Both Moderate and Heavy Alcohol Use Amplify the Adverse Cardiovascular Effects of Smoking in Young Patients with Hypertension
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Data Collection
2.3. Follow-up and Outcomes
2.4. Data Analysis
3. Results
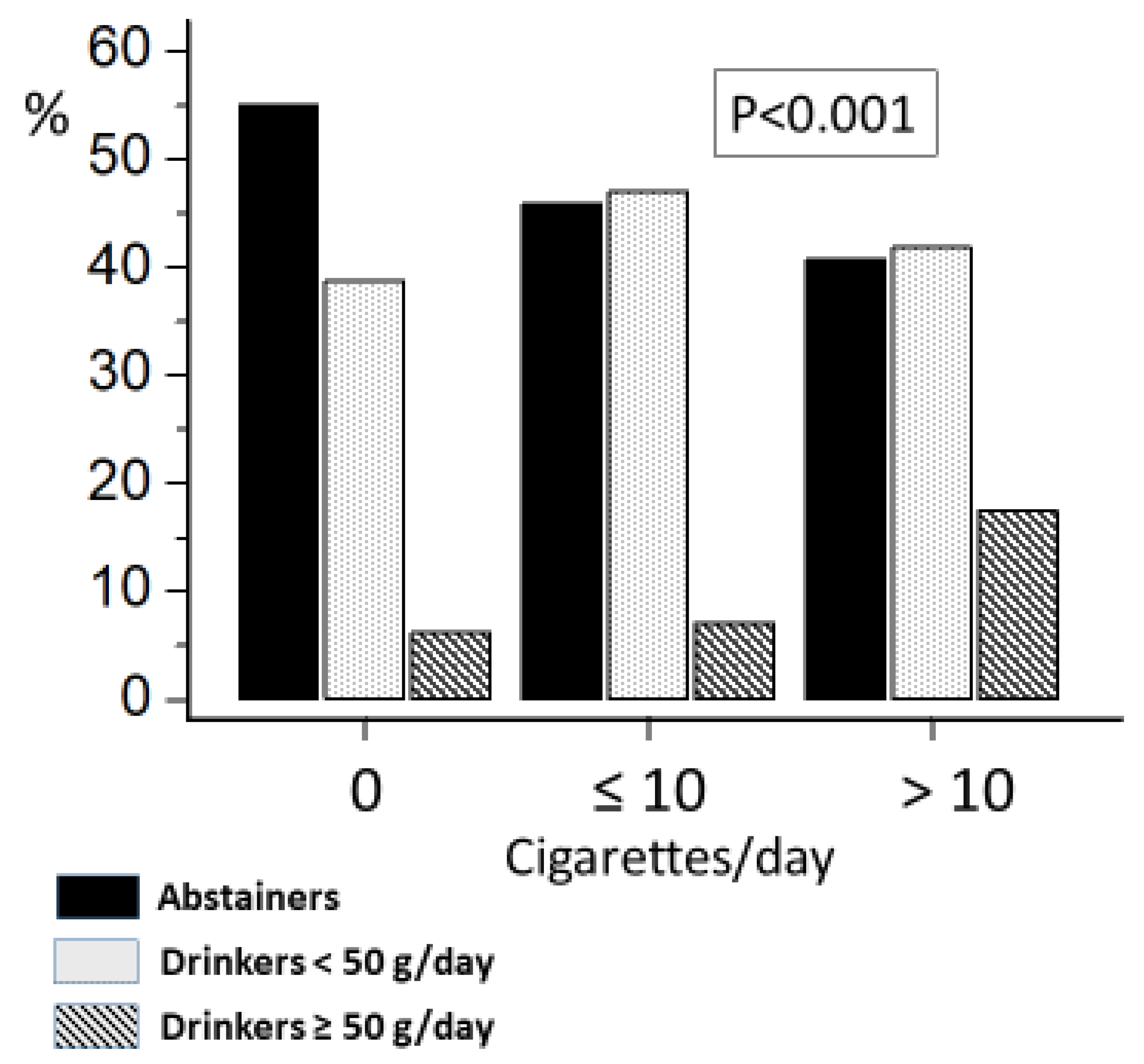
3.1. Relationship between Lifestyle Factors
3.2. Association with Cardiovascular and Renal Events
3.3. Risk in Smokers by Level of Alcohol Consumption
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doyle, J.T.; Dawber, T.R.; Kannel, W.B.; Heslin, A.S.; Kahn, H.A. Cigarette smoking and coronary heart disease. Combined experience of the Albany and Framingham studies. N. Engl. J. Med. 1962, 266, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 1226–1228. [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2004. [Google Scholar]
- Barua, R.S.; Ambrose, J.A. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arter. Thromb. Vasc. Biol. 2013, 33, 1460–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, B.D.; Abnet, C.C.; Feskanich, D.; Freedman, N.D.; Hartge, P.; Lewis, C.E.; Ockene, J.K.; Prentice, R.L.; Speizer, F.E.; Thun, M.J.; et al. Smoking and mortality—Beyond established causes. N. Engl. J. Med. 2015, 372, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products. 2021. Available online: https://www.who.int/publications/i/item/9789240032095 (accessed on 15 July 2022).
