Sex-Related Aspects in Diabetic Kidney Disease—An Update
Abstract
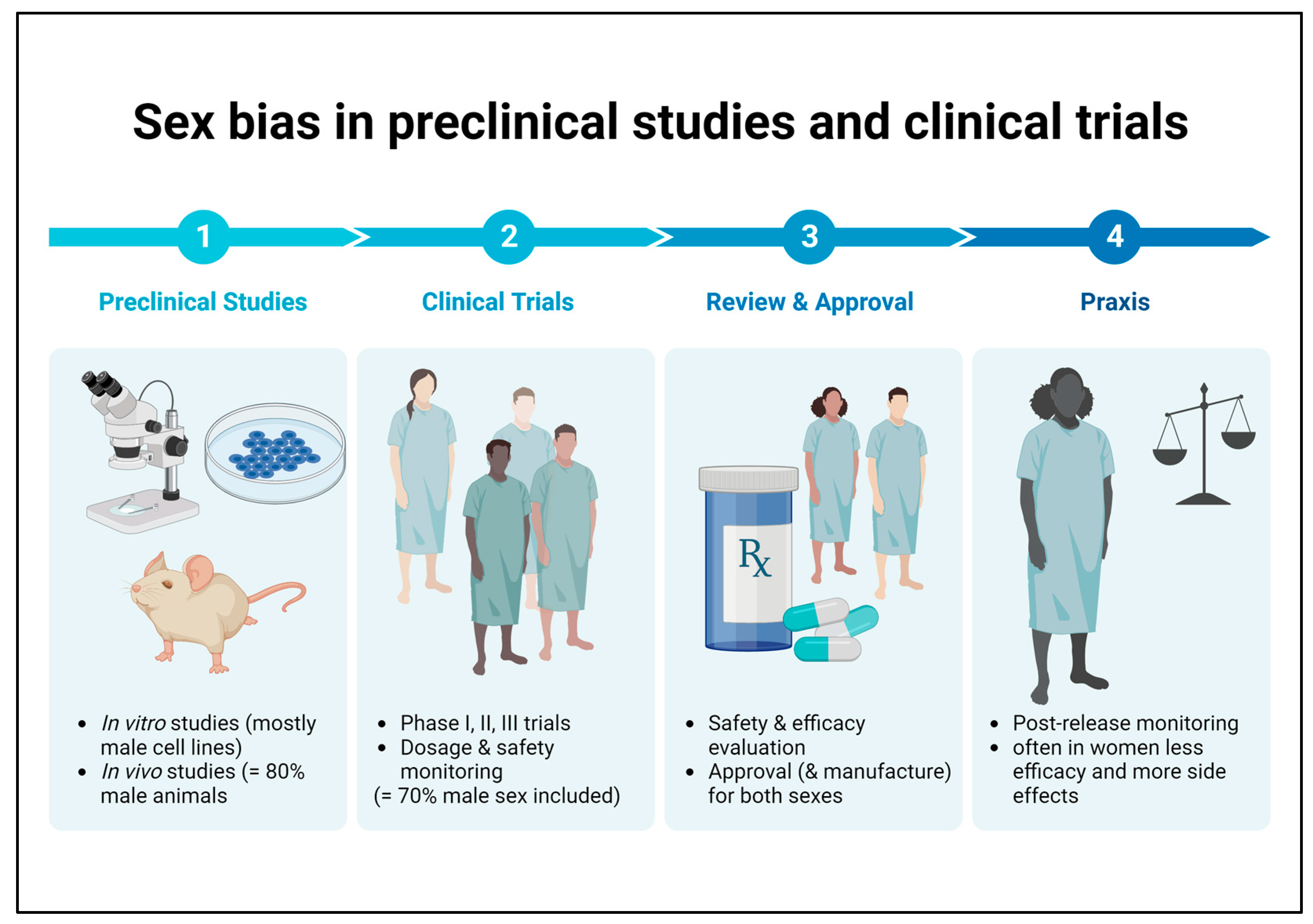
:1. Introduction
2. Sex Differences in Human DKD
2.1. Sex Differences in Development, Progression, and ESKF in DKD
2.2. Factors That May Influence Sex Differences in DKD
2.3. Data on Possible Underlying Mechanisms in Human DKD
2.4. Sex Aspects in Pharmacological Studies for Prevention and Treatment of DKD
2.4.1. Medications with Primary Reno-Protective Action
2.4.2. Antidiabetic Medications with Reno-Protective Effect
3. Findings from Preclinical Research Regarding Underlying Mechanisms
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ORWH. Sex & Gender. Available online: https://orwh.od.nih.gov/sex-gender (accessed on 12 February 2019).
