2D Strain Analysis in Myocarditis—Can We Be Any Closer to Diagnose the Acute Phase of the Disease?
Abstract
1. Introduction
2. Methods
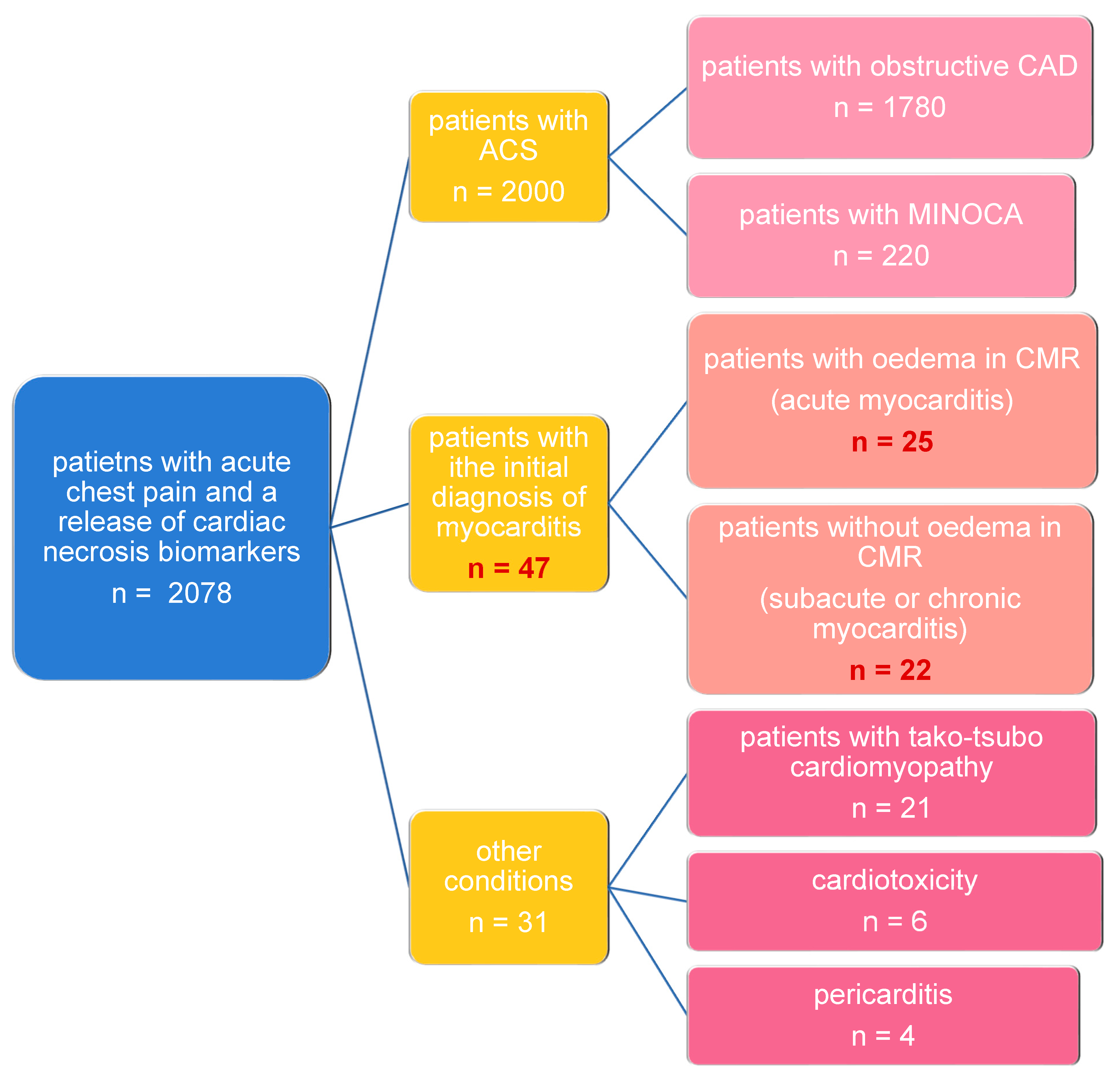
2.1. Study Population
2.2. Echocardiography
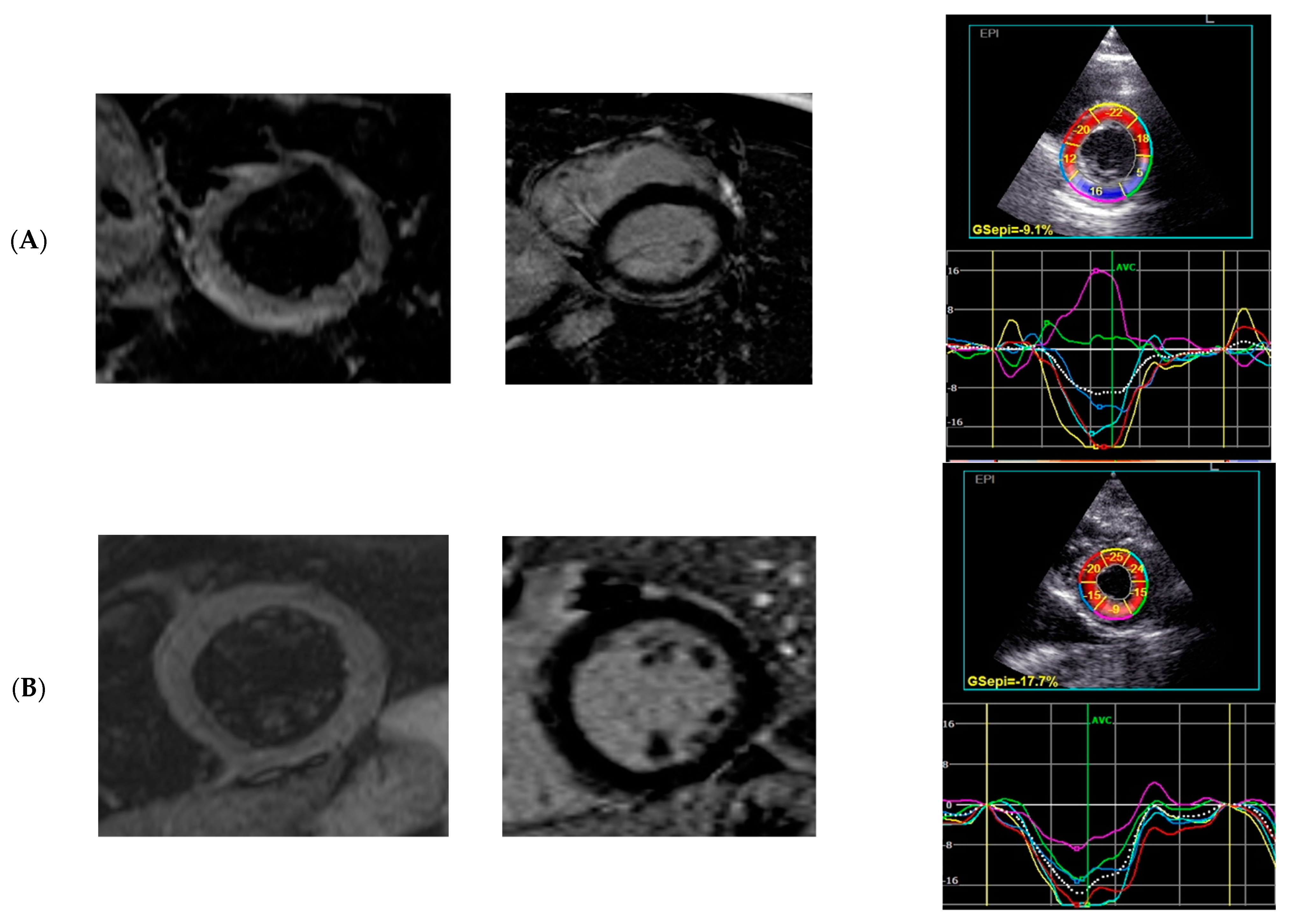
2.3. 2D STE: Two-Dimensional Speckle-Tracking Echocardiography
2.4. CMR Cardiac Magnetic Resonance
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. CMR Results
3.3. Echocardiographic Results
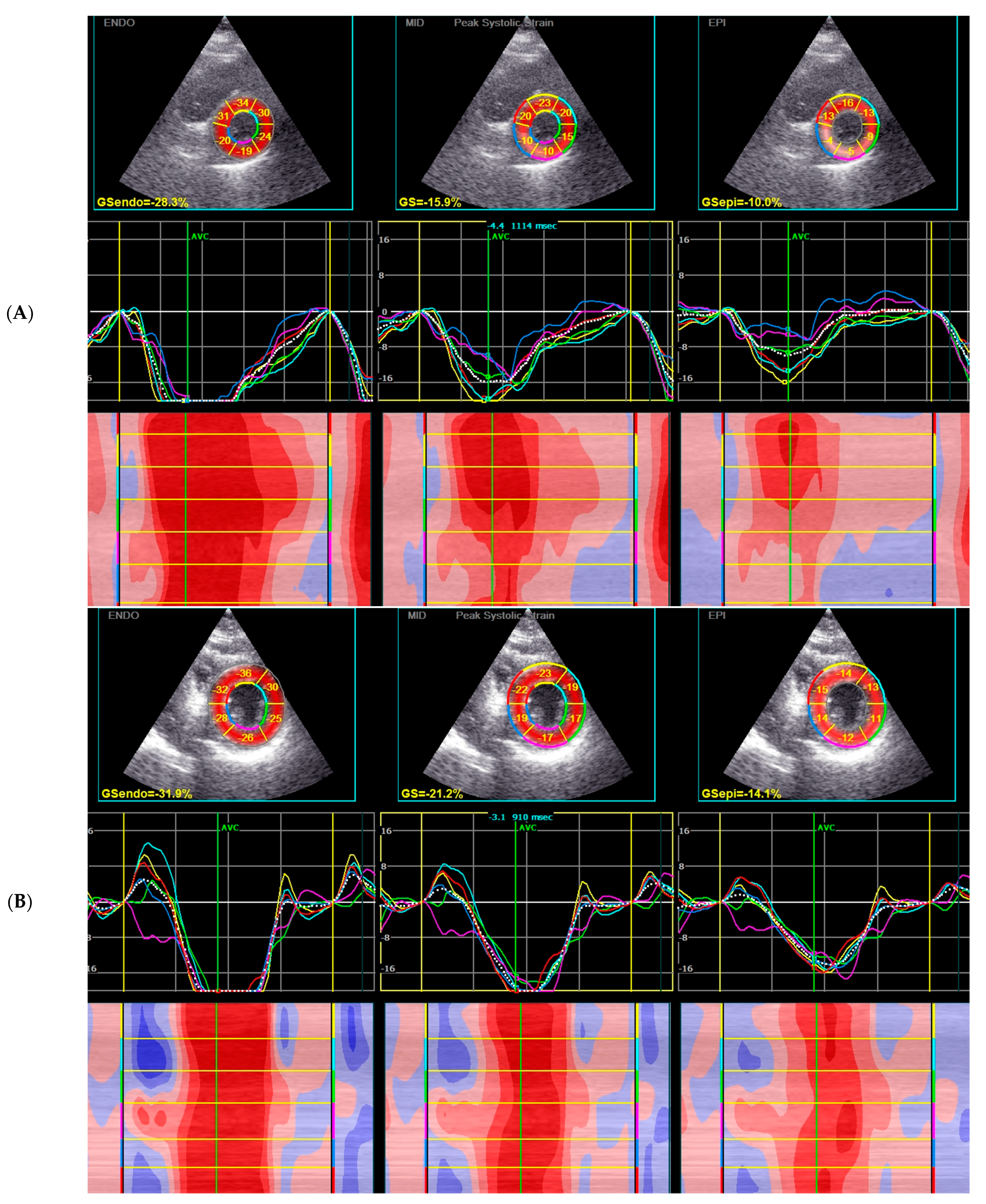
3.4. 2D STE Analysis
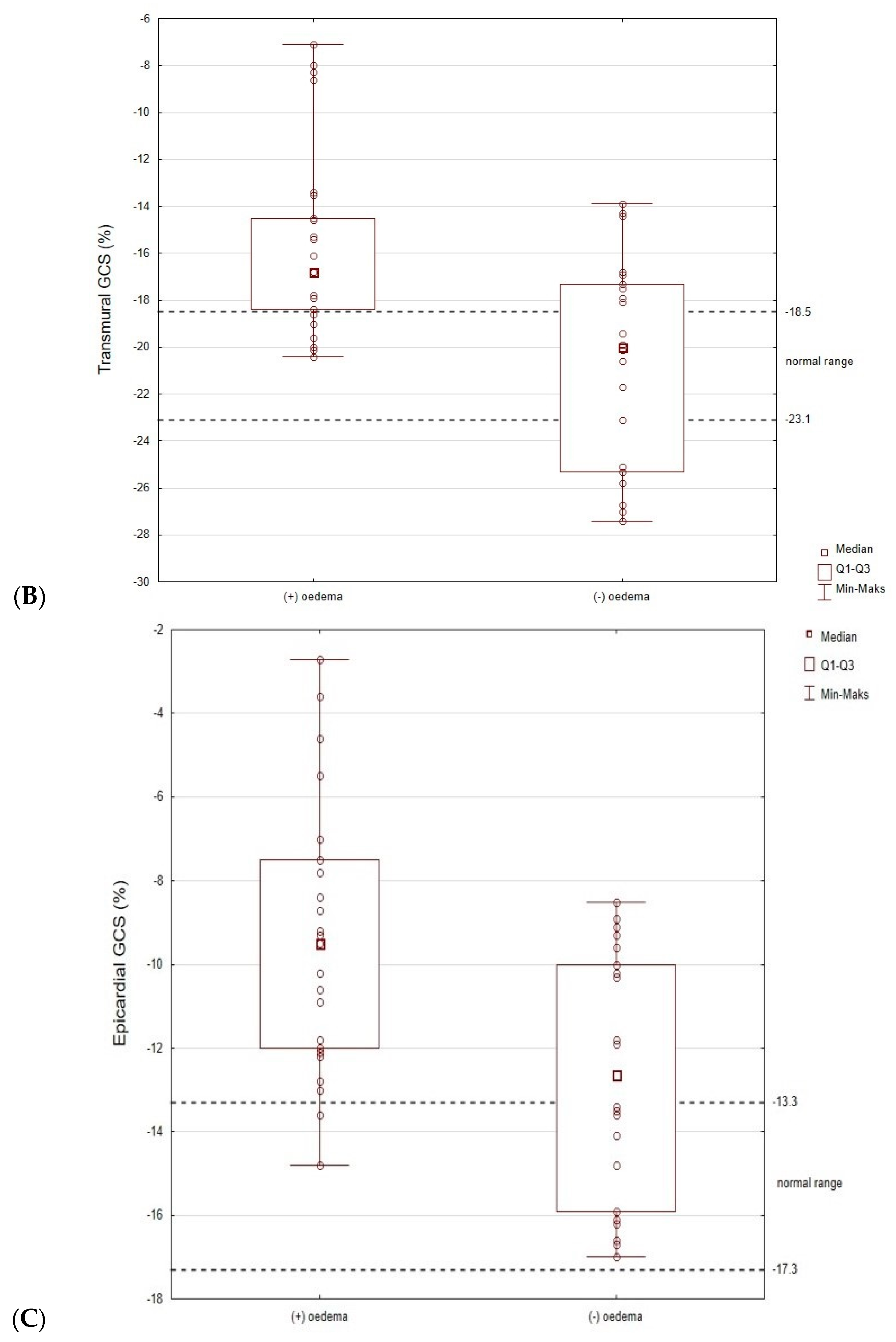
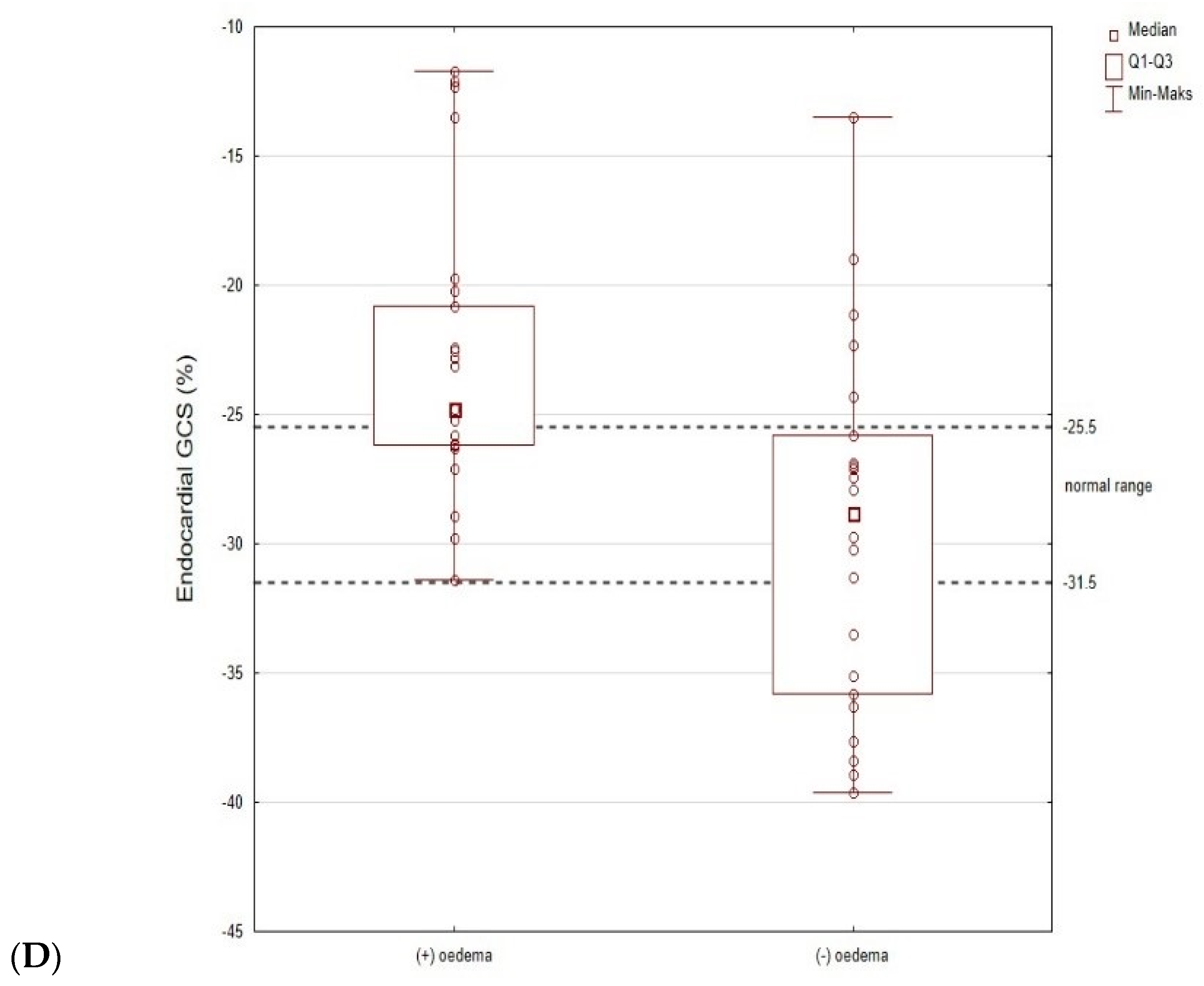
3.5. The Subgroup Analysis (Patients with and without Oedema)
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basso, C.; Calabrese, F.; Corrado, D.; Thiene, G. Postmortem diagnosis in sudden cardiac death victims: Macroscopic, microscopic and molecular findings. Cardiovasc. Res. 2001, 50, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC white paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Caspar, T.; Fichot, M.; Ohana, M.; Ghannudi, S.E.; Morel, O.; Ohlmann, P. Late detection of left ventricular dysfunction using two-dimensional and three-dimensional specle-tracking echocardiography in patients with history of nonsevere acute myocarditis. J. Am. Soc. Echocardiogr. 2017, 30, 756–762. [Google Scholar] [CrossRef]
