Microcirculatory and Rheological Adaptive Mechanisms at High Altitude in European Lowlander Hikers and Nepalese Highlanders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
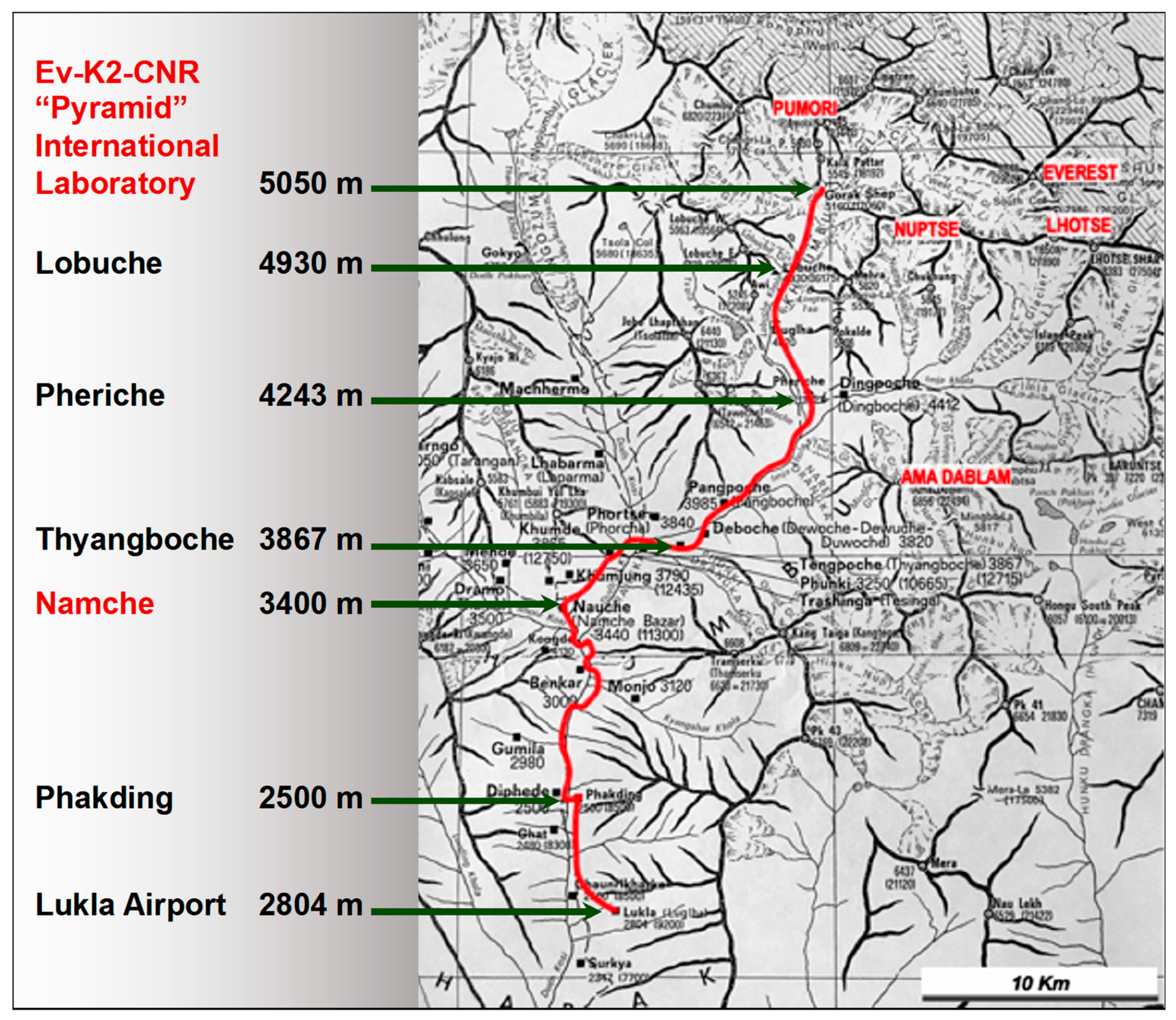
2.2. Protocol of the Study
- At 1350 m a.s.l., on the second day of permanence in Kathmandu;
- At 3400 m a.s.l., on the second day of permanence in Namche Bazar;
- At 5050 m. a.s.l., on the second day of permanence in Lobuche;
- And the 8th day of stay in this high-altitude laboratory.