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Walker, M. Smoking cessation and the risk of stroke in middle-aged men. JAMA 1995, 274, 155–160. [Google Scholar] [CrossRef]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Corrao, G.; Rubbiati, L.; Bagnardi, V.; Zambon, A.; Poikolainen, K. Alcohol and coronary heart disease: A meta-analysis. Addiction 2000, 95, 1505–1523. [Google Scholar] [CrossRef]
- Rehm, J.; Baliunas, D.; Borges, G.L.G.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V.; et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction 2010, 105, 817–843. [Google Scholar] [CrossRef] [Green Version]
- Freiberg, M.S.; Samet, J.H. Alcohol and coronary heart disease: The answer awaits a randomized controlled trial. Circulation 2005, 112, 1379–1381. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and Cardiovascular Health: The Razor-Sharp Double-Edged Sword. J. Am. Coll. Cardiol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef] [Green Version]
- Shaper, A.; Wannamethee, G.; Walker, M. Alcohol and Mortality in British Men: Explaining the U-Shaped Curve. Lancet 1988, 332, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.; Broad, J.; Connor, J.; Wells, S. Alcohol and ischaemic heart disease: Probably no free lunch. Lancet 2005, 366, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.E.; Ronksley, P.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: Systematic review and meta-analysis of interventional studies. BMJ 2011, 342, d636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronksley, P.E.; Brien, S.E.; Turner, B.J.; Mukamal, K.J.; Ghali, W.A. Association of alcohol consumption with selected cardiovascular disease outcomes: A systematic review and meta-analysis. BMJ 2011, 342, d671. [Google Scholar] [CrossRef] [Green Version]
- Grant, B.F. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abus. 1998, 10, 59–73. [Google Scholar] [CrossRef]
- King, A.C.; Epstein, A.M. Alcohol Dose—Dependent Increases in Smoking Urge in Light Smokers. Alcohol. Clin. Exp. Res. 2005, 29, 547–552. [Google Scholar] [CrossRef]
- McKee, S.A.; Falba, T.; O’Malley, S.S.; Sindelar, J.; O’Connor, P.G. Smoking Status as a Clinical Indicator for Alcohol Misuse in US Adults. Arch. Intern. Med. 2007, 167, 716–721. [Google Scholar] [CrossRef]
- Husky, M.M.; Paliwal, P.; Mazure, C.M.; McKee, S.A. Gender Differences in Association with Substance Use Diagnoses and Smoking. J. Addict. Med. 2007, 1, 161–164. [Google Scholar] [CrossRef]
- Rosengren, A.; Wilhelmsen, L.; Wedel, H. Separate and Combined Effects of Smoking and Alcohol Abuse in Middle-aged Men. Acta Medica Scand. 1988, 223, 111–118. [Google Scholar] [CrossRef]
- Madani, A.H.; Dikshit, M.; Bhaduri, D.; Aghamolaei, T.; Moosavy, S.H.; Azarpaykan, A. Interaction of Alcohol Use and Specific Types of Smoking on the Development of Oral Cancer. Int. J. High Risk Behav. Addict. 2014, 3, e12120. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, F.; Yamamoto, K.; Suzuki, S.; Inoue, H.; Tsurumaru, M.; Kajiyama, Y.; Kato, H.; Igaki, H.; Furuta, K.; Fujita, H.; et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut 2010, 59, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Cancer Risk Associated with Alcohol and Tobacco Use: Focus on Upper Aero-digestive Tract and Liver. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 193–198. [Google Scholar]
- McKee, S.A.; Krishnan-Sarin, S.; Shi, J.; Mase, T.; O’Malley, S.S. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology 2006, 189, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.A.; Drobes, D.J. Cognitive manifestations of drinking–smoking associations: Preliminary findings with a cross-primed Stroop task. Drug Alcohol Depend. 2014, 147, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, T.; Nogawa, K.; Sakata, K.; Morimoto, H.; Morita, K.; Watanabe, Y.; Suwazono, Y. Effects of Alcohol Consumption and Smoking on the Onset of Hypertension in a Long-Term Longitudinal Study in a Male Workers’ Cohort. Int. J. Environ. Res. Public Health 2021, 18, 11781. [Google Scholar] [CrossRef]
