Preoperative Predictors of Late Aortic Expansion in Acute Type B Aortic Dissection Treated with TEVAR
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Imaging Data
2.3. TEVAR Procedures
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Operative Details
3.3. Postoperative Complications
3.4. Univariable and Multivariable Logistic Regression Analysis of the Risk Factors of LAE
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.L.; Huang, H.Y.; Li, Z.; Yu, Y.S.; Hu, Y.Q.; Ye, W.X.; Hua, F.; Chen, Y.H.; Ni, H.; Ding, Q.W.; et al. Risk factors associated with aortic remodeling in patients with Stanford type B aortic dissection after thoracic endovascular aortic repair. Genet. Mol. Res. 2015, 14, 11692–11699. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, J.; Znaniecki, L.; Kaszubowski, M.; Rogowski, J. Late Aortic Remodeling after Endovascular Repair of Complicated Type B Aortic Dissection-TEVAR Protects Only the Covered Segment of Thoracic Aorta. Ann. Vasc. Surg. 2019, 55, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Kische, S.; Rousseau, H.; Eggebrecht, H.; Rehders, T.C.; Kundt, G.; Glass, A.; Scheinert, D.; Czerny, M.; Kleinfeldt, T.; et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013, 6, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.I.; Durham, C.; Clouse, W.D.; Patel, V.I.; Lancaster, R.T.; Cambria, R.P.; Conrad, M.F. Predictors of late aortic intervention in patients with medically treated type B aortic dissection. J. Vasc. Surg. 2018, 67, 78–84. [Google Scholar] [CrossRef]
- Tolenaar, J.L.; Kern, J.A.; Jonker, F.H.; Cherry, K.J.; Tracci, M.C.; Angle, J.F.; Sabri, S.; Trimarchi, S.; Strider, D.; Alaiwaidi, G.; et al. Predictors of false lumen thrombosis in type B aortic dissection treated with TEVAR. Ann. Cardiothorac. Surg. 2014, 3, 255–263. [Google Scholar] [CrossRef]
- Fattori, R.; Montgomery, D.; Lovato, L.; Kische, S.; Di Eusanio, M.; Ince, H.; Eagle, K.A.; Isselbacher, E.M.; Nienaber, C.A. Survival after endovascular therapy in patients with type B aortic dissection: A report from the International Registry of Acute Aortic Dissection (IRAD). JACC Cardiovasc. Interv. 2013, 6, 876–882. [Google Scholar] [CrossRef]
