Rheumatoid Arthritis from Easy to Complex Disease: From the “2022 GISEA International Symposium”
Abstract
1. Introduction
2. Is Seronegative RA Easy or Complex to Treat?
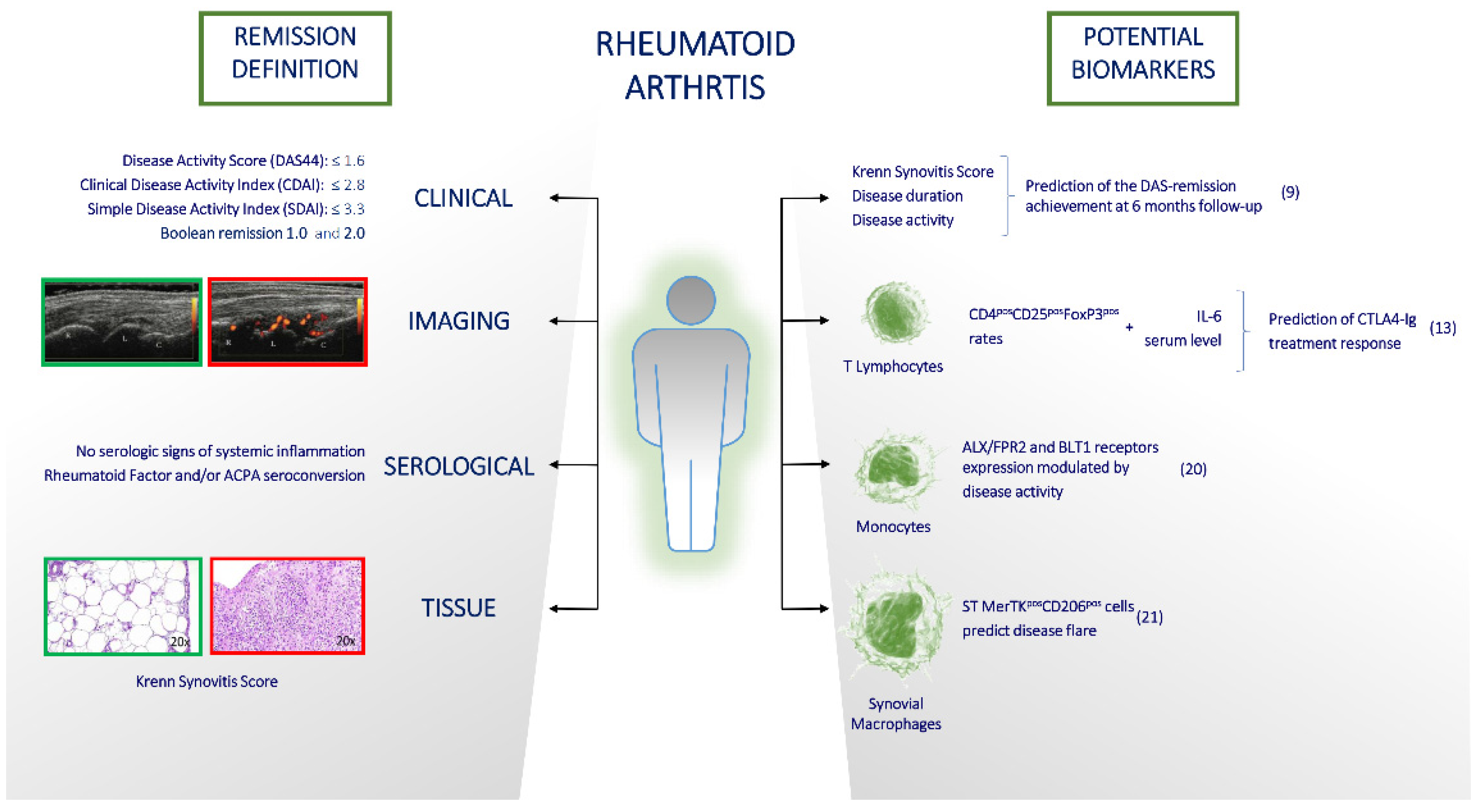
3. How Achievable Is Remission in Current RA Management and Is It Easy to Maintain?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lapadula, G.; Ferraccioli, G.; Ferri, C.; Punzi, L.; Trotta, F. GISEA: An Italian Biological Agents Registry in Rheumatology. Reumatismo 2011, 63, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2016, 68, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chatzidionysiou, K.; Fragoulis, G.E. Established Rheumatoid Arthritis—Redefining the Concept. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101476. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Roodenrijs, N.M.T.; Welsing, P.M.J.; Kedves, M.; Hamar, A.; Van Der Goes, M.C.; Kent, A.; Bakkers, M.; Blaas, E.; Senolt, L.; et al. EULAR Definition of Difficult-to-Treat Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Isaacs, J.D. Rheumatoid Arthritis: From Palliation to Remission in Two Decades. Clin. Med. 2014, 14 (Suppl. S6), s50–s55. [Google Scholar] [CrossRef] [PubMed]
- Aga, A.B.; Lie, E.; Uhlig, T.; Olsen, I.C.; Wierød, A.; Kalstad, S.; Rødevand, E.; Mikkelsen, K.; Kvien, T.K.; Haavardsholm, E.A. Time Trends in Disease Activity, Response and Remission Rates in Rheumatoid Arthritis during the Past Decade: Results from the NOR-DMARD Study 2000-2010. Ann. Rheum. Dis. 2015, 74, 381–388. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann. Rheum. Dis. 2020, 79, S685–S699. [Google Scholar] [CrossRef]
- Paalanen, K.; Rannio, K.; Rannio, T.; Asikainen, J.; Hannonen, P.; Sokka, T. Does Early Seronegative Arthritis Develop into Rheumatoid Arthritis? A 10-Year Observational Study. Clin. Exp. Rheumatol. 2019, 37, 37–43. [Google Scholar]
- Paalanen, K.; Puolakka, K.; Nikiphorou, E.; Hannonen, P.; Sokka, T. Erratum to: Is Seronegative Rheumatoid Arthritis True Rheumatoid Arthritis? A Nationwide Cohort Study. Rheumatology 2021, 60, 2493–2494. [Google Scholar] [CrossRef]
- Nordberg, L.B.; Lillegraven, S.; Lie, E.; Aga, A.B.; Olsen, I.C.; Hammer, H.B.; Uhlig, T.; Jonsson, M.K.; Van Der Heijde, D.; Kvien, T.K.; et al. Patients with Seronegative RA Have More Inflammatory Activity Compared with Patients with Seropositive RA in an Inception Cohort of DMARD-Naïve Patients Classified According to the 2010 ACR/EULAR Criteria. Ann. Rheum. Dis. 2017, 76, 341–345. [Google Scholar] [CrossRef]
- Coffey, C.M.; Crowson, C.S.; Myasoedova, E.; Matteson, E.L.; Davis, J.M. Evidence of Diagnostic and Treatment Delay in Seronegative Rheumatoid Arthritis: Missing the Window of Opportunity. Mayo Clin. Proc. 2019, 94, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kuwana, M.; Kameda, H.; Takeuchi, T. Sensitivity and Specificity of 2010 Rheumatoid Arthritis Classification Criteria. Rheumatology 2011, 50, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Biliavska, I.; Stamm, T.A.; Martinez-Avila, J.; Huizinga, T.W.J.; Landewé, R.B.M.; Steiner, G.; Aletaha, D.; Smolen, J.S.; MacHold, K.P. Application