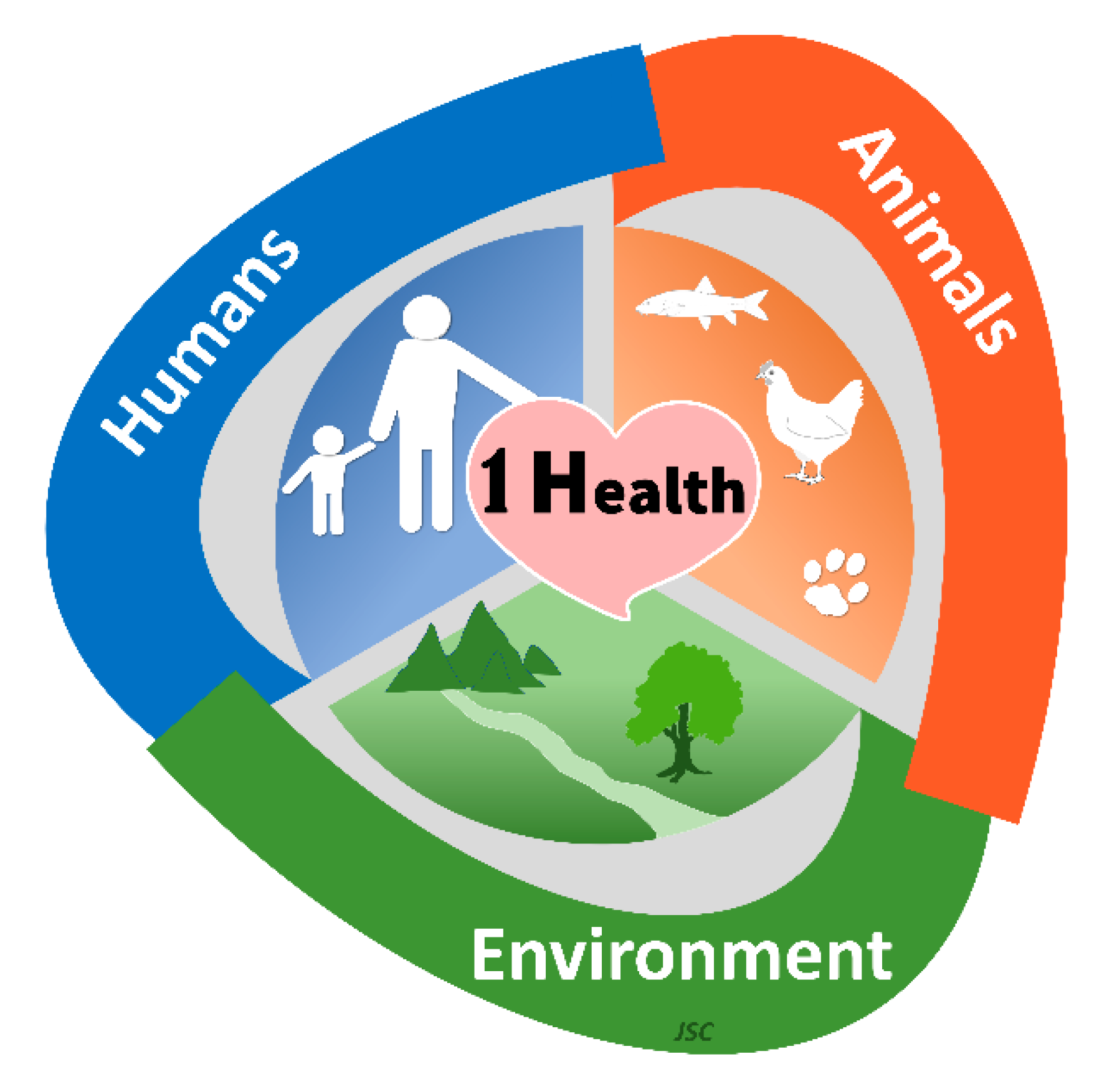
No Boundaries for Toxicology in Clinical Medicine: One Health, One Society and One Planet for All of Us
Conflicts of Interest
References
- Atlas, R.M. One Health: Its origins and future. Curr. Top Microbiol. Immunol. 2013, 365, 1–13. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.; Cullor, J. A one health perspective on dairy production and dairy food safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Wong, N.; Thomas, J.; Talkington, K.; Jungman, E.; Coukell, A. Antimicrobial drug use in food-producing animals and associated human health risks: What, and how strong, is the evidence? BMC Vet. Res. 2017, 13, 211. [Google Scholar]
- Rabinowitz, P.M.; Natterson-Horowitz, B.J.; Kahn, L.H.; Kock, R.; Pappaioanou, M. Incorporating one health into medical education. BMC Med. Educ. 2017, 17, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, A.; Balsari, S.; Nusbaum, J.; Heerboth, A.; Lemery, J. Perspective: Environment, biodiversity, and the education of the physician of the future. Acad. Med. 2013, 88, 168–172. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Quadripartite Call to Action for One Health for a Safer World. 23 March. Available online: https://www.who.int/news/item/27-03-2023-quadripartite-call-to-action-for-one-health-for-a-safer-world (accessed on 23 March 2023).