- Baccouche, H.; Mahrholdt, H.; Meinhard, G.; Merher, R.; Voehringer, M.; Hill, S.; Klingel, K.; Kandolf, R.; Sechtem, U.; Yilmaz, A. Diagnostic synergy of non- invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur. Heart J. 2009, 30, 2869–2879. [Google Scholar] [CrossRef]
- Manalo, R.; Bourget, L.; Garg, A. Does myocardial strain remain abnormal long after normalization of ejection fraction in patients with acute myocarditis? Echocardiography 2019, 36, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.; Sprinkart, A.; Thomas, D. Comparison of original and 2018 Lake Louise criteria for diagnosis of acute myocarditis: Results of a validation cohort. Radiol. Radiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef] [PubMed]
- Holzman, M.; Nicko, A.; Kuhl, U.; Poller, W.; Hoffmann, W.; Morguet, A.; Witzenbichler, B.; Tschöpe, C.; Schultheiss, H.P.; Pauschinger, M. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: A retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation 2008, 118, 1722–1728. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Otto, C.M. Textbook of Clinical Echocardiography; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–40. [Google Scholar]
- Nagata, Y.; Chien-Chia, W.; Otsuji, Y. Takeuchi Normal range of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography. PLoS ONE 2017, 12, e0180584. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dulgheru, R.; Bernard, A.; Ilardi, F.; Contu, L.; Addetia, K.; Cabalelero, L.; Akhaladze, N.; Athanassopoulos, G.D.; Barona, D.; et al. Echocardiographoc reference ranges for normal left ventricular 2D strain: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 833–840. [Google Scholar] [CrossRef]
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hubner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Monney, P.A.; Sekhri, N.; Burchell, T.; Knight, C.; Davies, C.; Deaner, A.; Sheaf, M.; Baithun, S.; Petersen, S.; Wragg, A.; et al. Acute myocarditis presenting as acute coronary syndrome: Role of early cardiac resonance in its diagnosis. Heart 2011, 97, 1312–1318. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Homsi, R.; Dabir, D.; Kuetting, D.L.; Marx Ch Doerner, J.; Schlesinger-Irsch, U.; Andrie, R.; Sprinkart, A.M.; Schmeel, F.C.; Stehning Ch Fimmers, R.; et al. Comprehensive Cardiac Magnetic Resonance for short-term follow-up in acute myocarditis. J. Am. Heart Assoc. 2016, 5, e003603. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.; Kontorovitch, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef]