of the 2010 ACR/EULAR Classification Criteria in Patients with Very Early Inflammatory Arthritis: Analysis of Sensitivity, Specificity and Predictive Values in the SAVE Study Cohort. Ann. Rheum. Dis. 2013, 72, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Davis, J.; Matteson, E.L.; Crowson, C.S. Is the Epidemiology of Rheumatoid Arthritis Changing? Results from a Population-Based Incidence Study, 1985-2014. Ann. Rheum. Dis. 2020, 79, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Kaipiainen-Seppanen, O.; Kautiainen, H. Declining Trend in the Incidence of Rheumatoid Factor-Positive Rheumatoid Arthritis in Finland 1980–2000. J. Rheumatol. 2006, 33, 2132–2138. [Google Scholar]
- Jacobsson, L.T.H.; Hanson, R.L.; Knowler, W.C.; Pillemer, S.; Pettitt, D.J.; Mccance, D.R.; Bennett, P.H. Decreasing Incidence and Prevalence of Rheumatoid Arthritis in Pima Indians over a Twenty-Five-Year Period. Arthritis Rheum. 1994, 37, 1158–1165. [Google Scholar] [CrossRef]
- Matthijssen, X.M.E.; Niemantsverdriet, E.; Huizinga, T.W.J.; van der Helm-Van Mil, A.H.M. Enhanced Treatment Strategies and Distinct Disease Outcomes among Autoantibody-Positive and -Negative Rheumatoid Arthritis Patients over 25 Years: A Longitudinal Cohort Study in the Netherlands. PLoS Med. 2020, 17, e1003296. [Google Scholar] [CrossRef]
- Mouterde, G.; Rincheval, N.; Lukas, C.; Daien, C.; Saraux, A.; Dieudé, P.; Morel, J.; Combe, B. Outcome of Patients with Early Arthritis without Rheumatoid Factor and ACPA and Predictors of Rheumatoid Arthritis in the ESPOIR Cohort. Arthritis Res. Ther. 2019, 21, 140. [Google Scholar] [CrossRef]
- Ogawa, Y.; Takahashi, N.; Kaneko, A.; Hirano, Y.; Kanayama, Y.; Yabe, Y.; Oguchi, T.; Fujibayashi, T.; Takagi, H.; Hanabayashi, M.; et al. Association between Seropositivity and Discontinuation of Tumor Necrosis Factor Inhibitors Due to Ineffectiveness in Rheumatoid Arthritis. Clin. Rheumatol. 2019, 38, 2757–2763. [Google Scholar] [CrossRef]
- Bugatti, S.; De Stefano, L.; Manzo, A.; Sakellariou, G.; Xoxi, B.; Montecucco, C. Limiting Factors to Boolean Remission Differ between Autoantibody-Positive and -Negative Patients in Early Rheumatoid Arthritis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211011826. [Google Scholar] [CrossRef] [PubMed]
- Haschka, J.; Englbrecht, M.; Hueber, A.J.; Manger, B.; Kleyer, A.; Reiser, M.; Finzel, S.; Tony, H.P.; Kleinert, S.; Feuchtenberger, M.; et al. Relapse Rates in Patients with Rheumatoid Arthritis in Stable Remission Tapering or Stopping Antirheumatic Therapy: Interim Results from the Prospective Randomised Controlled RETRO Study. Ann. Rheum. Dis. 2016, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fedele, A.L.; Petricca, L.; Tolusso, B.; Alivernini, S.; Canestri, S.; Di Mario, C.; Bosello, S.L.; Ferraccioli, G.; Gremese, E. Interleukin-6 and IgA-Rheumatoid Factor Are Crucial for Baseline Erosiveness, and Anti-Citrullinated Peptide Antibodies for Radiographic Progression in Early Rheumatoid Arthritis Treated According to a Treat-to-Target Strategy. Scand. J. Rheumatol. 2018, 47, 351–359. [Google Scholar] [CrossRef]