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, K. Diabetic nephropathy: Is it always there? Assumptions, weaknesses and pitfalls in the diagnosis. Hormones 2017, 16, 351–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. Methods Mol. Biol. 2020, 2067, 3–7. [Google Scholar] [CrossRef]
- Qi, C.; Mao, X.; Zhang, Z.; Wu, H. Classification and Differential Diagnosis of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 8637138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafian, B.; Alpers, C.E.; Fogo, A.B. Pathology of human diabetic nephropathy. Contrib. Nephrol. 2011, 170, 36–47. [Google Scholar] [CrossRef]
- Wolf, G.; Ritz, E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J. Am. Soc. Nephrol. 2003, 14, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Iwano, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 279–284. [Google Scholar] [CrossRef]
- Li, R.; Chung, A.C.; Dong, Y.; Yang, W.; Zhong, X.; Lan, H.Y. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int. 2013, 84, 1129–1144. [Google Scholar] [CrossRef] [Green Version]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329. [Google Scholar] [CrossRef]
- Coggins, C.H.; Breyer Lewis, J.; Caggiula, A.W.; Castaldo, L.S.; Klahr, S.; Wang, S.R. Differences between women and men with chronic renal disease. Nephrol. Dial. Transpl. 1998, 13, 1430–1437. [Google Scholar] [CrossRef] [Green Version]
- Silbiger, S.; Neugarten, J. Gender and human chronic renal disease. Gend. Med. 2008, 5 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, B.O.; Ingebretsen, O.C. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006, 69, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafar, T.H.; Schmid, C.H.; Stark, P.C.; Toto, R.; Remuzzi, G.; Ruggenenti, P.; Marcantoni, C.; Becker, G.; Shahinfar, S.; De Jong, P.E.; et al. The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol. Dial. Transpl. 2003, 18, 2047–2053. [Google Scholar] [CrossRef]
- Garovic, V.D.; August, P. Sex Differences and Renal Protection: Keeping in Touch with Your Feminine Side. J. Am. Soc. Nephrol. 2016, 27, 2921–2924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef]
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raile, K.; Galler, A.; Hofer, S.; Herbst, A.; Dunstheimer, D.; Busch, P.; Holl, R.W. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007, 30, 2523–2528. [Google Scholar] [CrossRef] [Green Version]
- Schultz, C.J.; Konopelska-Bahu, T.; Dalton, R.N.; Carroll, T.A.; Stratton, I.; Gale, E.A.; Neil, A.; Dunger, D.B. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Care 1999, 22, 495–502. [Google Scholar] [CrossRef]
- Zhang, L.; Krzentowski, G.; Albert, A.; Lefèbvre, P.J. Factors predictive of nephropathy in DCCT Type 1 diabetic patients with good or poor metabolic control. Diabet. Med. 2003, 20, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Katon, W.; Young, B.A. Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology 2015, 20, 451–458. [Google Scholar] [CrossRef]
- Finne, P.; Reunanen, A.; Stenman, S.; Groop, P.H.; Gronhagen-Riska, C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005, 294, 1782–1787. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Yanai, M.; Takeuchi, K.; Matsuyama, K.; Nitta, K.; Hayashi, K.; Takahashi, S. Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press Res. 2014, 39, 279–288. [Google Scholar] [CrossRef]
- Piani, F.; Melena, I.; Tommerdahl, K.L.; Nokoff, N.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.H.; Cherney, D.Z.; Johnson, R.J.; Nadeau, K.J.; et al. Sex-related differences in diabetic kidney disease: A review on the mechanisms and potential therapeutic implications. J. Diabetes Complicat. 2021, 35, 107841. [Google Scholar] [CrossRef]
- von Bundesärztekammer, E.P.; Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Typ-2-Diabetes—Teilpublikation der Langfassung, 2. Auflage; Version 1. Available online: www.leitlinien.de/diabetes (accessed on 13 September 2021).