- Uziębło-Życzkowska, B.; Mielniczuk, M.; Ryczek, R.; Krzesiński, P. Myocarditis successfully diagnosed and controlled with speckle tracking echocardiography. Cardiovasc. Ultrasound 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Leitman, M.; Vered, Z.; Tyomkin, V.; Macogon, B.; Moravscy, G.; Peleg, E.; Copel, L. Speckle tracking imaging in inflammatory heart diseases. Int. J. Cardiovasc. Imaging 2018, 34, 787–792. [Google Scholar] [CrossRef]
- Sperlongano, S.; D’Amato, A.; Tagliamonte, E.; Russo, V.; Desiderio, A.; Ilardi, F.; Muscogiuri, G.; Esposito, G.; Pontone, G.; Esposito, G.; et al. Acute myocarditis: Prognostic role of speckle tracking echocardiography and comparison with magnetic resonance features. Heart Vessel. 2021, 37, 121–131. [Google Scholar] [CrossRef]
- Trovato, R.L.; La Franca, E.; Nugara, C.; Di Lisi, D.; Zarcone, A.; Bellavia, D.; Carminia, G.; Clemenza, F.; Novo, S.; Novo, G. 1232 Acute Myocarditis: Prognostic role of speckle tracking echocardiography and cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2020, 21 (Suppl. S1), jez319.693. [Google Scholar] [CrossRef]
- Logstrup, B.B.; Nielsen, J.M.; Kim, W.Y.; Poulsen, S.H. Myocardial edema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Kostakou, P.M.; Kostopoulos, V.S.; Tryfou, E.S.; Giannaris, V.D.; Rodis, I.E.; Olympios, C.D.; Kouris, N.T. Subclinical left ventricular dysfunction and correlation with regional strain analysis in myocarditis with normal ejection fraction. A new diagnostic criterion. Int. J. Cardiol. 2018, 259, 116–121. [Google Scholar] [CrossRef] [PubMed]