- Dey, M.; Nagy, G.; Nikiphorou, E. Comorbidities and Extra-Articular Manifestations in Difficult-to-Treat Rheumatoid Arthritis: Different Sides of the Same Coin? Rheumatology 2022, keac584. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Kelly, C.; Eggleton, P.; De Soyza, A.; Hutchinson, D. The Lung in ACPA-Positive Rheumatoid Arthritis: An Initiating Site of Injury? Rheumatology 2014, 53, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.R.; Pecani, A.; Ciciarello, F.; Colasanti, T.; Di Franco, M.; Miranda, F.; Conti, F.; Valesini, G.; Alessandri, C. Association between Antibodies to Carbamylated Proteins and Subclinical Atherosclerosis in Rheumatoid Arthritis Patients. BMC Musculoskelet. Disord. 2017, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid Arthritis: Extra-Articular Manifestations and Comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bobadilla, N.; Soto-Fajardo, C.; Amezcua-Guerra, L.M.; Batres-Marroquín, A.B.; Vargas, T.; Hernández-Diazcouder, A.; Jiménez-Rojas, V.; Medina-García, A.C.; Pineda, C.; Silveira, L.H. Patients with Seronegative Rheumatoid Arthritis Have a Different Phenotype than Seropositive Patients: A Clinical and Ultrasound Study. Front. Med. 2022, 9, 978351. [Google Scholar] [CrossRef]
- Kamiya, H.; Panlaqui, O.M. Systematic Review and Meta-Analysis of the Risk of Rheumatoid Arthritis-Associated Interstitial Lung Disease Related to Anti-Cyclic Citrullinated Peptide (CCP) Antibody. BMJ Open 2021, 11, e048409. [Google Scholar] [CrossRef]
- De Stefano, L.; D’Onofrio, B.; Manzo, A.; Montecucco, C.; Bugatti, S. The Genetic, Environmental, and Immunopathological Complexity of Autoantibody-Negative Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 12386. [Google Scholar] [CrossRef]
- Ohta, R.; Sano, C. Differentiating between Seronegative Elderly-Onset Rheumatoid Arthritis and Polymyalgia Rheumatica: A Qualitative Synthesis of Narrative Reviews. Int. J. Environ. Res. Public Health 2023, 20, 1789. [Google Scholar] [CrossRef] [PubMed]
- Kaeley, G.S.; Bakewell, C.; Deodhar, A. The Importance of Ultrasound in Identifying and Differentiating Patients with Early Inflammatory Arthritis: A Narrative Review. Arthritis Res. Ther. 2020, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Zhao, M.; Zhang, Y.; Xie, Y.; Cao, J.; Pan, Y. Seronegative Rheumatic Arthritis Has Milder Inflammation and Bone Erosion in an Ultrasound Study of Disease-Modifying Anti-Rheumatic Drugs (DMARDs)-Naïve Chinese Cohort. Ann. Transl. Med. 2022, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Grisar, J.C.; Stamm, T.A.; Sharp, J.T. Estimation of a Numerical Value for Joint Damage-Related Physical Disability in Rheumatoid Arthritis Clinical Trials. Ann. Rheum. Dis. 2010, 69, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, E.; Mesina, F.; Caporali, R. Clinical Remission in Rheumatoid Arthritis and Psoriatic Arthritis. Clin. Exp. Rheumatol. 2018, 36, 900–910. [Google Scholar]