- Qin, Y.L.; Deng, G.; Li, T.X.; Jing, R.W.; Teng, G.J. Risk factors of incomplete thrombosis in the false lumen after endovascular treatment of extensive acute type B aortic dissection. J. Vasc. Surg. 2012, 56, 1232–1238. [Google Scholar] [CrossRef]
- Evangelista, A.; Salas, A.; Ribera, A.; Ferreira-Gonzalez, I.; Cuellar, H.; Pineda, V.; Gonzalez-Alujas, T.; Bijnens, B.; Permanyer-Miralda, G.; Garcia-Dorado, D. Long-term outcome of aortic dissection with patent false lumen: Predictive role of entry tear size and location. Circulation 2012, 125, 3133–3141. [Google Scholar] [CrossRef]
- Chen, I.M.; Chen, P.L.; Huang, C.Y.; Weng, S.H.; Chen, W.Y.; Shih, C.C. Factors Affecting Optimal Aortic Remodeling After Thoracic Endovascular Aortic Repair of Type B (IIIb) Aortic Dissection. Cardiovasc. Interv. Radiol. 2017, 40, 671–681. [Google Scholar] [CrossRef]
- Immer, F.F.; Krahenbuhl, E.; Hagen, U.; Stalder, M.; Berdat, P.A.; Eckstein, F.S.; Schmidli, J.; Carrel, T.P. Large area of the false lumen favors secondary dilatation of the aorta after acute type A aortic dissection. Circulation 2005, 112, I249–I252. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Zhang, Y.; Shi, D.; Shen, Y.; Bao, J.; Zhao, Z.; Feng, X.; Feng, R.; Zhou, J.; et al. Should the distal tears of aortic dissection be treated? The risk of distal tears after proximal repair of aortic dissection. Int. J. Cardiol. 2018, 261, 162–166. [Google Scholar] [CrossRef]
- Lombardi, J.V.; Hughes, G.C.; Appoo, J.J.; Bavaria, J.E.; Beck, A.W.; Cambria, R.P.; Charlton-Ouw, K.; Eslami, M.H.; Kim, K.M.; Leshnower, B.G.; et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J. Vasc. Surg. 2020, 71, 723–747. [Google Scholar] [CrossRef]
- Burris, N.S.; Patel, H.J.; Hope, M.D. Retrograde flow in the false lumen: Marker of a false lumen under stress? J. Thorac. Cardiovasc. Surg. 2019, 157, 488–491. [Google Scholar] [CrossRef]
- Wan Ab Naim, W.N.; Ganesan, P.B.; Sun, Z.; Lei, J.; Jansen, S.; Hashim, S.A.; Ho, T.K.; Lim, E. Flow pattern analysis in type B aortic dissection patients after stent-grafting repair: Comparison between complete and incomplete false lumen thrombosis. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2961. [Google Scholar] [CrossRef]
