Abstract
Cardiovascular disease still represents the main cause of mortality worldwide. Despite huge improvements, atherosclerosis persists as the principal pathological condition, both in stable and acute presentation. Specifically, acute coronary syndromes have received substantial research and clinical attention in recent years, contributing to improve overall patients’ outcome. The identification of different evolution patterns of the atherosclerotic plaque and coronary artery disease has suggested the potential need of different treatment approaches, according to the mechanisms and molecular elements involved. In addition to traditional risk factors, the finer portrayal of other metabolic and lipid-related mediators has led to higher and deep knowledge of atherosclerosis, providing potential new targets for clinical management of the patients. Finally, the impressive advances in genetics and non-coding RNAs have opened a wide field of research both on pathophysiology and the therapeutic side that are extensively under investigation.
1. Introduction
A number of substantial improvements has been achieved in the diagnosis and treatment of acute coronary syndrome (ACS) in the last decades, especially in terms of antithrombotic therapies [1,2] and percutaneous intervention [3,4,5,6]. However, cardiovascular diseases persist as the leading cause of mortality worldwide, with ischemic heart disease accounting for the majority of cardiovascular deaths [7,8].
Angiographically identifiable atherosclerotic lesions are considered the key elements in the determinism of coronary artery disease (CAD): historically, clinically significant (flow-limiting) coronary stenoses were the primary lesions for chronic coronary syndrome (CCS), with their future progression causing acute cardiac events, including unstable angina, myocardial infarction (MI) and cardiac death.
Although the revascularization of severe coronary artery stenoses certainly reduces symptoms and ameliorates quality of life, several reports have found no substantial prognostic improvements [9,10] of interventional approaches, while optimal medical therapy has demonstrated a reduction in cardiac death and MI [9,11]. Despite guideline-based treatment, a proportion of patients with CCS progress to acute event with detrimental consequences in overall patient outcome.
Thus, research efforts have shifted to the identification of features and characteristics of atherosclerotic plaques, aiming to better patient risk stratification [12,13,14,15]. Alongside that, advances in understanding pathogenic pathways and genetic role have provided new insights into the atherothrombotic process.
2. Definition and Clinical Presentation of Acute Coronary Syndrome
The clinical spectrum of ACS includes unstable angina, non-ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI). These three clinical entities are graduated according to the severity and rapidity of the required action for their treatment. At the bottom of severity is placed unstable angina, in which the referred symptoms suggest an acute myocardial injury, but the biochemical evidence is lacking. NSTEMI and STEMI are grouped in the nosological condition of type 1 MI, according to the Fourth Universal Definition of Myocardial Infarction, requiring appreciable troponin level changes that both rise or fall together with clinical evidence of ischemia [16].
Even if the proportion of STEMI among ACS is decreasing in Western countries, thanks to improvements in contrasting well-established risk factors, the rates of in-hospital mortality and morbidity remain high [17]. Moreover, the previous paradigm of coronary atherosclerotic plaque rupture as the singular cause of STEMI or NSTEMI, recently re-named non-ST-segment elevation acute coronary syndrome (NSTE-ACS), has fallen in the last decade [18]. The availability of intravascular ultrasonography (IVUS) and optical coherence tomography (OCT) has allowed for the study of the in vivo characteristics and morphology of plaques and their composition [19,20,21,22,23]. The majority of ACS has been caused by the rupture of an atherosclerotic plaque with thrombus formation. Studies among patients referred for an ACS have documented the rupture of lipid-rich plaque in two thirds of cases [24]. However, a non-negligible proportion of patients experience ACS due to plaque erosion, calcific nodules, coronary spasm, and spontaneous coronary artery dissection (Figure 1).
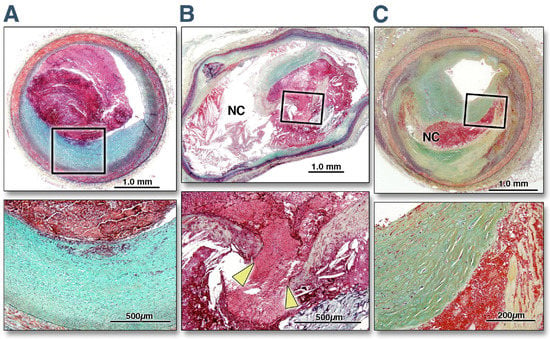
Figure 1.