- Gao, N.; Liu, T.; Wang, Y.; Chen, M.; Yu, L.; Fu, C.; Xu, K. Assessing the association between smoking and hypertension: Smoking status, type of tobacco products, and interaction with alcohol consumption. Front. Cardiovasc. Med. 2023, 10, 1027988. [Google Scholar] [CrossRef]
- Aggarwal, R.; Yeh, R.W.; Maddox, K.E.J.; Wadhera, R.K. Cardiovascular Risk Factor Prevalence, Treatment, and Control in US Adults Aged 20 to 44 Years, 2009 to March 2020. JAMA 2023, 329, 899. [Google Scholar] [CrossRef]
- Mehta, N.K.; Abrams, L.R.; Myrskylä, M. US life expectancy stalls due to cardiovascular disease, not drug deaths. Proc. Natl. Acad. Sci. USA 2020, 117, 6998–7000. [Google Scholar] [CrossRef] [Green Version]
- Ariss, R.W.; Minhas, A.M.K.; Lang, J.; Ramanathan, P.K.; Khan, S.U.; Kassi, M.; Warraich, H.J.; Kolte, D.; Alkhouli, M.; Nazir, S. Demographic and regional trends in stroke-related mortality in young adults in the United States, 1999 to 2019. J. Am. Heart Assoc. 2022, 11, e025903. [Google Scholar] [CrossRef]
- Palatini, P.; Fania, C.; Mos, L.; Mazzer, A.; Saladini, F.; Casiglia, E. Alcohol intake more than doubles the risk of early cardiovascular events in young hypertensive smokers. Am. J. Med. 2017, 130, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Palatini, P.; Graniero, G.R.; Mormino, P.; Nicolosi, L.; Mos, L.; Visentin, P.; Pessina, A.C. Relation between physical training and ambulatory blood pressure in stage I hypertensive subjects. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Circulation 1994, 90, 2870–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatini, P.; Canali, C.; Graniero, G.R.; Rossi, G.; De Toni, R.; Santonastaso, M.; Follo, M.D.; Zanata, G.; Ferrarese, E.; Mormino, P.; et al. Relationship of plasma renin activity with caffeine intake and physical training in mild hypertensive men. Eur. J. Epidemiol. 1996, 12, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Mormino, P.; Dorigatti, F.; Santonastaso, M.; Mos, L.; De Toni, R.; Winnicki, M.; Follo, M.D.; Biasion, T.; Garavelli, G.; et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: The Harvest. Kidney Int. 2006, 70, 578–584. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, E. Life’s Simple 7: Vital But Not Easy. J. Am. Heart Assoc. 2018, 7, e009324. [Google Scholar] [CrossRef]
- Shin, J.; Paik, H.Y.; Joung, H.; Shin, S. Smoking and alcohol consumption influence the risk of cardiovascular diseases in Korean adults with elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2187–2194. [Google Scholar] [CrossRef]
- Chudzińska, M.; Wołowiec, Ł.; Banach, J.; Rogowicz, D.; Grześk, G. Alcohol and Cardiovascular Diseases—Do the Consumption Pattern and Dose Make the Difference? J. Cardiovasc. Dev. Dis. 2022, 9, 317. [Google Scholar] [CrossRef]
- Xu, W.-H.; Zhang, X.-L.; Gao, Y.-T.; Xiang, Y.-B.; Gao, L.-F.; Zheng, W.; Shu, X.-O. Joint effect of cigarette smoking and alcohol consumption on mortality. Prev. Med. 2007, 45, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Verplaetse, T.L.; McKee, S.A. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am. J. Drug Alcohol Abus. 2016, 43, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fan, X.; Wei, L.; Yang, K.; Jiao, M. The impact of high-risk lifestyle factors on all-cause mortality in the US non-communicable disease population. BMC Public Health 2023, 23, 422. [Google Scholar] [CrossRef]
- Venza, I.; Visalli, M.; Oteri, R.; Teti, D.; Venza, M. Combined effects of cigarette smoking and alcohol consumption on antioxidant/oxidant balance in age-related macular degeneration. Aging Clin. Exp. Res. 2012, 24, 530–536. [Google Scholar] [PubMed]