- Tsai, M.T.; Wu, H.Y.; Roan, J.N.; Tsai, Y.S.; Hsieh, P.C.; Yang, Y.J.; Luo, C.Y. Effect of false lumen partial thrombosis on repaired acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2014, 148, 2140–2146.e2143. [Google Scholar] [CrossRef]
- Tolenaar, J.L.; van Keulen, J.W.; Jonker, F.H.W.; van Herwaarden, J.A.; Verhagen, H.J.; Moll, F.L.; Muhs, B.E.; Trimarchi, S. Morphologic predictors of aortic dilatation in type B aortic dissection. J. Vasc. Surg. 2013, 58, 1220–1225. [Google Scholar] [CrossRef]
- Sueyoshi, E.; Sakamoto, I.; Hayashi, K.; Yamaguchi, T.; Imada, T. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation 2004, 110, II256–II261. [Google Scholar] [CrossRef]
- Kamman, A.V.; Brunkwall, J.; Verhoeven, E.L.; Heijmen, R.H.; Trimarchi, S.; Trialists, A. Predictors of aortic growth in uncomplicated type B aortic dissection from the Acute Dissection Stent Grafting or Best Medical Treatment (ADSORB) database. J. Vasc. Surg. 2017, 65, 964–971.e963. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B. Aortic Dissection Risk in Marfan Syndrome. J. Am. Coll. Cardiol. 2020, 75, 854–856. [Google Scholar] [CrossRef]
- Koo, H.-K.; Lawrence, K.A.; Musini, V.M. Beta-blockers for preventing aortic dissection in Marfan syndrome. Cochrane Database Syst. Rev. 2017, 11, CD011103. [Google Scholar] [CrossRef]
- Armour, C.H.; Menichini, C.; Milinis, K.; Gibbs, R.G.J.; Xu, X.Y. Location of Reentry Tears Affects False Lumen Thrombosis in Aortic Dissection Following TEVAR. J. Endovasc. Ther. 2020, 27, 396–404. [Google Scholar] [CrossRef]
- Van Bogerijen, G.H.W.; Tolenaar, J.L.; Rampoldi, V.; Moll, F.L.; van Herwaarden, J.A.; Jonker, F.H.W.; Eagle, K.A.; Trimarchi, S. Predictors of aortic growth in uncomplicated type B aortic dissection. J. Vasc. Surg. 2014, 59, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Bartnes, K.; Tsai, T.T.; Eagle, K.A.; Evangelista, A.; Nienaber, C.A.; Suzuki, T.; Fattori, R.; Froehlich, J.B.; Hutchison, S.; et al. Extent of Preoperative False Lumen Thrombosis Does Not Influence Long-Term Survival in Patients with Acute Type A Aortic Dissection. J. Am. Heart Assoc. 