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808. [Google Scholar] [CrossRef]
- Looker, H.C.; Krakoff, J.; Funahashi, T.; Matsuzawa, Y.; Tanaka, S.; Nelson, R.G.; Knowler, W.C.; Lindsay, R.S.; Hanson, R.L. Adiponectin concentrations are influenced by renal function and diabetes duration in Pima Indians with type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4010–4017. [Google Scholar] [CrossRef] [Green Version]
- Crook, E.D.; Patel, S.R. Diabetic nephropathy in African-American patients. Curr. Diabetes Rep. 2004, 4, 455–461. [Google Scholar] [CrossRef]
- Orchard, T.J.; Dorman, J.S.; Maser, R.E.; Becker, D.J.; Drash, A.L.; Ellis, D.; LaPorte, R.E.; Kuller, L.H. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990, 39, 1116–1124. [Google Scholar] [CrossRef]
- Maric, C.; Sullivan, S. Estrogens and the diabetic kidney. Gend. Med. 2008, 5 (Suppl. S1), S103–S113. [Google Scholar] [CrossRef] [Green Version]
- Parving, H.H.; Gall, M.A.; Skøtt, P.; Jørgensen, H.E.; Løkkegaard, H.; Jørgensen, F.; Nielsen, B.; Larsen, S. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int. 1992, 41, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Ravid, M.; Brosh, D.; Ravid-Safran, D.; Levy, Z.; Rachmani, R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch. Intern. Med. 1998, 158, 998–1004. [Google Scholar] [CrossRef]
- Nakano, S.; Ogihara, M.; Tamura, C.; Kitazawa, M.; Nishizawa, M.; Kigoshi, T.; Uchida, K. Reversed circadian blood pressure rhythm independently predicts endstage renal failure in non-insulin-dependent diabetes mellitus subjects. J. Diabetes Complicat. 1999, 13, 224–231. [Google Scholar] [CrossRef]
- Lewis, E.J.; Hunsicker, L.G.; Rodby, R.A.; The Collaborative Study Group. A clinical trial in type 2 diabetic nephropathy. Am. J. Kidney Dis. 2001, 38, S191–S194. [Google Scholar] [CrossRef]
- Keane, W.F.; Brenner, B.M.; de Zeeuw, D.; Grunfeld, J.P.; McGill, J.; Mitch, W.E.; Ribeiro, A.B.; Shahinfar, S.; Simpson, R.L.; Snapinn, S.M.; et al. The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney Int. 2003, 63, 1499–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breyer, J.A.; Bain, R.P.; Evans, J.K.; Nahman, N.S., Jr.; Lewis, E.J.; Cooper, M.; McGill, J.; Berl, T. Predictors of the progression of renal insufficiency in patients with insulin-dependent diabetes and overt diabetic nephropathy. The Collaborative Study Group. Kidney Int. 1996, 50, 1651–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossing, P.; Hougaard, P.; Parving, H.H. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: A 10-year prospective observational study. Diabetes Care 2002, 25, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Torffvit, O.; Agardh, C.D. The impact of metabolic and blood pressure control on incidence and progression of nephropathy: A 10-year study of 385 type 2 diabetic patients. J. Diabetes Complicat. 2001, 15, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Laron-Kenet, T.; Shamis, I.; Weitzman, S.; Rosen, S.; Laron, Z.V. Mortality of patients with childhood onset (0–17 years) Type I diabetes in Israel: A population-based study. Diabetologia 2001, 44 (Suppl. S3), B81–B86. [Google Scholar] [CrossRef]
- Dahlquist, G.; Källén, B. Mortality in childhood-onset type 1 diabetes: A population-based study. Diabetes Care 2005, 28, 2384–2387. [Google Scholar] [CrossRef] [Green Version]
- Hackett, G.; Cole, N.; Mulay, A.; Strange, R.C.; Ramachandran, S. Long-term testosterone therapy in type 2 diabetes is associated with reduced mortality without improvement in conventional cardiovascular risk factors. BJU Int. 2019, 123, 519–529. [Google Scholar] [CrossRef]