- DiBella, G.; Gaeta, M.; Pingitore, A.; Oreto, G.; Zito, C.; Minutoli, F.; Anfuso, C.; Dattilo, G.; Lamari, A.; Coglitore, S.; et al. Myocardial deformation in acute myocarditis with normal left ventricular wall motion–a cardiac magnetic resonance and 2-dimensional strain echocardiographic study. Circ. J. 2010, 74, 1205–1213. [Google Scholar]
- Erley, J.; Genovese, D.; Tapaskar, N.; Alvi, N.; Rashesi, N.; Besser, S.A.; Kawaji, K.; Goyal, N.; Kelle, S.; Lang, R.M.; et al. Echocardiography and cardiovascular magnetic resonance based evaluation of myocardial strain and relationship with late gadolinium enhancement. J. Cardioavsc. Magn. Reson. 2019, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Luetkens, J.A.; Schlesinger-Irsch, U.; Kuetting, D.L.; Dabir, D.; Homsi, R.; Doerner, J.; Schmeel, F.C.; Fimmers, R.; Sprinkart, A.M.; Naehle, C.P.; et al. Feature-tracking myocardial strain analysis in acute myocarditis: Diagnostic value and association with myocardial edema. Eur. Radiol. 2017, 27, 4661–4671. [Google Scholar] [CrossRef] [PubMed]



| Parameters | All Patients Me (IQR) | Oedema (+) Me (IQR) | Oedema (−) Me (IQR) | p |
|---|---|---|---|---|
| Age, years | 32 (22–43) | 29 (21–41) | 32.5 (24–45) | 0.321 |
| Systolic blood pressure, mmHg | 129 (119–138) | 130 (120–135) | 124 (115–140) | 0.601 |