- Kahn, L.H.; Kaplan, B.; Steele, J.H. Confronting zoonoses through closer collaboration between medicine and veterinary medicine (as ‘one medicine’). Vet. Ital. 2007, 43, 5–19. [Google Scholar]
- Beasley, V. ‘One toxicology’, ‘ecosystem health’ and ‘one health’. Vet. Ital. 2009, 45, 97–110. [Google Scholar]
- Rumbeiha, W.K. Toxicology and “One Health”: Opportunities for Multidisciplinary Collaborations. J. Med. Toxicol. 2012, 8, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Polivka, B.J. The Great London Smog of 1952. Am. J. Nurs. 2018, 118, 57–61. [Google Scholar] [CrossRef]
- Buttke, D.E. Toxicology, environmental health, and the “One Health” concept. J. Med. Toxicol. 2011, 7, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Jarman, W.M.; Ballschmiter, K. From coal to DDT: The history of the development of the pesticide DDT from synthetic dyes till Silent Spring. Endeavour 2012, 36, 131–142. [Google Scholar] [CrossRef] [PubMed]
- King, L.J.; Anderson, L.R.; Blackmore, C.G.; Blackwell, M.J.; Lautner, E.A.; Marcus, L.C.; Meyer, T.E.; Monath, T.P.; Nave, J.E.; Ohle, J.; et al. Executive summary of the AVMA One Health Initiative Task Force report. J. Am. Vet. Med. Assoc. 2008, 233, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; de Souza Dias, B.F.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation-Lancet Commission on planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Wadsworth, E.; Drummond, C.; Deluca, P. The Dynamic Environment of Crypto Markets: The Lifespan of New Psychoactive Substances (NPS) and Vendors Selling NPS. Brain Sci. 2018, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Welz, A.; Koba, M.; Kośliński, P.; Siódmiak, J. Rapid Targeted Method of Detecting Abused Piperazine Designer Drugs. J. Clin. Med. 2021, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Welz, A.; Koba, M.; Kośliński, P.; Siódmiak, J. Comparison of LC-MS and LC-DAD Methods of Detecting Abused Piperazine Designer Drugs. J. Clin. Med. 2022, 11, 1758. [Google Scholar] [CrossRef]
- Poyatos, L.; Torres, A.; Papaseit, E.; Pérez-Mañá, C.; Hladun, O.; Núñez-Montero, M.; de la Rosa, G.; Torrens, M.; Fuster, D.; Muga, R.; et al. Abuse Potential of Cathinones in Humans: A Systematic Review. J. Clin. Med. 2022, 11, 1004. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. The Genesis of a New Open-Access Journal Focused on the Latest Scientific Advances in Psychoactive Substances. Psychoactives 2022, 1, 1–6. [Google Scholar] [CrossRef]
- Nižnanský, Ľ.; Nižnanská, Ž.; Kuruc, R.; Szórádová, A.; Šikuta, J.; Zummerová, A. Ayahuasca as a Decoction Applied to Human: Analytical Methods, Pharmacology and Potential Toxic Effects. J. Clin. Med. 2022, 11, 1147. [Google Scholar] [CrossRef]
- Dacosta-Sánchez, D.; Díaz-Batanero, C.; Fernandez-Calderon, F.; Lozano, Ó.M. Impact of Cluster B Personality Disorders in Drugs Therapeutic Community Treatment Outcomes: A Study Based on Real World Data. J. Clin. Med. 2021, 10, 2572. [Google Scholar] [CrossRef]
- Marsch, L.A.; Campbell, A.; Campbell, C.; Chen, C.H.; Ertin, E.; Ghitza, U.; Lambert-Harris, C.; Hassanpour, S.; Holtyn, A.F.; Hser, Y.I.; et al. The application of digital health to the assessment and treatment of substance use disorders: The past, current, and future role of the National Drug Abuse Treatment Clinical Trials Network. J. Subst. Abuse Treat 2020, 112s, 4–11. [Google Scholar] [CrossRef]
- Magalhães, T.; Dinis-Oliveira, R.J.; Taveira-Gomes, T. Digital Health and Big Data Analytics: Implications of Real-World Evidence for Clinicians and Policymakers. Int. J. Environ. Res. Public Health 2022, 19, 8364. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Oliveira, N.G.; Dinis-Oliveira, R.J. Pharmacokinetic and pharmacodynamic of bupropion: Integrative overview of relevant clinical and forensic aspects. Drug Metab. Rev. 2019, 51, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J. Metabolic Profiles of Propofol and Fospropofol: Clinical and Forensic Interpretative Aspects. Biomed. Res. Int. 2018, 2018, 6852857. [Google Scholar] [CrossRef] [Green Version]