- Alwani, M.; Al-Zoubi, R.M.; Al-Qudimat, A.; Yassin, A.; Aboumarzouk, O.; Al-Rumaihi, K.; Talib, R.; Al-Ansari, A. The impact of long-term Testosterone Therapy (TTh) in renal function (RF) among hypogonadal men: An observational cohort study. Ann. Med. Surg. 2021, 69, 102748. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Holl, R.W.; Grabert, M.; Thon, A.; Heinze, E. Urinary excretion of albumin in adolescents with type 1 diabetes: Persistent versus intermittent microalbuminuria and relationship to duration of diabetes, sex, and metabolic control. Diabetes Care 1999, 22, 1555–1560. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Handisurya, A. Metabolic diseases and associated complications: Sex and gender matter! Eur. J. Clin. Investig. 2009, 39, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Maric, C. Sex, diabetes and the kidney. Am. J. Physiol. Ren. Physiol. 2009, 296, F680–F688. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.N. The influence of sex and puberty on the progression of diabetic nephropathy and retinopathy. Diabetologia 2011, 54, 1943–1945. [Google Scholar] [CrossRef] [Green Version]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [Green Version]
- Wannamethee, S.G.; Papacosta, O.; Lawlor, D.A.; Whincup, P.H.; Lowe, G.D.; Ebrahim, S.; Sattar, N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012, 55, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.K.; Owusu Adjah, E.S.; Samanta, M.; Patel, K.; Bellary, S.; Hanif, W.; Khunti, K. Comparison of body mass index at diagnosis of diabetes in a multi-ethnic population: A case-control study with matched non-diabetic controls. Diabetes Obes. Metab. 2017, 19, 1014–1023. [Google Scholar] [CrossRef] [Green Version]
- Olivarius, N.d.F.; Vestbo, E.; Andreasen, A.H.; Mogensen, C.E. Renal involvement is related to body height in newly diagnosed diabetic women aged 40 years or over. Diabetes Metab. 2001, 27, 14–18. [Google Scholar]
- Livingstone, C.; Collison, M. Sex steroids and insulin resistance. Clin. Sci. 2002, 102, 151–166. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibley, S.D.; Thomas, W.; de Boer, I.; Brunzell, J.D.; Steffes, M.W. Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: Role of central obesity. Am. J. Kidney Dis. 2006, 47, 223–232. [Google Scholar] [CrossRef]
- Shepard, B.D. Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am. J. Physiol. Ren. Physiol. 2019, 317, F456–F462. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Schultz, C.; Ong, K.; Frystyk, J.; Dalton, R.N.; Perry, L.; Ørskov, H.; Dunger, D.B. Low IGF-I and elevated testosterone during puberty in subjects with type 1 diabetes developing microalbuminuria in comparison to normoalbuminuric control subjects: The Oxford Regional Prospective Study. Diabetes Care 2003, 26, 1456–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maric, C.; Forsblom, C.; Thorn, L.; Wadén, J.; Groop, P.H. Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids 2010, 75, 772–778. [Google Scholar] [CrossRef] [Green Version]
- Harjutsalo, V.; Maric-Bilkan, C.; Forsblom, C.; Groop, P.H.; FinnDiane Study Group. Age at menarche and the risk of diabetic microvascular complications in patients with type 1 diabetes. Diabetologia 2016, 59, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Wells, C.C.; Garman, J.H.; Asico, L.; Escano, C.S.; Maric, C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension 2008, 51, 1218–1224. [Google Scholar] [CrossRef]
- Stamataki, K.E.; Spina, J.; Rangou, D.B.; Chlouverakis, C.S.; Piaditis, G.P. Ovarian function in women with non-insulin dependent diabetes mellitus. Clin. Endocrinol. 1996, 45, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Isono, M.; Isshiki, K.; Araki, S.; Sugimoto, T.; Guo, B.; Sato, H.; Haneda, M.; Kashiwagi, A.; Koya, D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am. J. Pathol. 2005, 166, 1629–1636. [Google Scholar] [CrossRef] [Green Version]
