induced Damage in an Animal Model of Emphysema. Stem Cells Int. 2018, 2018, 9492038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, J.; Johansson, E.; Bernstein, J.A.; Chakraborty, R.; Khurana Hershey, G.K.; Rothenberg, M.E.; Mersha, T.B. Resolving the Etiology of Atopic Disorders by Using Genetic Analysis of Racial Ancestry. J. Allergy Clin. Immunol. 2016, 138, 676–699. [Google Scholar] [CrossRef] [Green Version]
- Perri, M.; Lucente, M.; Cannataro, R.; De Luca, I.F.; Gallelli, L.; Moro, G.; De Sarro, G.; Caroleo, M.C.; Cione, E. Variation in Immune-Related microRNAs Profile in Human Milk Amongst Lactating Women. MicroRNA 2018, 7, 107–114. [Google Scholar] [CrossRef]
- Uekert, S.; Akan, G.; Evans, M.; Li, Z.; Roberg, K.; Tisler, C.; Dasilva, D.; Anderson, E.; Gangnon, R.; Allen, D. Sex-Related Differences in Immune Development and the Expression of Atopy in Early Childhood. J. Allergy Clin. Immunol. 2006, 118, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Matteis, M.; Polverino, F.; Spaziano, G.; Roviezzo, F.; Santoriello, C.; Sullo, N.; Bucci, M.R.; Rossi, F.; Polverino, M.; Owen, C.A.; et al. Effects of Sex Hormones on Bronchial Reactivity during the Menstrual Cycle. BMC Pulm. Med. 2014, 14, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, S.; Werz, O. Impact of Androgens on Inflammation-Related Lipid Mediator Biosynthesis in Innate Immune Cells. Front. Immunol. 2020, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Roviezzo, F.; Sorrentino, R.; Iacono, V.M.; Brancaleone, V.; Terlizzi, M.; Riemma, M.A.; Bertolino, A.; Rossi, A.; Matteis, M.; Spaziano, G.; et al. Disodium Cromoglycate Inhibits Asthma-like Features Induced by Sphingosine-1-Phosphate. Pharmacol. Res. 2016, 113, 626–635. [Google Scholar] [CrossRef]
- Rossi, A.; Pergola, C.; Pace, S.; Rådmark, O.; Werz, O.; Sautebin, L. In Vivo Sex Differences in Leukotriene Biosynthesis in Zymosan-Induced Peritonitis. Pharmacol. Res. 2014, 87, 1–7. [Google Scholar] [CrossRef]
- Pace, S.; Pergola, C.; Dehm, F.; Rossi, A.; Gerstmeier, J.; Troisi, F.; Pein, H.; Schaible, A.M.; Weinigel, C.; Rummler, S.; et al. Androgen-Mediated Sex Bias Impairs Efficiency of Leukotriene Biosynthesis Inhibitors in Males. J. Clin. Investig. 2017, 127, 3167–3176. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef]
- Pergola, C.; Rogge, A.; Dodt, G.; Northoff, H.; Weinigel, C.; Barz, D.; Rådmark, O.; Sautebin, L.; Werz, O. Testosterone Suppresses Phospholipase D, Causing Sex Differences in Leukotriene Biosynthesis in Human Monocytes. FASEB J. 2011, 25, 3377–3387. [Google Scholar] [CrossRef]
- Volf, N.V.; Belousova, L.V.; Knyazev, G.G.; Kulikov, A.V. Gender Differences in Association between Serotonin Transporter Gene Polymorphism and Resting-State EEG Activity. Neuroscience 2015, 284, 513–521. [Google Scholar] [CrossRef]
- Bi, D.; Chen, M.; Zhang, X.; Wang, H.; Xia, L.; Shang, Q.; Li, T.; Zhu, D.; Blomgren, K.; He, L.; et al. The Association between Sex-Related Interleukin-6 Gene Polymorphisms and the Risk for Cerebral Palsy. J. Neuroinflamm. 2014, 11, 100. [Google Scholar] [CrossRef] [Green Version]
| Atopic | Healthy | p Value | |
|---|---|---|---|
| Total participants (N) | 36 | 114 | |
| Mean Age (SD) | 39.3(21.8) | 59.6(24.5) | NS |