- Carvalho, P.D.; Ferreira, R.J.O.; Landewé, R.; Vega-Morales, D.; Salomon-Escoto, K.; Veale, D.J.; Chopra, A.; Da Silva, J.A.P.; MacHado, P.M. Association of 17 Definitions of Remission with Functional Status in a Large Clinical Practice Cohort of Patients with Rheumatoid Arthritis. J. Rheumatol. 2020, 47, 20–27. [Google Scholar] [CrossRef]
- Studenic, P.; Aletaha, D.; De Wit, M.; Stamm, T.A.; Alasti, F.; Lacaille, D.; Smolen, J.S.; Felson, D.T. American College of Rheumatology/EULAR Remission Criteria for Rheumatoid Arthritis: 2022 Revision. Ann. Rheum. Dis. 2023, 82, 74–80. [Google Scholar] [CrossRef]
- Smolen, J.S.; Breedveld, F.C.; Burmester, G.R.; Bykerk, V.; Dougados, M.; Emery, P.; Kvien, T.K.; Navarro-Compán, M.V.; Oliver, S.; Schoels, M.; et al. Treating Rheumatoid Arthritis to Target: 2014 Update of the Recommendations of an International Task Force. Ann. Rheum. Dis. 2016, 75, 3–15. [Google Scholar] [CrossRef]
- Han, J.; Geng, Y.; Deng, X.; Zhang, Z. Subclinical Synovitis Assessed by Ultrasound Predicts Flare and Progressive Bone Erosion in Rheumatoid Arthritis Patients with Clinical Remission: A Systematic Review and Metaanalysis. J. Rheumatol. 2016, 43, 2010–2018. [Google Scholar] [CrossRef]
- Peluso, G.; Michelutti, A.; Bosello, S.; Gremese, E.; Tolusso, B.; Ferraccioli, G. Clinical and Ultrasonographic Remission Determines Different Chances of Relapse in Early and Long Standing Rheumatoid Arthritis. Ann. Rheum. Dis. 2011, 70, 172–175. [Google Scholar] [CrossRef]
- Orange, D.E.; Agius, P.; DiCarlo, E.F.; Mirza, S.Z.; Pannellini, T.; Szymonifka, J.; Jiang, C.S.; Figgie, M.P.; Frank, M.O.; Robinson, W.H.; et al. Histologic and Transcriptional Evidence of Subclinical Synovial Inflammation in Patients With Rheumatoid Arthritis in Clinical Remission. Arthritis Rheumatol. 2019, 71, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Kinne, R.W.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis Score: Discrimination between Chronic Low-Grade and High-Grade Synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; Tolusso, B.; Gessi, M.; Gigante, M.R.; Mannocci, A.; Petricca, L.; Perniola, S.; Di Mario, C.; Bui, L.; Fedele, A.L.; et al. Inclusion of Synovial Tissue-Derived Characteristics in a Nomogram for the Prediction of Treatment Response in Treatment-Naive Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2021, 73, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Elisa, G.; Tolusso, B.; Petricca, L.; Di Mario, C.; Gigante, M.R.; Ferraccioli, G.; Alivernini, S. Peripheral Blood CD4posCD25posFoxP3pos Cells and Inflammatory Cytokines as Biomarkers of Response in Rheumatoid Arthritis Patients Treated with CTLA4-Ig. Arthritis Res. Ther. 2022, 24, 143. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Clish, C.B.; Brannon, J.; Colgan, S.P.; Chiang, N.; Gronert, K. Novel Functional Sets of Lipid-Derived Mediators with Antiinflammatory Actions Generated from Omega-3 Fatty Acids via Cyclooxygenase 2-Nonsteroidal Antiinflammatory Drugs and Transcellular Processing. J. Exp. Med. 2000, 192, 1197–1204. [Google Scholar] [CrossRef]