- Lamon-Fava, S.; Barnett, J.B.; Woods, M.N.; McCormack, C.; McNamara, J.R.; Schaefer, E.J.; Longcope, C.; Rosner, B.; Gorbach, S.L. Differences in serum sex hormone and plasma lipid levels in Caucasian and African-American premenopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 4516–4520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Osada, S.; Matsushita, M.; Abe, K.; Kogo, H. Changes in ovarian steroidogenesis in insulin-resistant, type 2 diabetic Goto-Kakizaki rats after thyroidectomy and gonadotropin treatment. Eur. J. Pharmacol. 2005, 513, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.C.; Riazi, S.; Mankhey, R.W.; Bhatti, F.; Ecelbarger, C.; Maric, C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend. Med. 2005, 2, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.N.; Stankovic, M.; Cushman, T.T.; Goldstein, I.; Munarriz, R.; Traish, A.M. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salonia, A.; Lanzi, R.; Scavini, M.; Pontillo, M.; Gatti, E.; Petrella, G.; Licata, G.; Nappi, R.E.; Bosi, E.; Briganti, A.; et al. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care 2006, 29, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Devish, K.; Langer, W.J.; Carmines, P.K.; Lane, P.H. Testosterone treatment promotes tubular damage in experimental diabetes in prepubertal rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F1681–F1690. [Google Scholar] [CrossRef]
- Prabhu, A.; Xu, Q.; Manigrasso, M.B.; Biswas, M.; Flynn, E.; Iliescu, R.; Lephart, E.D.; Maric, C. Expression of aromatase, androgen and estrogen receptors in peripheral target tissues in diabetes. Steroids 2010, 75, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, M.E.; Mizuno, N.K.; Bonorden, M.J.; Ray, A.; Sokolchik, I.; Narasimhan, M.L.; Cleary, M.P. Role of the adiponectin leptin ratio in prostate cancer. Oncol. Res. 2009, 18, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Panagiotopolous, S.; Sharpe, K.; MacIsaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G.; Thomas, M.C. Low testosterone and anaemia in men with type 2 diabetes. Clin. Endocrinol. 2009, 70, 547–553. [Google Scholar] [CrossRef]
- Grossmann, M.; Thomas, M.C.; Panagiotopoulos, S.; Sharpe, K.; Macisaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1834–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maric-Bilkan, C.; Galis, Z.S. Trends in NHLBI-Funded Research on Sex Differences in Hypertension. Circ. Res. 2016, 119, 591–595. [Google Scholar] [CrossRef]
- Maric-Bilkan, C.; Arnold, A.P.; Taylor, D.A.; Dwinell, M.; Howlett, S.E.; Wenger, N.; Reckelhoff, J.F.; Sandberg, K.; Churchill, G.; Levin, E.; et al. Report of the National Heart, Lung, and Blood Institute Working Group on Sex Differences Research in Cardiovascular Disease: Scientific Questions and Challenges. Hypertension 2016, 67, 802–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, B.; Mårin, P.; Lissner, L.; Vermeulen, A.; Björntorp, P. Testosterone concentrations in women and men with NIDDM. Diabetes Care 1994, 17, 405–411. [Google Scholar] [CrossRef]
- Stellato, R.K.; Feldman, H.A.; Hamdy, O.; Horton, E.S.; McKinlay, J.B. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: Prospective results from the Massachusetts male aging study. Diabetes Care 2000, 23, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Tsai, E.C.; Matsumoto, A.M.; Fujimoto, W.Y.; Boyko, E.J. Association of bioavailable, free, and total testosterone with insulin resistance: Influence of sex hormone-binding globulin and body fat. Diabetes Care 2004, 27, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clotet, S.; Riera, M.; Pascual, J.; Soler, M.J. RAS and sex differences in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F945–F957. [Google Scholar] [CrossRef] [Green Version]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef]
- Loeffler, I.; Wolf, G. Transforming growth factor-beta and the progression of renal disease. Nephrol. Dial. Transpl. 2014, 29 (Suppl. S1), i37–i45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond-Stanic, M.K.; You, Y.H.; Sharma, K. Sugar, sex, and TGF-β in diabetic nephropathy. Semin. Nephrol. 2012, 32, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, I.; Hanyu, A.; Wayama, M.; Goto, N.; Katsuno, Y.; Kawasaki, S.; Nakajima, Y.; Kajiro, M.; Komatsu, Y.; Fujimura, A.; et al. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J. Biol. Chem. 2010, 285, 14747–14755. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Ruan, M.; Clifton, K.; Syed, F.; Khosla, S.; Oursler, M.J. TGF-β mediates suppression of adipogenesis by estradiol through connective tissue growth factor induction. Endocrinology 2012, 153, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Lane, P.H.; Snelling, D.M.; Babushkina-Patz, N.; Langer, W.J. Sex differences in the renal transforming growth factor-beta 1 system after puberty. Pediatr. Nephrol. 2001, 16, 61–68. [Google Scholar] [CrossRef]