2.3. Complete Blood Count
2.4. Blood Viscosity
2.5. Blood Filterability
2.6. In Vivo Microcirculatory Study
- Morphology of the capillary loops: normally the capillary loops have a “hairpin” appearance, the afferent branch being thinner than the efferent branch, the latter being slightly sinuous. The presence of capillaries characterised by repeated curves and twists (kinking) of ectasias and abnormal dilations is reported.
- Presence of pericapillary oedema: normally the capillary loops are well defined, and the blood flow is evident and easy to analyse. In the presence of pericapillary oedema, the definition of the capillary loops is reduced. A soft-focus effect (“flou effect”) is therefore observed on biomicroscopy, in which the contours of the capillary loops are less defined. This soft-focus effect can also be heavy, and in this case the loops appear poorly defined. Severe degrees of oedema do not allow visualisation of the capillary loops.
- Blood flow assessment: normally on biomicroscopy, the flow appears continuous, with regular and periodic variations in the flow velocity depending on the vasomotor activity, in particular by the slow wave of vasomotion [7]. Functional alterations of flow can be documented by the presence of interruption of the flow, sometimes followed by the inversion of the flow. In the presence of erythrocyte aggregates, the flow is discontinuous, with the progression of red blood cells in packets.
2.7. Oxygen Saturation
2.8. Haemodynamic Parameters
2.9. Statistical Analysis
3. Results
4. Discussion
4.1. Haemorheological Changes with Altitude
4.2. Microcircolatory Changes with Altitude
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grocott, M.; Montgomery, H.; Vercueil, A. High-altitude physiology and pathophysiology: Implications and relevance for intensive care medicine. Crit. Care 2007, 11, 203. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Rennie, D.; Levine, H.D. The incidence, importance, and prophylaxis of acute mountain sickness. Lancet 1976, 2, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Rennie, D. Avoiding mountain sickness. Lancet 1978, 2, 938. [Google Scholar] [CrossRef]
- Selland, M.A.; Stelzner, T.J.; Stevens, T.; Mazzeo, R.S.; McCullough, R.E.; Reeves, J.T. Pulmonary function and hypoxic ventilatory response in subjects susceptible to high-altitude pulmonary edema. Chest 1993, 103, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef]
- Reid, H.L.; Barnes, A.J.; Lock, P.J.; Dormandy, J.A.; Dormandy, T.L. A simple method for measuring erythrocyte deformability. J. Clin. Pathol. 1976, 29, 855–858. [Google Scholar] [CrossRef]
- Salvi, P.; Faini, A.; Castiglioni, P.; Brunacci, F.; Montaguti, L.; Severi, F.; Gautier, S.; Pretolani, E.; Benetos, A.; Parati, G. Increase in slow-wave vasomotion by hypoxia and ischemia in lowlanders and highlanders. J. Appl. Physiol. 2018, 125, 780–789. [Google Scholar] [CrossRef]
- Skalak, R.; Hanss, M.; Chien, S. Indices of filterability of red blood cell suspensions. Biorheology 1983, 20, 311–316. [Google Scholar] [CrossRef]
- Weed, R.I. The importance of erythrocyte deformability. Am. J. Med. 1970, 49, 147–150. [Google Scholar] [CrossRef]