| Diastolic blood pressure, mmHg | 75 (70–85) | 77 (70–84) | 71 (70–85) | 0.424 |
| Heart rate, bpm | 80 (70–90) | 82 (70–90) | 75 (64–90) | 0.316 |
| Peak C-reactive protein, mg/L | 42.4 (12–102.7) | 43.3 (25.3–112) | 37.8 (11–101.2) | 0.348 |
| WBC, 106/L | 9.5 (7.91–12.06) | 9.5 (8.1–11.9) | 9.3 (7.2–12.4) | 0.725 |
| Hgb, g/dL | 14.5 (13.8–15.4) | 14.6 (14.1–15.4) | 14.5 (13.4–15.2) | 0.741 |
| HCT, % | 42.6 (40.1–45.6) | 43 (41–45.6) | 41.9 (38.6–45.3) | 0.301 |
| Peak TnT hs, ng/L | 639.5 (118–1252) | 741 (376–1315) | 322 (10–949) | 0.025 |
| Peak CK-MB max, ng/L | 23.59 (4.2–102.7) | 26.3 (15.1–52.5) | 11.7 (1.3–38.7) | 0.049 |
| Plasma glucose, mmol/L | 6.17 (5.51– 6.96) | 5.9 (5.51–6.96) | 6.2 (5.66–6.82) | 0.877 |
| eGFR, mL/min/1.73 m2 | 112.1 (96.1–118.3) | 112.8 (104.6–117.9) | 104.5 (94.9–118.3) | 0.326 |
| Parameters | All Patients Me (IQR) | Oedema (+) Me (IQR) | Oedema (−) Me (IQR) | p |
|---|---|---|---|---|
| EF, % | 58.0 (52–60) | 57.0 (51–60) | 58 (57–62) | 0.028 |
| LV segments with oedema, n | - | 2 (1–2) | 0 (−) | 0.000 |
| LV segments with LGE, n | 4 (4–6) | 4 (2–4) | 0.016 | |
| Pericardial effusions/pericardial abnormalities, n | 0 | 0 | 0 | - |
| Parameters | All Patients Me (IQR) | Oedema (+) Me (IQR) | Oedema (−) Me (IQR) | p |
|---|---|---|---|---|