2013, 2, e000112. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Evangelista, A.; Nienaber, C.A.; Myrmel, T.; Meinhardt, G.; Cooper, J.V.; Smith, D.E.; Suzuki, T.; Fattori, R.; Llovet, A.; et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N. Engl. J. Med. 2007, 357, 349–359. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, T.E.; Gleason, T.G.; Patel, H.J.; Aldea, G.S.; Bavaria, J.E.; Beaver, T.M.; Chen, E.P.; Czerny, M.; Estrera, A.L.; Firestone, S.; et al. The Society of Thoracic Surgeons/American Association for Thoracic Surgery Clinical Practice Guidelines on the Management of Type B Aortic Dissection. Ann. Thorac. Surg. 2022, 113, 1073–1092. [Google Scholar] [CrossRef]
- Higashigaito, K.; Sailer, A.M.; van Kuijk, S.M.J.; Willemink, M.J.; Hahn, L.D.; Hastie, T.J.; Miller, D.C.; Fischbein, M.P.; Fleischmann, D. Aortic growth and development of partial false lumen thrombosis are associated with late adverse events in type B aortic dissection. J. Thorac. Cardiovasc. Surg. 2021, 161, 1184–1190.e1182. [Google Scholar] [CrossRef]
- Gao, Z.; Qin, Z.; Qian, D.; Pan, W.; Zhou, G.; An, Z.; Hou, C.; Wang, L.; Zhang, L.; Gu, T.; et al. Risk factors for incomplete thrombosis in false lumen in sub-acute type B aortic dissection post-TEVAR. Heart Vessel. 2022, 37, 505–512. [Google Scholar] [CrossRef]
- Trimarchi, S.; Tolenaar, J.L.; Jonker, F.H.; Murray, B.; Tsai, T.T.; Eagle, K.A.; Rampoldi, V.; Verhagen, H.J.; van Herwaarden, J.A.; Moll, F.L.; et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J. Thorac. Cardiovasc. Surg. 2013, 145, S208–S212. [Google Scholar] [CrossRef]
- Naim, W.N.W.A.; Ganesan, P.B.; Sun, Z.; Liew, Y.M.; Qian, Y.; Lee, C.-J.; Jansen, S.; Hashim, S.A.; Lim, E. Prediction of thrombus formation using vortical structures presentation in Stanford type B aortic dissection: A preliminary study using CFD approach. Appl. Math. Model. 2016, 40, 3115–3127. [Google Scholar] [CrossRef]