- Wells, R. Syndromes of hyperviscosity. N. Engl. J. Med. 1970, 283, 183–186. [Google Scholar] [CrossRef]
- Weed, R.I.; LaCelle, P.L.; Merrill, E.W. Metabolic dependence of red cell deformability. J. Clin. Investig. 1969, 48, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Pretolani, E.; Zoli, I.; Battistini, G.; Salvi, P. Blood filterability in ischemic heart disease. Boll. Soc. Ital. Biol. Sper. 1983, 59, 1819–1824. [Google Scholar] [PubMed]
- Pretolani, E.; Zoli, I.; Battistini, G.; Salvi, P. Blood filterability in cerebral vascular disease. Boll. Soc. Ital. Biol. Sper. 1983, 59, 1807–1811. [Google Scholar] [PubMed]
- Docci, D.; del Vecchio, C.; Salvi, P.; Turci, F.; Salvi, G.; Cenciotti, L.; Pretolani, E. Osmotic fragility of erythrocytes, cell deformability and secondary hyperparathyroidism in uremic patients on maintenance hemodialysis. Clin. Nephrol. 1985, 23, 68–73. [Google Scholar]
- Mao, T.Y.; Fu, L.L.; Wang, J.S. Hypoxic exercise training causes erythrocyte senescence and rheological dysfunction by depressed Gardos channel activity. J. Appl. Physiol. 2011, 111, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Bor-Kucukatay, M.; Colak, R.; Erken, G.; Kilic-Toprak, E.; Kucukatay, V. Altitude training induced alterations in erythrocyte rheological properties: A controlled comparison study in rats. Clin. Hemorheol. Microcirc. 2014, 58, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Takeoka, M.; Sakai, A.; Kobayashi, T. Microcirculation and high altitude edema. Int. J. Sports Med. 1992, 13 (Suppl. S1), S52–S54. [Google Scholar] [CrossRef]
- Ernst, E.; Altman, C.; Saradeth, T. Hemorheological changes following exposure to moderately high altitude. Clin. Hemorheol. Microcirc. 1991, 11, 303–307. [Google Scholar] [CrossRef]
- Stauffer, E.; Loyrion, E.; Hancco, I.; Waltz, X.; Ulliel-Roche, M.; Oberholzer, L.; Robach, P.; Pichon, A.; Brugniaux, J.V.; Bouzat, P.; et al. Blood viscosity and its determinants in the highest city in the world. J. Physiol. 2020, 598, 4121–4130. [Google Scholar] [CrossRef]
- Tremblay, J.C.; Hoiland, R.L.; Carter, H.H.; Howe, C.A.; Stembridge, M.; Willie, C.K.; Gasho, C.; MacLeod, D.B.; Pyke, K.E.; Ainslie, P.N. UBC-Nepal expedition: Upper and lower limb conduit artery shear stress and flow-mediated dilation on ascent to 5,050 m in lowlanders and Sherpa. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1532–H1543. [Google Scholar] [CrossRef]
- Davies, T.; Gilbert-Kawai, E.; Wythe, S.; Meale, P.; Mythen, M.; Levett, D.; Mitchell, K.; Grocott, M.; Clough, G.; Martin, D.; et al. Sustained vasomotor control of skin microcirculation in Sherpas versus altitude-naive lowlanders: Experimental evidence from Xtreme Everest 2. Exp. Physiol. 2018, 103, 1494–1504. [Google Scholar] [CrossRef]
- Carey, D.; Thanaj, M.; Davies, T.; Gilbert-Kawai, E.; Mitchell, K.; Levett, D.Z.H.; Mythen, M.G.; Martin, D.S.; Grocott, M.P.; Chipperfield, A.J.; et al. Enhanced flow-motion complexity of skin microvascular perfusion in Sherpas and lowlanders during ascent to high altitude. Sci. Rep. 2019, 9, 14391. [Google Scholar] [CrossRef]