- Virdis, A.; Giannarelli, C.; Fritsch Neves, M.; Taddei, S.; Ghiadoni, L. Cigarette Smoking and Hypertension. Curr. Pharm. Des. 2010, 16, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bartlett, S.E. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol. Disord. Drug Targets 2010, 9, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Freedman, N.D.; Albert, P.S.; Huxley, R.R.; Shiels, M.S.; Withrow, D.R.; Spillane, S.; Powell-Wiley, T.M.; De González, A.B. Association of Cardiovascular Disease With Premature Mortality in the United States. JAMA Cardiol. 2019, 4, 1230–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridget, M.; Kuehn, M.S.J. Tobacco use remains high in middle and high schools. JAMA 2022, 328, 2389–2390. [Google Scholar]
- Babb, S.; Malarcher, A.; Schauer, G.; Asman, K.; Jamal, A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 65, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Swales, J.D.; Ramsey, L.E.; Coope, J.R. Treating mild hypertension. Report of the British Hypertension Society working party. BMJ 1989, 298, 694–698. [Google Scholar]
- Sever, P.; Beevers, G.; Bulpitt, C.; Lever, A.; Ramsay, L.; Reid, J.; Swales, J. Management guidelines in essential hypertension: Report of the second working party of the British Hypertension Society. BMJ 1993, 306, 983–987. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.; MacMahon, S.; Mancia, G.; Whitworth, J.; Beilin, L.; Hansson, L.; Neal, B.; Rodgers, A.; Mhurchu, C.N.; Clark, T. 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Sub-Committee. Blood Press Suppl. 1999, 1, 9–43. [Google Scholar]
- European Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003, 21, 1011–1053. [Google Scholar] [CrossRef]




| Variable | Alcohol No Smoking No (N = 525) | Alcohol Yes Smoking No (N = 429) | Alcohol No Smoking Yes (N = 112) | Alcohol Yes Smoking Yes (N = 142) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p-Value | |
| Age, years | 30.8 | 8.8 | 35.4 * | 7.7 | 32.4 | 8.0 | 34.8 * | 7.9 | <0.001 |
| BMI, kg/m2 | 25.0 | 3.7 | 25.6 | 2.9 | 25.7 | 3.4 | 25.4 | 3.1 | 0.07 |
| Office SBP, mmHg | 145.9 | 10.7 | 145.6 | 10.0 | 144.5 | 10.7 | 144.5 | 11.2 | 0.37 |
| Office DBP, mmHg | 92.9 | 6.1 | 94.4 | 5.1 | 93.3 | 5.4 | 93.3 | 6.1 | 0.26 |
| Heart rate, bpm | 75.7 | 9.6 | 73.4 | 9.1 | 75.3 | 9.6 | 73.1 | 9.7 | 0.13 |
| Cholesterol, mg/dL | 193.4 | 36.7 | 201.2 | 39.0 | 197.2 | 38.2 | 200.2 | 39.9 | 0.92 |
| 24-h SBP, mmHg | 130.3 | 10.9 | 131.2 | 10.2 | 131.4 | 12.5 | 133.8 † | 10.6 | 0.014 |
| 24-h DBP, mmHg | 81.7 | 8.2 | 81.8 | 8.0 | 81.3 | 8.4 | 82.3 | 8.1 | 0.60 |
| Sex, male % | 64.8 | -- | 81.2 | -- | 60.7 | -- | 85.2 | -- | <0.001 |
| Coffee use, yes % | 62.7 | -- | 83.5 | -- | 70.5 | -- | 88.7 | -- | <0.001 |
| Physical activity, yes % | 43.0 | -- | 35.7 | -- | 29.5 | -- | 39.4 | -- | 0.02 |
| MACE, yes % | 6.3 | -- | 8.6 | -- | 9.8 | -- | 18.3 | -- | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palatini, P.; Mos, L.; Saladini, F.; Vriz, O.; Fania, C.; Ermolao, A.; Battista, F.; Canevari, M.; Rattazzi, M. Both Moderate and Heavy Alcohol Use Amplify the Adverse Cardiovascular Effects of Smoking in Young Patients with Hypertension. J. Clin. Med. 2023, 12, 2792. https://doi.org/10.3390/jcm12082792
Palatini P, Mos L, Saladini F, Vriz O, Fania C, Ermolao A, Battista F, Canevari M, Rattazzi M. Both Moderate and Heavy Alcohol Use Amplify the Adverse Cardiovascular Effects of Smoking in Young Patients with Hypertension. Journal of Clinical Medicine. 2023; 12(8):2792. https://doi.org/10.3390/jcm12082792
Chicago/Turabian StylePalatini, Paolo, Lucio Mos, Francesca Saladini, Olga Vriz, Claudio Fania, Andrea Ermolao, Francesca Battista, Mattia Canevari, and Marcello Rattazzi. 2023. "Both Moderate and Heavy Alcohol Use Amplify the Adverse Cardiovascular Effects of Smoking in Young Patients with Hypertension" Journal of Clinical Medicine 12, no. 8: 2792. https://doi.org/10.3390/jcm12082792