- Lee, S.K. Sex as an important biological variable in biomedical research. BMB Rep. 2018, 51, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Sarafidis, P.A.; Memmos, E.; Alexandrou, M.E.; Papagianni, A. Mineralocorticoid Receptor Antagonists for Nephroprotection: Current Evidence and Future Perspectives. Curr. Pharm. Des. 2018, 24, 5528–5536. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Filippatos, G.; Nowack, C.; Kolkhof, P.; Joseph, A.; Mentenich, N.; Pitt, B.; et al. Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial. Am. J. Nephrol. 2019, 50, 345–356. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Fassi, A.; Ilieva, A.P.; Bruno, S.; Iliev, I.P.; Brusegan, V.; Rubis, N.; Gherardi, G.; Arnoldi, F.; Ganeva, M.; et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2004, 351, 1941–1951. [Google Scholar] [CrossRef] [Green Version]
- Mauer, M.; Zinman, B.; Gardiner, R.; Suissa, S.; Sinaiko, A.; Strand, T.; Drummond, K.; Donnelly, S.; Goodyer, P.; Gubler, M.C.; et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 2009, 361, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Ito, S.; Izzo, J.L., Jr.; Januszewicz, A.; Katayama, S.; Menne, J.; Mimran, A.; Rabelink, T.J.; Ritz, E.; Ruilope, L.M.; et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2011, 364, 907–917. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Parving, H.H.; Andress, D.L.; Bakris, G.; Correa-Rotter, R.; Hou, F.F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947. [Google Scholar] [CrossRef]
- Rossing, P.; Persson, F.; Frimodt-Møller, M. Prognosis and treatment of diabetic nephropathy: Recent advances and perspectives. Nephrol. Ther. 2018, 14 (Suppl. S1), S31–S37. [Google Scholar] [CrossRef]
- Giugliano, D.; De Nicola, L.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Type 2 diabetes and the kidney: Insights from cardiovascular outcome trials. Diabetes Obes. Metab. 2019, 21, 1790–1800. [Google Scholar] [CrossRef]
- Marso, S.P.; Poulter, N.R.; Nissen, S.E.; Nauck, M.A.; Zinman, B.; Daniels, G.H.; Pocock, S.; Steinberg, W.M.; Bergenstal, R.M.; Mann, J.F.; et al. Design of the liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (LEADER) trial. Am. Heart J. 2013, 166, 823–830.e825. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Desai, M.; Jardine, M.; Balis, D.; Meininger, G.; Perkovic, V. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J. Am. Soc. Nephrol. 2017, 28, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Takashima, H.; Yoshida, Y.; Nagura, C.; Furukawa, T.; Tei, R.; Maruyama, T.; Maruyama, N.; Abe, M. Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: A randomized open-label prospective trial. Diabetes Vasc. Dis. Res. 2018, 15, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Davidson, J.A. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: Overview of current evidence. Postgrad. Med. 2019, 131, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Singh, R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: A systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab. Syndr. 2020, 14, 181–187. [Google Scholar] [CrossRef]
- Sabolic, I.; Vrhovac, I.; Eror, D.B.; Gerasimova, M.; Rose, M.; Breljak, D.; Ljubojevic, M.; Brzica, H.; Sebastiani, A.; Thal, S.C.; et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Cell Physiol. 2012, 302, C1174–C1188. [Google Scholar] [CrossRef] [Green Version]
- Brosius, F.C., 3rd; Alpers, C.E.; Bottinger, E.P.; Breyer, M.D.; Coffman, T.M.; Gurley, S.B.; Harris, R.C.; Kakoki, M.; Kretzler, M.; Leiter, E.H.; et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2503–2512. [Google Scholar] [CrossRef] [Green Version]