- Sailer, A.M.; Nelemans, P.J.; Hastie, T.J.; Chin, A.S.; Huininga, M.; Chiu, P.; Fischbein, M.P.; Dake, M.D.; Miller, D.C.; Schurink, G.W.; et al. Prognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma. J. Thorac. Cardiovasc. Surg. 2017, 154, 1192–1200. [Google Scholar] [CrossRef]
- Sueyoshi, E.; Sakamoto, I.; Uetani, M. Growth rate of affected aorta in patients with type B partially closed aortic dissection. Ann. Thorac. Surg. 2009, 88, 1251–1257. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakakibara, M.; Ishii, H.; Hayashida, R.; Jinno, Y.; Okumura, S.; Okada, K.; Murohara, T. Influence of the false lumen status on clinical outcomes in patients with acute type B aortic dissection. J. Vasc. Surg. 2014, 59, 321–326. [Google Scholar] [CrossRef]
- Hata, M.; Sezai, A.; Niino, T.; Yoda, M.; Wakui, S.; Unosawa, S.; Umeda, T.; Shimura, K.; Osaka, S.; Furukawa, N.; et al. Prognosis for patients with type B acute aortic dissection: Risk analysis of early death and requirement for elective surgery. Circ. J. 2007, 71, 1279–1282. [Google Scholar] [CrossRef]
- Miller, C.C.; Sandhu, H.K.; Charlton-Ouw, K.M.; Azizzadeh, A.; Estrera, A.L.; Leake, S.; Safi, H.J. Patient height as a risk factor for poor outcome in acute type B aortic dissection. J. Cardiovasc. Surg. 2017, 58, 551–556. [Google Scholar] [CrossRef]
- Yuan, X.; Mitsis, A.; Semple, T.; Castro Verdes, M.; Cambronero-Cortinas, E.; Tang, Y.; Nienaber, C.A. False lumen intervention to promote remodelling and thrombosis-The FLIRT concept in aortic dissection. Catheter. Cardiovasc. Interv. 2018, 92, 732–740. [Google Scholar] [CrossRef]
- Pellenc, Q.; Roussel, A.; De Blic, R.; Girault, A.; Cerceau, P.; Ben Abdallah, I.; Milleron, O.; Jondeau, G.; Castier, Y. False lumen embolization in chronic aortic dissection promotes thoracic aortic remodeling at midterm follow-up. J. Vasc. Surg. 2019, 70, 710–717. [Google Scholar] [CrossRef]
- Hofferberth, S.C.; Nixon, I.K.; Mossop, P.J. Aortic false lumen thrombosis induction by embolotherapy (AFTER) following endovascular repair of aortic dissection. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2012, 19, 538–545. [Google Scholar] [CrossRef]


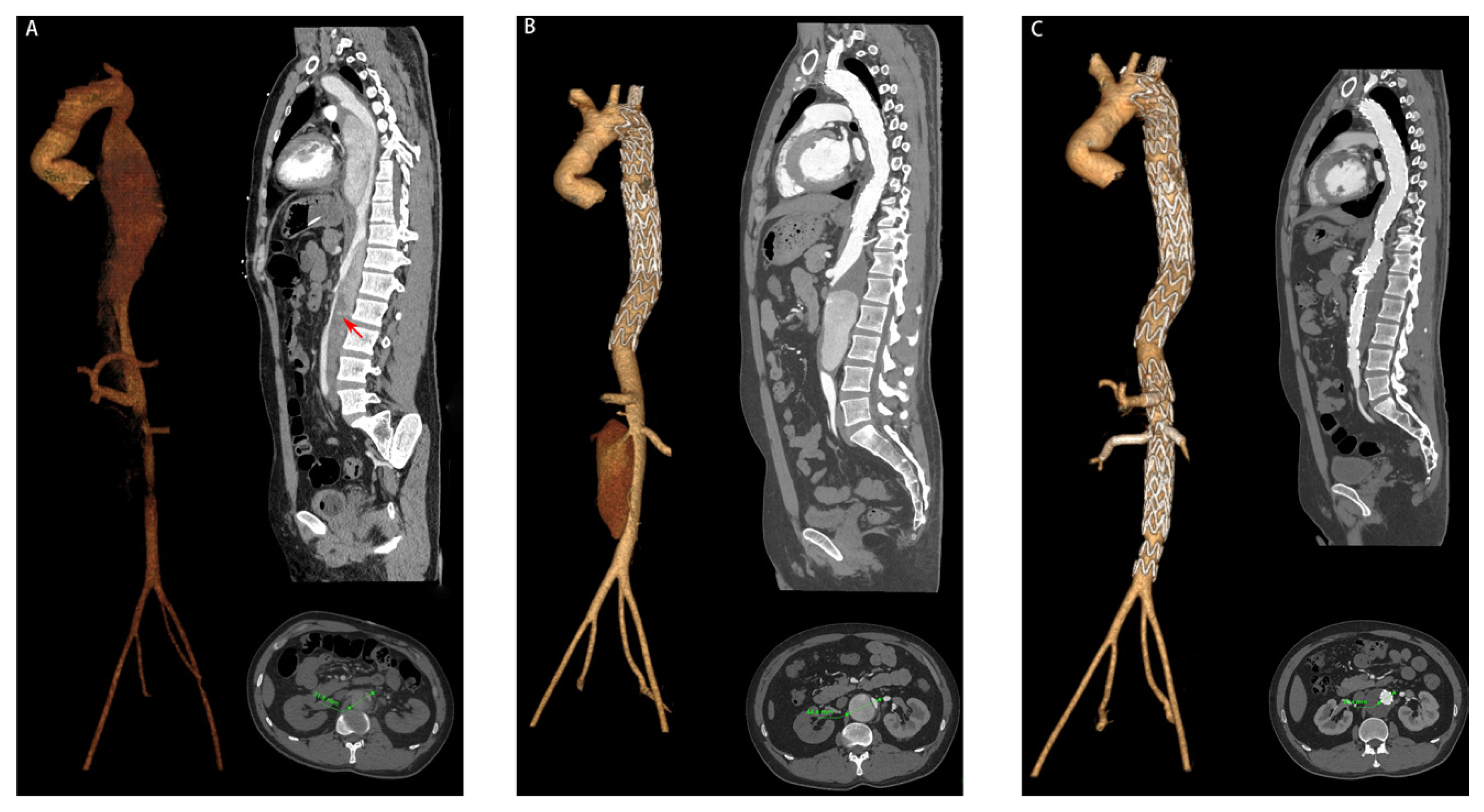
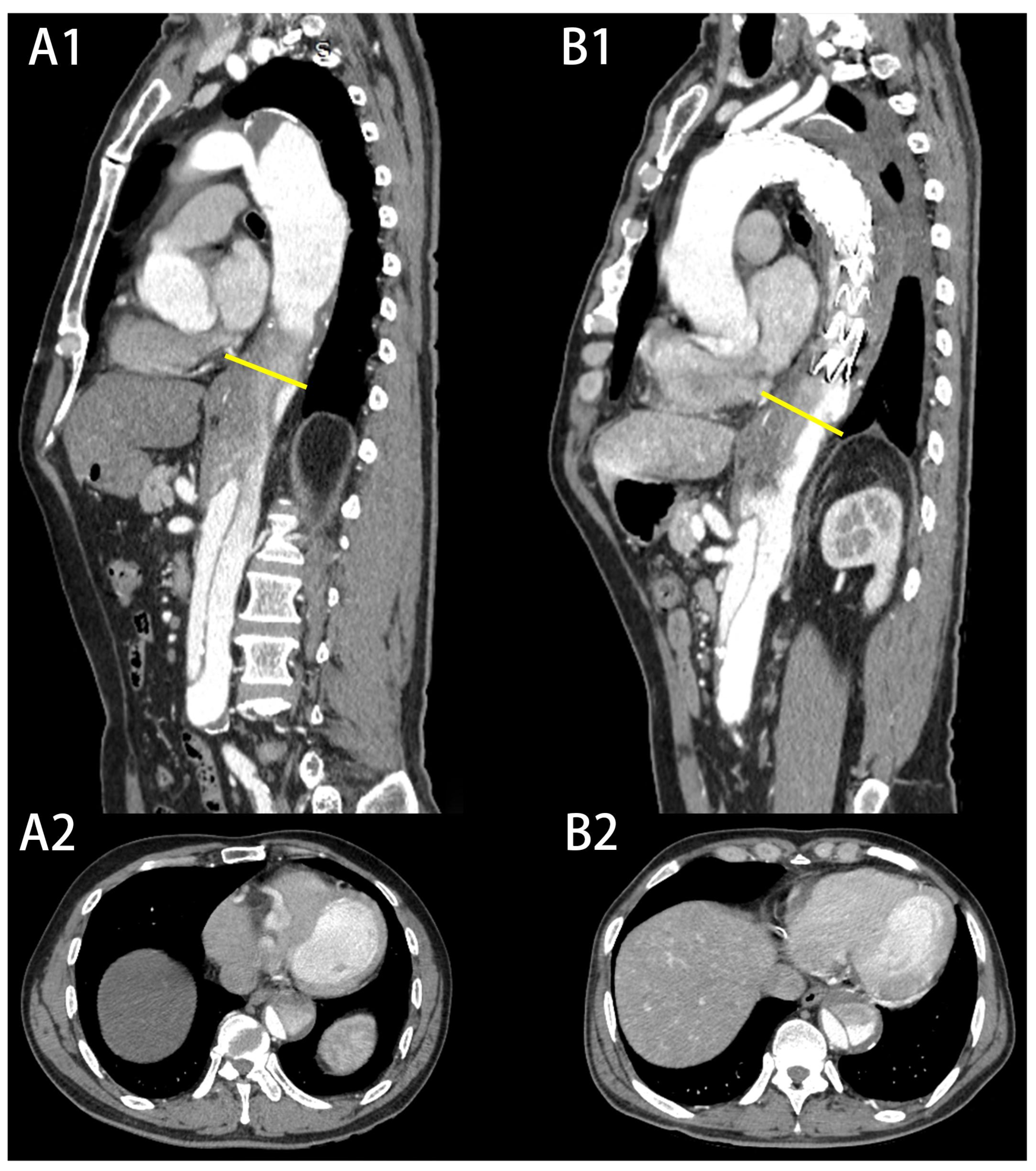