| EF, % | 58 (52–60) | 53.0 (48–60) | 60 (57–61) | 0.009 |
| LVIDD, mm | 50 (46–53) | 51 (47–53) | 49 (45–51) | 0.082 |
| LVIDS, mm | 34 (30–36) | 35 (30–37) | 32 (29–35) | 0.314 |
| LV EDV, mL | 110 (105–118) | 114 (107–123) | 107.5 (101–115) | 0.038 |
| LV ESV, mL | 55 (53–58) | 55 (53–59) | 55 (53–58) | 0.759 |
| PWd, mm | 10 (9–11) | 10 (9–11) | 9.5 (9–10) | 0.907 |
| PWs, mm | 14 (13–15) | 14 (13–15) | 14 (13–15) | 0.649 |
| IVSd, mm | 10 (10–11) | 10 (9–11) | 10 (10–12) | 0.680 |
| IVSs, mm | 14 (13–15) | 14 (13–15) | 14,5 (13–15) | 0.519 |
| LAVI, mL/m2 | 30 (27–35) | 29 (27–40) | 31 (27–35) | 0.872 |
| E/A ratio | 1.4 (1.2–1.7) | 1.5 (1.2–1.7) | 1.4 (1.2–1.5) | 0.563 |
| E/E’ ratio | 7 (6–8) | 7 (5–7) | 7 (7–9) | 0.058 |
| TAPSE, mm | 23 (21–26) | 23 (20–25) | 23,5 (22–26) | 0.191 |
| Global deformation analysis | ||||
| GLPS Avg, % | −19.1 (−20.4–−16.2) | −17.7 (−20.1–−16.0) | −19.5 (−21.51–−18.5) | 0.074 |
| GLPS LAX, % | −18.7 (−20.5–−16.5) | −17.8 (−19.8–−15.9) | −19.7 (−23.2–−17.8) | 0.041 |
| GLPS A2C, % | −19.85 (−21.2–−16.9) | −18.35 (−21.0–−16.6) | −20.3 (−21.4–−18.2) | 0.25 |
| GLPS A4C, % | −18.3 (−20–−15.7) | −17.0 (−19.4–−15.6) | −19.4 (−20.1–−17.7) | 0.055 |
| GRS, % | 29.2 (24.6–38.8) | 28.6 (24.3–36.5) | 33.5 (26.1–40.6) | 0.17 |
| GCS, % | −17.9 (−20.1–−15.3) | −16.8 (−18.4–−14.5) | −20.0 (−25.3–−17.3) | 0.001 |