- Phillips, A.; Janssen, U.; Floege, J. Progression of diabetic nephropathy. Insights from cell culture studies and animal models. Kidney Blood Press Res. 1999, 22, 81–97. [Google Scholar] [CrossRef]
- Loeffler, I. Pathophysiology of diabetic nephropathy. In Diabetes and Kidney Disease; Wolf, G., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. S45–S61. [Google Scholar]
- Alpers, C.E.; Hudkins, K.L. Mouse models of diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2011, 20, 278–284. [Google Scholar] [CrossRef]
- Noshahr, Z.S.; Salmani, H.; Khajavi Rad, A.; Sahebkar, A. Animal Models of Diabetes-Associated Renal Injury. J. Diabetes Res. 2020, 2020, 9416419. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Koya, D. Rodent models of diabetic nephropathy: Their utility and limitations. Int. J. Nephrol. Renov. Dis. 2016, 9, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziller, N.; Kotolloshi, R.; Esmaeili, M.; Liebisch, M.; Mrowka, R.; Baniahmad, A.; Liehr, T.; Wolf, G.; Loeffler, I. Sex Differences in Diabetes- and TGF-β1-Induced Renal Damage. Cells 2020, 9, 2236. [Google Scholar] [CrossRef]
- Lane, P.H. Diabetic kidney disease: Impact of puberty. Am. J. Physiol. Ren. Physiol. 2002, 283, F589–F600. [Google Scholar] [CrossRef]
- Shah, T.A.; Rogers, M.B. Unanswered Questions Regarding Sex and BMP/TGF-beta Signaling. J. Dev. Biol. 2018, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Lane, P.H.; Snelling, D.M.; Langer, W.J. Streptozocin diabetes elevates all isoforms of TGF-beta in the rat kidney. Int. J. Exp. Diabetes Res. 2001, 2, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Prabhu, A.; Xu, S.; Manigrasso, M.B.; Maric, C. Dose-dependent effects of dihydrotestosterone in the streptozotocin-induced diabetic rat kidney. Am. J. Physiol. Ren. Physiol. 2009, 297, F307–F315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambineri, A.; Pelusi, C. Sex hormones, obesity and type 2 diabetes: Is there a link? Endocr. Connect. 2019, 8, R1–R9. [Google Scholar] [CrossRef]
- Cheung, K.K.; Luk, A.O.; So, W.Y.; Ma, R.C.; Kong, A.P.; Chow, F.C.; Chan, J.C. Testosterone level in men with type 2 diabetes mellitus and related metabolic effects: A review of current evidence. J. Diabetes Investig. 2015, 6, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.B.; Sawyer, R.T.; Hutchens, Z.M., Jr.; Flynn, E.R.; Maric-Bilkan, C. Combined inhibition of aromatase activity and dihydrotestosterone supplementation attenuates renal injury in male streptozotocin (STZ)-induced diabetic rats. Am. J. Physiol. Ren. Physiol. 2012, 302, F1203–F1209. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Ramesh, S. Sex hormones in women with kidney disease. Nephrol. Dial. Transplant. 2016, 31, 1787–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mankhey, R.W.; Bhatti, F.; Maric, C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2005, 288, F399–F405. [Google Scholar] [CrossRef] [Green Version]

| Type of Diabetes (Studies’ Number) | Albuminuria | Low eGFR | ESKF | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| T1DM (21) | 64.7% | 35.3% | 50% | 50% | 85.7% | 14.3% |
| T2DM (15) | 71.4% | 28.6% | 38.5% | 61.5% | 42.9% | 57.1% |
| T1DM/T2DM (14) | 80% | 20% | 44.5% | 55.5% | 20% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeffler, I.; Ziller, N. Sex-Related Aspects in Diabetic Kidney Disease—An Update. J. Clin. Med. 2023, 12, 2834. https://doi.org/10.3390/jcm12082834
Loeffler I, Ziller N. Sex-Related Aspects in Diabetic Kidney Disease—An Update. Journal of Clinical Medicine. 2023; 12(8):2834. https://doi.org/10.3390/jcm12082834
Chicago/Turabian StyleLoeffler, Ivonne, and Nadja Ziller. 2023. "Sex-Related Aspects in Diabetic Kidney Disease—An Update" Journal of Clinical Medicine 12, no. 8: 2834. https://doi.org/10.3390/jcm12082834
APA StyleLoeffler, I., & Ziller, N. (2023). Sex-Related Aspects in Diabetic Kidney Disease—An Update. Journal of Clinical Medicine, 12(8), 2834. https://doi.org/10.3390/jcm12082834