| Characteristic | Expanded Group (n = 15) | Non-Expanded Group (n = 81) | p Value |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 14 (93.3) | 71 (87.7) | 0.526 |
| Age (year), mean ± SD | 53.1 ± 10.6 | 54.8 ± 11.9 | 0.598 |
| Smoking, n (%) | 5 (33.3) | 25 (30.9) | 0.850 |
| Hyperlipidemia, n (%) | 4 (26.7) | 16 (19.8) | 0.545 |
| BMI (kg/m2), mean ± SD | 27.1 ± 4.4 | 25.9 ± 4.3 | 0.336 |
| Hypertension, n (%) | 10 (66.7) | 68 (84.0) | 0.115 |
| Diabetes, n (%) | 2 (13.3) | 9 (11.1) | 0.804 |
| CAD, n (%) | 1 (6.7) | 9 (11.1) | 0.605 |
| Atherosclerosis, n (%) | 7 (46.7) | 32 (39.5) | 0.604 |
| CVD, n (%) | 0 (0.0) | 4 (4.9) | 0.379 |
| VHD, n (%) | 1 (6.7) | 4 (4.9) | 0.782 |
| CKD, n (%) | 1 (6.7) | 7 (8.6) | 0.799 |
| Symptoms | 0.488 | ||
| Chest pain, n (%) | 9 (60.0) | 44 (54.3) | |
| Abdominal pain, n (%) | 3 (20.0) | 9 (11.1) | |
| Back pain, n (%) | 1 (6.7) | 18 (22.2) | |
| Asymptomatic, n (%) | 2 (13.3) | 10 (12.3) | |
| Chronicity classification of AD | 0.521 | ||
| Hyperacute, n (%) | 0 (0.0) | 6 (7.4) | |
| Acute, n (%) | 11 (73.3) | 58 (71.6) | |
| Subacute, n (%) | 4 (26.7) | 17 (21.0) | |
| ASA Classification | 0.407 | ||
| I, n (%) | 0 (0.0) | 8 (9.9) | |
| II, n (%) | 2 (13.3) | 10 (12.3) | |
| III, n (%) | 9 (60.0) | 53 (65.4) | |
| IV, n (%) | 4 (26.7) | 9 (11.1) | |
| V, n (%) | 0 (0.0) | 1 (1.2) | |
| Onset to TEVAR (days), mean ± SD | 12.8 ± 6.9 | 12.4 ± 6.6 | 0.823 |
| Characteristic | Expanded Group (n = 15) | Non-Expanded Group (n = 81) | p Value |
|---|---|---|---|
| Annular tear, n (%) | 2 (13.3) | 6 (7.4) | 0.446 |
| Dissection extends to aortic arch, n (%) | 2 (13.3) | 13 (16.0) | 0.790 |
| Untreated tears, mean ± SD | 1.87 ± 0.99 | 1.78 ± 0.85 | 0.748 |
| Diameter of stents (mm), range | 32.0 (32.0, 34.0) | 32.0 (31.5, 36.0) | 0.761 |
| Length of stents (mm), median (IQR) | 192.0 (189.0, 197.0) | 194.0 (189.5, 200) | 0.223 |
| Dissection extends to abdominal branches | |||
| Coeliac trunk, n (%) | 6 (40.0) | 43 (53.1) | 0.352 |
| SMA, n (%) | 1 (6.7) | 11 (13.6) | 0.457 |
| RA, n (%) | 7 (46.7) | 38 (46.9) | 0.986 |
| Dissection extends to CIA, n (%) | 12 (80.0) | 60 (74.1) | 0.626 |
| TL compression, n (%) | 14 (93.3) | 60 (74.1) | 0.103 |