- Hilty, M.P.; Merz, T.M.; Hefti, U.; Ince, C.; Maggiorini, M.; Pichler Hefti, J. Recruitment of non-perfused sublingual capillaries increases microcirculatory oxygen extraction capacity throughout ascent to 7126 m. J. Physiol. 2019, 597, 2623–2638. [Google Scholar] [CrossRef]
- Capaldo, G.; Ince, C.; Hilty, M. The clinical relevance of high-altitude microcirculation studies: The example of COVID-19. In Annual Update in Intensive Care and Emergency Medicine 2021; Vincent, J.L., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 103–110. [Google Scholar]
- Martin, D.S.; Ince, C.; Goedhart, P.; Levett, D.Z.; Grocott, M.P.; Caudwell Xtreme Everest Research, G. Abnormal blood flow in the sublingual microcirculation at high altitude. Eur. J. Appl. Physiol. 2009, 106, 473–478. [Google Scholar] [CrossRef]
- Martin, D.S.; Goedhart, P.; Vercueil, A.; Ince, C.; Levett, D.Z.; Grocott, M.P.; Caudwell Xtreme Everest Research, G. Changes in sublingual microcirculatory flow index and vessel density on ascent to altitude. Exp. Physiol. 2010, 95, 880–891. [Google Scholar] [CrossRef]
- Gilbert-Kawai, E.; Coppel, J.; Court, J.; van der Kaaij, J.; Vercueil, A.; Feelisch, M.; Levett, D.; Mythen, M.; Grocott, M.P.; Martin, D.; et al. Sublingual microcirculatory blood flow and vessel density in Sherpas at high altitude. J. Appl. Physiol. 2017, 122, 1011–1018. [Google Scholar] [CrossRef]
- West, J.B.; Mathieu-Costello, O. High altitude pulmonary edema is caused by stress failure of pulmonary capillaries. Int. J. Sports Med. 1992, 13 (Suppl. S1), S54–S58. [Google Scholar] [CrossRef] [PubMed]
- Arias-Stella, J.; Saldana, M. The Terminal Portion of the Pulmonary Arterial Tree in People Native to High Altitudes. Circulation 1963, 28, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Penaloza, D.; Arias-Stella, J.; Sime, F.; Recavarren, S.; Marticorena, E. The Heart and Pulmonary Circulation in Children at High Altitudes: Physiological, Anatomical, and Clinical Observations. Pediatrics 1964, 34, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.; Banchero, N.; Penaloza, D.; Gamboa, R.; Cruz, J.; Marticorena, E. Pulmonary hypertension in children born and living at high altitudes. Am. J. Cardiol. 1963, 11, 143–149. [Google Scholar] [CrossRef]
- Penaloza, D.; Sime, F. Circulatory dynamics during high altitude pulmonary edema. Am. J. Cardiol. 1969, 23, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.; Heath, D.; Gosney, J.; Williams, D. Altitude-related deaths in seven trekkers in the Himalayas. Thorax 1983, 38, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Nayak, N.C.; Roy, S.; Narayanan, T.K. Pathologic Features of Altitude Sickness. Am. J. Pathol. 1964, 45, 381–391. [Google Scholar] [PubMed]

| Parameter | Lowlanders | Highlanders | p-Value |
|---|---|---|---|
| Sex, m/f | 6/2 | 11/0 | |
| Age, years | 34.6 ± 3.5 | 27.8 ± 6.6 | 0.017 |
| Height, cm | 173.4 ± 6.3 | 160.8 ± 7.7 | 0.002 |
| Weight, Kg | 76.8 ± 7.6 | 52.7 ± 5.6 | <0.001 |
| BMI, Kg/m2 | 25.5 ± 1.8 | 20.4 ± 1.8 | <0.001 |
| BSA, m2 | 1.91 ± 0.12 | 1.54 ± 0.11 | <0.001 |
| Parameters | Ethnicity | Altitude, m a.s.l. | Trend | |||
|---|---|---|---|---|---|---|