| GCS MID systolic strain | −17 (−14.4–−19.4) | −15.7 (−17.2–−13.7) | −19.6 (−23.8–−16.6) | 0.000 |
| GCS EPI systolic strain | −10.6 (−13.5–−8.9) | −9.5 (−12.0–−7.5) | −12.7 (−15.9–−10.0) | 0.003 |
| GCS ENDO systolic strain | −26.2 (−29.8–−22.4) | −24.8 (−26.2–−20.8) | −28.8 (−35.8–−25.8) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supeł, K.; Wieczorkiewicz, P.; Przybylak, K.; Zielińska, M. 2D Strain Analysis in Myocarditis—Can We Be Any Closer to Diagnose the Acute Phase of the Disease? J. Clin. Med. 2023, 12, 2777. https://doi.org/10.3390/jcm12082777
Supeł K, Wieczorkiewicz P, Przybylak K, Zielińska M. 2D Strain Analysis in Myocarditis—Can We Be Any Closer to Diagnose the Acute Phase of the Disease? Journal of Clinical Medicine. 2023; 12(8):2777. https://doi.org/10.3390/jcm12082777
Chicago/Turabian StyleSupeł, Karolina, Paulina Wieczorkiewicz, Katarzyna Przybylak, and Marzenna Zielińska. 2023. "2D Strain Analysis in Myocarditis—Can We Be Any Closer to Diagnose the Acute Phase of the Disease?" Journal of Clinical Medicine 12, no. 8: 2777. https://doi.org/10.3390/jcm12082777
APA StyleSupeł, K., Wieczorkiewicz, P., Przybylak, K., & Zielińska, M. (2023). 2D Strain Analysis in Myocarditis—Can We Be Any Closer to Diagnose the Acute Phase of the Disease? Journal of Clinical Medicine, 12(8), 2777. https://doi.org/10.3390/jcm12082777