| Partial thrombosis of FL, n (%) | 9 (60.0) | 7 (8.6) | <0.001 |
| Maximum descending aortic diameter (mm), mean ± SD | 41.3 ± 4.5 | 37.5 ± 3.0 | <0.001 |
| Level of Aorta | Group | Before TEVAR | Follow-Up |
|---|---|---|---|
| Mean ± SD (mm) | Mean ± SD (mm) | ||
| T10 | Expanded group | 40.1 ± 4.2 | 44.0 ± 4.0 |
| Non-expanded group | 36.3 ± 3.8 | 36.1 ± 4.4 | |
| T12 | Expanded group | 35.8 ± 3.7 | 40.6 ± 5.3 |
| Non-expanded group | 34.4 ± 2.8 | 35.1 ± 3.2 | |
| L2 | Expanded group | 30.0 ± 3.3 | 32.5 ± 2.7 |
| Non-expanded group | 28.8 ± 2.4 | 29.3 ± 2.4 | |
| L4 | Expanded group | 27.5 ± 2.9 | 28.7 ± 3.0 |
| Non-expanded group | 24.2 ± 2.5 | 24.7 ± 2.7 |
| Characteristic | OR | 95%CI | p Value |
|---|---|---|---|
| Hypertension | 0.495 | 0.102–2.395 | 0.382 |
| TL compression | 6.301 | 0.340–116.920 | 0.217 |
| Maximum descending aortic diameter (mm) | 1.385 | 1.100–1.743 | 0.006 |
| Partial thrombosis of FL | 10.989 | 2.295–48.403 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Yang, H.; Li, G.; Xu, X.; Liu, H.; Gu, J.; Li, M.; Gu, W.; Shao, Y.; Ni, B. Preoperative Predictors of Late Aortic Expansion in Acute Type B Aortic Dissection Treated with TEVAR. J. Clin. Med. 2023, 12, 2826. https://doi.org/10.3390/jcm12082826
Dong Z, Yang H, Li G, Xu X, Liu H, Gu J, Li M, Gu W, Shao Y, Ni B. Preoperative Predictors of Late Aortic Expansion in Acute Type B Aortic Dissection Treated with TEVAR. Journal of Clinical Medicine. 2023; 12(8):2826. https://doi.org/10.3390/jcm12082826
Chicago/Turabian StyleDong, Zhiqiang, He Yang, Gang Li, Xinyang Xu, Hong Liu, Jiaxi Gu, Minghui Li, Weidong Gu, Yongfeng Shao, and Buqing Ni. 2023. "Preoperative Predictors of Late Aortic Expansion in Acute Type B Aortic Dissection Treated with TEVAR" Journal of Clinical Medicine 12, no. 8: 2826. https://doi.org/10.3390/jcm12082826
APA StyleDong, Z., Yang, H., Li, G., Xu, X., Liu, H., Gu, J., Li, M., Gu, W., Shao, Y., & Ni, B. (2023). Preoperative Predictors of Late Aortic Expansion in Acute Type B Aortic Dissection Treated with TEVAR. Journal of Clinical Medicine, 12(8), 2826. https://doi.org/10.3390/jcm12082826