| 1350 | 3400 | 5050 | 5050 (8th Day) | p-Value | ||
| SaO2, % | Lowlanders | 95.9 (95.4–97.0) | 92.6 (89.0–94.5) * | 80.5 (77.0–84.0) * | 83.5 (77.8–89.5) * | <0.001 |
| Highlanders | 94.0 (92.4–95.5) | 85.0 (83.0–91.0) *† | ||||
| White Blood Cells, | Lowlanders | 6.77 (6.29–7.11) | 6.15 (5.77–6.39) * | 6.32 (5.97–6.89) | 7.30 (6.60–7.52) | 0.391 |
| ×103/μL | Highlanders | 10.22 (8.66–11.64) † | 9.40 (7.11–10.00) † | |||
| Platelets, ×103/μL | Lowlanders | 258 (236–289) | 239 (217–255) * | 318 (290–358) * | 327 (297–386) * | <0.001 |
| Highlanders | 345 (283–365) † | 382 (338–451) † | ||||
| Plateletcrit, % | Lowlanders | 0.21 (0.18–0.24) | 0.20 (0.18–0.22) | 0.25 (0.25–0.27) * | 0.26 (0.25–0.29) * | <0.001 |
| Highlanders | 0.27 (0.21–0.29) † | 0.28 (0.27–0.31) | ||||
| Red Blood Cells, ×106/μL | Lowlanders | 4.82 (4.65–5.12) | 4.48 (4.32–4.56) * | 5.11 (4.71–5.27) | 5.19 (4.90–5.38) * | 0.040 |
| Highlanders | 5.36 (5.01–5.55) † | 5.15 (4.89–5.50) | ||||
| Haemoglobin, g/dL | Lowlanders | 14.6 (13.8–15.0) | 14.1 (13.6–14.4) | 15.5 (14.6–16.6) * | 16.3 (15.3–16.9) * | 0.002 |
| Highlanders | 17.2 (16.2–17.6) † | 16.3 (15.8–16.8) | ||||
| Haematocrit, % | Lowlanders | 43.7 (41.9–44.4) | 39.5 (38.4–40.9) * | 45.3 (42.8–48.0) | 46.6 (43.4–48.5) * | 0.055 |
| Highlanders | 48.0 (46.0–50.1) † | 47.8 (45.0–48.7) | ||||
| MCV, fl | Lowlanders | 90.1 (86.8–92.3) | 89.2 (85.6–91.6) | 89.5 (87.2–91.6) | 88.8 (87.4–91.3) | 0.650 |
| Highlanders | 89.9 (88.5–93.6) | 89.0 (88.4–94.2) | ||||
| MCH, pg | Lowlanders | 30.0 (28.4–31.1) | 31.6 (30.6–32.4) * | 30.6 (30.4–31.5) | 31.2 (30.2–32.2) * | 0.139 |
| Highlanders | 32.0 (31.2–33.3) | 30.6 (30.1–32.7) * | ||||
| MCHC, g/dL | Lowlanders | 32.9 (32.5–33.0) | 35.3 (35.1–36.3) * | 34.4 (33.9–34.8) * | 34.9 (34.6–35.3) * | 0.034 |
| Highlanders | 35.4 (34.9–35.8) | 34.4 (33.9–34.8) * | ||||
| RDW, % | Lowlanders | 13.0 (12.8–13.6) | 13.0 (12.6–13.8) | 13.6 (13.3–14.3) * | 14.2 (13.6–14.7) * | 0.002 |
| Highlanders | 13.4 (12.8–13.6) | 13.5 (13.0–13.6) | ||||
| Blood Filtration Time, | Lowlanders | 22.0 (20.6–25.2) | 23.7 (21.1–28.6) | 37.2 (35.1–44.5) * | 36.5 (33.5–45.5) * | <0.001 |
| s/mL | Highlanders | 39.0 (37.9–62.9) † | 41.0 (34.0–56.7) | |||
| Blood Filterability, VRBC | Lowlanders | 113.7 (102.5–128.3) | 100.0 (88.4–108.5) | 71.5 (61.2–81.8) * | 75.5 (62.2–84.6) * | <0.001 |
| Highlanders | 72.8 (43.9–77.2) † | 67.7 (41.2–87.3) | ||||
| Blood Viscosity SR 450, | Lowlanders | 3.87 (3.59–4.07) | 3.24 (3.15–3.42) * | 3.70 (3.64–4.03) | 4.33 (4.08–4.67) * | 0.008 |
| mPa·s | Highlanders | 4.08 (3.74–4.45) † | 4.29 (3.94–4.81) *† | |||
| Blood Viscosity SR 225, | Lowlanders | 4.50 (3.96–4.60) | 4.12 (3.72–4.35) | 4.74 (4.54–4.96) * | 5.22 (4.93–5.45) * | <0.001 |
| mPa·s | Highlanders | 4.46 (4.29–4.60) † | 5.13 (4.70–5.32) *† | |||
| Blood Viscosity SR 90, | Lowlanders | 4.56 (4.47–4.76) | 3.68 (3.43–3.95) * | 4.72 (4.42–5.02) | 5.28 (5.01–5.74) * | 0.003 |
| mPa∙s | Highlanders | 4.83 (4.37–5.18) † | 5.02 (4.87–5.68) *† | |||
| Blood Viscosity SR 45, | Lowlanders | 4.19 (3.64–4.34) | 3.18 (2.24–4.39) | 7.92 (7.39–8.72) * | 7.81 (5.35–5.96) * | <0.001 |