- Dinis-Oliveira, R.J. Metabolism and metabolomics of opiates: A long way of forensic implications to unravel. J. Forensic. Leg. Med. 2019, 61, 128–140. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Pereira, C.L.; da Silva, D.D. Pharmacokinetic and Pharmacodynamic Aspects of Peyote and Mescaline: Clinical and Forensic Repercussions. Curr. Mol. Pharmacol. 2019, 12, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Lobato-Freitas, C.; Brito-da-Costa, A.M.; Dinis-Oliveira, R.J.; Carmo, H.; Carvalho, F.; Silva, J.P.; Dias-da-Silva, D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals 2021, 14, 186. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, L.; Dinis-Oliveira, R.J. The synthetic cathinone α-pyrrolidinovalerophenone (α-PVP): Pharmacokinetic and pharmacodynamic clinical and forensic aspects. Drug Metab. Rev. 2018, 50, 125–139. [Google Scholar] [CrossRef]
- Oliveira, N.G.; Dinis-Oliveira, R.J. Drugs of abuse from a different toxicological perspective: An updated review of cocaine genotoxicity. Arch. Toxicol. 2018, 92, 2987–3006. [Google Scholar] [CrossRef]
- Silva, A.R.; Dinis-Oliveira, R.J. Pharmacokinetics and pharmacodynamics of dextromethorphan: Clinical and forensic aspects. Drug Metab. Rev. 2020, 52, 258–282. [Google Scholar] [CrossRef]
- Sousa, A.; Dinis-Oliveira, R.J. Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: Relevant clinical and forensic aspects. Subst. Abus. 2020, 41, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.; Alonso, S.; Prieto, E.; Ascaso-Del-Rio, A.; Ortuño, J.; Fernandez, N.; Portolés, A. Single and Multiple Dose PK-PD Characterization for Carisoprodol. Part I: Pharmacokinetics, Metabolites, and 2C19 Phenotype Influence. Double-Blind, Placebo-Controlled Clinical Trial in Healthy Volunteers. J. Clin. Med. 2022, 11, 858. [Google Scholar] [CrossRef]
- Calvo, A.; González-Hidalgo, M.; Terleira, A.; Fernández, N.; Portolés, A. Carisoprodol Single and Multiple Dose PK-PD. Part II: Pharmacodynamics Evaluation Method for Central Muscle Relaxants. Double-Blind Placebo-Controlled Clinical Trial in Healthy Volunteers. J. Clin. Med. 2022, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Delcher, C.; Brown, J.D.; Wei, Y.J.; Reisfield, G.M.; Winterstein, A.G. Impact of Schedule IV controlled substance classification on carisoprodol utilization in the United States: An interrupted time series analysis. Drug Alcohol Depend. 2019, 202, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Vincze, I.; Czermann, R.; Nagy, Z.; Kovács, M.; Neely, M.; Farkas, R.; Kocsis, I.; Karvaly, G.B.; Kopitkó, C. Assessment of Antibiotic Pharmacokinetics, Molecular Biomarkers and Clinical Status in Critically Ill Adults Diagnosed with Community-Acquired Pneumonia and Receiving Intravenous Piperacillin/Tazobactam and Hydrocortisone over the First Five Days of Intensive Care: An Observational Study (STROBE Compliant). J. Clin. Med. 2022, 11, 4140. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinis-Oliveira, R.J. No Boundaries for Toxicology in Clinical Medicine: One Health, One Society and One Planet for All of Us. J. Clin. Med. 2023, 12, 2808. https://doi.org/10.3390/jcm12082808
Dinis-Oliveira RJ. No Boundaries for Toxicology in Clinical Medicine: One Health, One Society and One Planet for All of Us. Journal of Clinical Medicine. 2023; 12(8):2808. https://doi.org/10.3390/jcm12082808
Chicago/Turabian StyleDinis-Oliveira, Ricardo Jorge. 2023. "No Boundaries for Toxicology in Clinical Medicine: One Health, One Society and One Planet for All of Us" Journal of Clinical Medicine 12, no. 8: 2808. https://doi.org/10.3390/jcm12082808