| Gender (M/F) (N) | 18/18 | 56/58 | NS |
| Ethnicity | |||
| Caucasian | 36 | 114 | NS |
| BMI (SD) | 23.2(3.9) | 24.5(1.2) | NS |
| Smoking habit (current/former smoker) (N) | 8/3 | 15/7 | <0.05 |
| Comorbidities | |||
| - Hypertension (%) N | 5 (13.9%) | 16(14%) | NS |
| - Diabetes N (%) | 1 (2.8%) | 0 | NS |
| - Other cardiovascular diseases (%) | 3 (8.3%) | 0 | <0.0001 |
| Medications | |||
| - Corticosteroids (%) N | 14 (38.9%) | 21(18.4%) | <0.0001 |
| - B-blocked (%) N | 2 (5.5%) | 5(4.4%) | NS |
| - Ca antagonists (%) N | 3 (8.3%) | 2(1.7%) | <0.0001 <0.0001 |
| - Antihistamines (%) N | 9(25%) | 1(0.9%) | <0.0001 |
| rs2029253 ALOX5 | Men | Women | p Value |
|---|---|---|---|
| Tot. with mutation | 28/74 (37.9%) | 34/76 (44.7%) | <0.01 |
| Atopic with mutation | 6/17 (35.3%) | 8/19 (42.1%) | <0.05 |
| Healthy with mutation | 22/57 (38.6%) | 26/57 (45.6%) | 0.01 |
| rs2115819 ALOX5 | Men | Women | p Value |
|---|---|---|---|
| Tot. with mutation | 34/74 (45.9%) | 35/76 (46.1%) | NS |
| Atopic with mutation | 6/17 (35.3%) | 13/19 (68.4%) | <0.0001 |
| Healthy with mutation | 28/57 (49.1%) | 22/57 (38.6%) | <0.001 |
| A | |||
| rs2029253 ALOX5 | Healthy Men | Healthy Women | p Value |
| Without mutation | 35/57 (61.4%) | 31/57 (54.4%) | <0.001 |
| Homozygous wild-type | 7/57 (12.3%) | 11/57 (19.3%) | <0.001 |
| Homozygous variant | 10/57 (17.5%) | 4/57 (7%) | <0.0001 |
| Heterozygous | 5/57 (8.8%) | 11/57 (19.3%) | <0.0001 |
| B | |||
| rs2029253 ALOX5 | Atopic Men | Atopic Women | p Value |
| Without mutation | 11/17 (64.7%) | 11/19 (57.9%) | <0.001 |
| Homozygous wild-type | 0/17 (0%) | 4/19 (21.05%) | <0.0001 |
| Homozygous variant | 0/17 (0%) | 1/19 (5.3%) | <0.0001 |
| Heterozygous | 6/17 (35.3%) | 3/19 (15.8%) | <0.0001 |
| A | |||
| rs2115819 ALOX5 | Healthy Men | Healthy Women | p Value |
| Without mutation | 29/57 (50.8%) | 35/57 (61.4%) | <0.0001 |
| Homozygous wild-type | 9/57 (15.8%) | 6/57 (10.5%) | <0.001 |
| Homozygous variant | 11/57 (19.3%) | 2/57 (3.5%) | <0.0001 |
| Heterozygous | 8/57 (14.1%) | 14/57 (24.6%) | <0.0001 |
| B | |||
| rs2115819 ALOX5 | Atopic Men | Atopic Women | p Value |
| Without mutation | 11/17 (64.7%) | 6/19 (31.6%) | <0.0001 |
| Homozygous wild-type | 0/17 (0%) | 4/19 (21.05%) | <0.0001 |
| Homozygous variant | 0/17 (0%) | 4/19 (21.05%) | <0.0001 |
| Heterozygous | 6/17 (35.3%) | 5/19 (26.3%) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirra, D.; Esposito, R.; Spaziano, G.; Rafaniello, C.; Iovino, P.; Cione, E.; Gallelli, L.; D’Agostino, B. Association between Sex-Related ALOX5 Gene Polymorphisms and Lung Atopy Risk. J. Clin. Med. 2023, 12, 2775. https://doi.org/10.3390/jcm12082775
Mirra D, Esposito R, Spaziano G, Rafaniello C, Iovino P, Cione E, Gallelli L, D’Agostino B. Association between Sex-Related ALOX5 Gene Polymorphisms and Lung Atopy Risk. Journal of Clinical Medicine. 2023; 12(8):2775. https://doi.org/10.3390/jcm12082775
Chicago/Turabian StyleMirra, Davida, Renata Esposito, Giuseppe Spaziano, Concetta Rafaniello, Pasquale Iovino, Erika Cione, Luca Gallelli, and Bruno D’Agostino. 2023. "Association between Sex-Related ALOX5 Gene Polymorphisms and Lung Atopy Risk" Journal of Clinical Medicine 12, no. 8: 2775. https://doi.org/10.3390/jcm12082775