| mPa∙s | Highlanders | 5.42 (5.22–6.49) † | 7.43 (6.97–8.15) * | |||
| Blood Viscosity SR 22, | Lowlanders | 5.69 (5.36–5.96) | 4.32 (3.03–4.46) * | 6.12 (5.51–6.19) | 6.49 (6.30–6.75) * | 0.001 |
| mPa∙s | Highlanders | 4.53 (4.03–4.76) | 6.14 (5.66–6.39) * | |||
| Plasma Viscosity, mPa∙s | Lowlanders | 1.49 (1.32–1.60) | 1.50 (1.34–1.62) | |||
| Parameters | Ethnicity | Altitude, m a.s.l. | |||
|---|---|---|---|---|---|
| 1350 | 3400 | 5050 | 5050 (8th Day) | ||
| Heart Rate, b.p.m. | Lowlanders | 67.2 (59.8–74.9) | 71.2 (54.8–86.7) | 78.3 (74.6–82.4) | 76.5 (63.8–83.7) |
| Highlanders | 62.7 (57.0–69.7) | 64.3 (60.7–79.0) | |||
| Systolic Blood Pressure, | Lowlanders | 112.7 (109.1–116.4) | 118.5 (112.2–120.9) | 119.2 (114.5–122.2) | 121.0 (111.5–133.0) |
| mmHg mmHg × 103/μL | Highlanders | 115.0 (109.7–118.7) | 118.3 (102.7–121.3) | ||
| Diastolic Blood Pressure, mmHg | Lowlanders | 66.7 (62.6–71.3) | 68.0 (64.7–77.1) | 70.5 (66.2–75.6) | 70.5 (63.1–80.2) |
| Highlanders | 70.0 (66.7–76.0) | 72.3 (70.7–78.7) | |||
| European Lowlanders | ||||||||
| N. | Oedema * | Flow and Morphology | ||||||
| 1350 m | 3400 m | 5050 m | 1350 m a.s.l. | 3400 m a.s.l. | 5050 m a.s.l. | |||
| 01 | 0 | ++ | ++ | continuous flow | continuous flow | tortuous loops | ||
| 02 | 0 | + | + | continuous flow | very slow flow | very slow flow, with stops | ||
| 03 | 0/+ | + | ++ | slightly reduced flow | continuous flow | tortuous loops | ||
| 04 | 0 | +++ | +++ | continuous flow | not evaluable (oedema) | not evaluable (oedema) | ||
| 05 | 0/+ | +/++ | +/++ | slightly reduced flow | continuous flow | slow intermittent flow | ||
| 06 | 0 | 0/+ | + | continuous flow | slow flow | slow intermittent flow | ||
| 07 | 0 | + | +++ | continuous flow | continuous flow | not evaluable (oedema) | ||
| 08 | 0/+ | +/++ | ++/+++ | continuous flow | slow flow | not evaluable (oedema) | ||
| Nepalese Highlanders | ||||||||
| N. | Oedema * | Flow and Morphology | ||||||
| 3400 m | 5050 m | 3400 m a.s.l. | 5050 m a.s.l. | |||||
| 01 | 0/+ | + | dilated loops | low flow | ||||
| 02 | 0 | + | slow flow, dilated loops | slow flow, dilated loops | ||||
| 03 | + | ++ | continuous flow | continuous flow | ||||
| 04 | +++ | +++ | not evaluable (oedema) | not evaluable (oedema) | ||||
| 05 | 0 | ++ | very slow flow, dilated loops | very slow flow | ||||
| 06 | ++ | + | continuous flow, dilated loops | continuous flow, dilated loops | ||||
| 07 | + | +/++ | continuous flow, dilated loops | continuous flow, dilated loops | ||||
| 08 | ++ | ++ | continuous flow, dilated loops | continuous flow, dilated loops | ||||
| 09 | + | ++ | continuous flow, dilated loops | continuous flow, dilated loops | ||||
| 10 | + | ++ | continuous flow, dilated loops | slow flow, dilated loops | ||||
| 11 | 0 | 0/+ | slow flow | slow flow | ||||
| European Lowlanders | ||||
| N. | Flow and Morphology | |||
| 1350 m a.s.l. | 3400 m a.s.l. | 5050 m a.s.l. | ||
| 01 | regular morphology and flow | dilation and improved perfusion | dilation and improved perfusion | |
| 02 | irregular calibre of venules | arteriolar constriction | dilation and improved perfusion | |