- Young, S.P.; Kapoor, S.R.; Viant, M.R.; Byrne, J.J.; Filer, A.; Buckley, C.D.; Kitas, G.D.; Raza, K. The Impact of Inflammation on Metabolomic Profiles in Patients with Arthritis. Arthritis Rheum. 2013, 65, 2015–2023. [Google Scholar] [CrossRef]
- Ormseth, M.J.; Swift, L.L.; Fazio, S.; Linton, M.F.; Chung, C.P.; Raggi, P.; Rho, Y.H.; Solus, J.; Oeser, A.; Bian, A.; et al. Free Fatty Acids Are Associated with Insulin Resistance but Not Coronary Artery Atherosclerosis in Rheumatoid Arthritis. Atherosclerosis 2011, 219, 869–874. [Google Scholar] [CrossRef]
- Chen, M.; Lam, B.K.; Kanaoka, Y.; Nigrovic, P.A.; Audoly, L.P.; Austen, K.F.; Lee, D.M. Neutrophil-Derived Leukotriene B4 Is Required for Inflammatory Arthritis. J. Exp. Med. 2006, 203, 837–842. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Pathogenetic Insights from the Treatment of Rheumatoid Arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef]
- Perniola, S.; Bizzoca, R.; Fornaro, M.; Alivernini, S.; Gremese, E.; Iannone, F. High CD14+ Peripheral Monocytes Expression of ALX/FPR2 and BLT1 in Low Disease Activity and Remission Status in Rheumatoid Arthritis: A Pilot Study. Clin. Exp. Rheumatol. 2022, 40, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Perniola, S.; Tolusso, B.; Elmesmari, A.; Gessi, M.; Mario, C.D.; Gigante, M.R.; Petricca, L.; Bruno, D.; Somma, D.; Paglionico, A.; et al. OP0084 Digital Spatial Profiling Reveals Distinct Synovial Tissue Macrophage Transcriptomic Signature of Sustained Remission in Rheumatoid Arthritis Patients at Risk of Disease Flare after Treatment Cessation. Ann. Rheum. Dis. 2022, 81, 56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perniola, S.; Chimenti, M.S.; Spinelli, F.R.; Frediani, B.; Foti, R.; Ferrigno, S.; Garufi, C.; Cassone, G.; Venerito, V.; Atzeni, F.; et al. Rheumatoid Arthritis from Easy to Complex Disease: From the “2022 GISEA International Symposium”. J. Clin. Med. 2023, 12, 2781. https://doi.org/10.3390/jcm12082781
Perniola S, Chimenti MS, Spinelli FR, Frediani B, Foti R, Ferrigno S, Garufi C, Cassone G, Venerito V, Atzeni F, et al. Rheumatoid Arthritis from Easy to Complex Disease: From the “2022 GISEA International Symposium”. Journal of Clinical Medicine. 2023; 12(8):2781. https://doi.org/10.3390/jcm12082781
Chicago/Turabian StylePerniola, Simone, Maria Sole Chimenti, Francesca Romana Spinelli, Bruno Frediani, Rosario Foti, Sara Ferrigno, Cristina Garufi, Giulia Cassone, Vincenzo Venerito, Fabiola Atzeni, and et al. 2023. "Rheumatoid Arthritis from Easy to Complex Disease: From the “2022 GISEA International Symposium”" Journal of Clinical Medicine 12, no. 8: 2781. https://doi.org/10.3390/jcm12082781
APA StylePerniola, S., Chimenti, M. S., Spinelli, F. R., Frediani, B., Foti, R., Ferrigno, S., Garufi, C., Cassone, G., Venerito, V., Atzeni, F., Caporali, R., Conti, F., Favalli, E. G., Iannone, F., Sebastiani, M., Ferraccioli, G. F., Lapadula, G., & Gremese, E. (2023). Rheumatoid Arthritis from Easy to Complex Disease: From the “2022 GISEA International Symposium”. Journal of Clinical Medicine, 12(8), 2781. https://doi.org/10.3390/jcm12082781