| 03 | regular morphology and flow | slight reduction of the overall flow | dilation and improved perfusion | |
| 04 | n.d. | n.d. | n.d. | |
| 05 | regular morphology and flow | dilation and improved perfusion | dilation and improved perfusion | |
| 06 | arteriolar and capillary rarefaction | slight reduction of the overall flow | dilation and improved perfusion | |
| 07 | regular morphology and flow | regular morphology and flow | dilation and improved perfusion | |
| 08 | slightly granular flow | slight reduction of the overall flow | dilation and improved perfusion | |
| Nepalese Highlanders | ||||
| N. | Flow and Morphology | |||
| 3400 m a.s.l. | 5050 m a.s.l. | |||
| 01 | n.d. | n.d. | ||
| 02 | granular flow, arteriolar rarefaction, kinking * | slight dilation in the arteriolar network | ||
| 03 | regular flow and arteriolar morphology, kinking * | slight dilation in the arteriolar network | ||
| 04 | regular flow and arteriolar morphology, kinking * | reduced arteriolar calibre | ||
| 05 | regular flow and arteriolar morphology, kinking * | n.d. | ||
| 06 | regular flow, slight arteriolar reduction, kinking * | slight reduction of the overall flow | ||
| 07 | regular flow and arteriolar morphology, kinking * | slight dilation and improved perfusion | ||
| 08 | regular flow and arteriolar morphology, kinking * | arteriolar dilation and improved perfusion | ||
| 09 | regular flow and arteriolar morphology, kinking * | slight dilation and improved perfusion | ||
| 10 | regular flow and arteriolar morphology, kinking * | arteriolar dilation and improved perfusion | ||
| 11 | regular flow, transient arteriolar spasms | arteriolar dilation and improved perfusion | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvi, P.; Grillo, A.; Brunacci, F.; Severi, F.; Montaguti, L.; Gautier, S.; Salvi, L.; Pretolani, E.; Parati, G.; Benetos, A. Microcirculatory and Rheological Adaptive Mechanisms at High Altitude in European Lowlander Hikers and Nepalese Highlanders. J. Clin. Med. 2023, 12, 2872. https://doi.org/10.3390/jcm12082872
Salvi P, Grillo A, Brunacci F, Severi F, Montaguti L, Gautier S, Salvi L, Pretolani E, Parati G, Benetos A. Microcirculatory and Rheological Adaptive Mechanisms at High Altitude in European Lowlander Hikers and Nepalese Highlanders. Journal of Clinical Medicine. 2023; 12(8):2872. https://doi.org/10.3390/jcm12082872
Chicago/Turabian StyleSalvi, Paolo, Andrea Grillo, Fausto Brunacci, Francesca Severi, Luca Montaguti, Sylvie Gautier, Lucia Salvi, Enzo Pretolani, Gianfranco Parati, and Athanase Benetos. 2023. "Microcirculatory and Rheological Adaptive Mechanisms at High Altitude in European Lowlander Hikers and Nepalese Highlanders" Journal of Clinical Medicine 12, no. 8: 2872. https://doi.org/10.3390/jcm12082872
APA StyleSalvi, P., Grillo, A., Brunacci, F., Severi, F., Montaguti, L., Gautier, S., Salvi, L., Pretolani, E., Parati, G., & Benetos, A. (2023). Microcirculatory and Rheological Adaptive Mechanisms at High Altitude in European Lowlander Hikers and Nepalese Highlanders. Journal of Clinical Medicine, 12(8), 2872. https://doi.org/10.3390/jcm12082872