Histomorphological Characteristics of plaque erosion, plaque rupture, and a stable plaque. The top three photos report the cross-sectional area of a culprit lesion displaying a plaque erosion in a patient with acute coronary syndrome (A), a culprit lesion displaying a plaque disruption in a patient with acute coronary syndrome (B), and a stable unrelated plaque from a non-culprit site in another patient dying from sudden cardiac death (C). The boxed areas in the top panels are magnified in the bottom panels for better histological characterization. (A) The eroded plaque shows subcritical stenosis, an unremarkable necrotic core, and overlying thrombus on an intact fibrous cap. The cap is rich in smooth muscle cells and proteoglycans, and there is minimal inflammation at the base of the thrombus. The plaque does not show any positive remodeling. (B) Conversely, a positively remodeled, critically occlusive atherosclerotic plaque with a cholesterol crystal-rich large necrotic core (NC) covered by a very thin and inflamed fibrous cap, which is disrupted (area between the arrowheads). Smooth muscle cells are visible in the medial layer and thin fibrous cap and are minimally present at the base of the neointima. A large thrombogenic necrotic core is in communication with the vessel lumen with an occlusive thrombus. (C) A stable plaque shows smooth muscle and collagen-rich histology. The hemorrhagic necrotic core in the middle that separates the collagen of two separate ages represents a healed rupture site. The lesion is critically narrowed but does not show any positive remodeling or overlying thrombus. Reprinted from [25], with permission from Elsevier.
3. Physiopathology of Acute Coronary Syndrome
3.1. Plaque Disruption
The principal and more frequent event that leads to an acute presentation of coronary atherosclerosis is constituted by the luminal rupture of a “vulnerable” plaque [25]. Its features include a large lipid core mixed with foam cells, macrophages. That atheroma is covered by a thin fibrotic cap including extracellular matrix components. The acute rupture of the protective cap releases prothrombotic substances and material from the plaque, activating the coagulative cascade, thrombus formation with consequent ischemia [26]. For decades, research has focused their efforts on understanding the pathophysiology of the rupture of atheroma’s thin cap. Several inflammatory mediators, including interferon-γ and interleukin (IL)-1, block the production of extracellular matrix elements [27] and stimulate macrophages and other cells to release proteases that degrade extracellular molecules [28]. The same triggers that deteriorate the atheroma’s thin cap stimulate the production of prothrombotic elements, fibrin and plasminogen activator inhibitor-1, enhancing clot formation [29]. The exposed core elements, including von Willebrand factor, collagen and tissue factor, activate the circulating platelets, which in turn potentiate the coagulative process, resulting in a rapid thrombus formation and acute onset of heart ischemia [30,31].
3.2. Plaque Erosion
A relatively new concept is represented by the superficial erosion of atherosclerotic plaque as the cause of ACS. Historical data suggest a prevalence of 20% of ACS patients characterized by plaque erosion in the culprit lesions, while more recent reports indicate about 40% of ACS patients displaying plaque erosion [32,33,34]. Improvements in technological instruments, particularly the OCT, have helped to identify this condition in vivo. In general, patients with plaque erosion are younger than subjects admitted for ACS due to plaque rupture. Data from a large OCT systematic study showed 53.8 vs. 65.1 years, respectively, for patients with plaque erosion compared to those with plaque rupture [35]. The distribution of traditional cardiovascular risk factors is unbalanced between these two conditions. Patients with plaque erosion usually display lower prevalence of diabetes mellitus and hypertension, lower levels of low-density lipoprotein cholesterol and C-reactive protein, and a higher concentration of hemoglobin [36,37]. In addition, the complexity and severity of CAD are lower in patients with plaque erosion than among those with plaque rupture [38].
Considering the substantial differences observed from the clinical side, it is essential to understand the molecular mechanisms to tailor patient management and to explore new potential targets of treatment.
The first crucial issue is represented by the local shear stress corresponding to the plaque. Human studies have shown that a thrombus occurs in the zones of high endothelial shear stress [39]. The fluid dynamic impact leads to the degradation of the basement membrane, endothelial cell desquamation and death [40]. A contribution by innate immunity has been suggested [41]. Inflammation and minimally oxidized low-density lipoprotein (LDL) promote the expression of matrix metalloproteinase (MMP)-14 and MMP-2, which degrade type IV collagen, the main component of the basement membrane [42,43]. Furthermore, the enhanced activation of toll-like receptor (TLR)-2 has been found to maintain the underlying inflammation [44] and to favor the recruitment of granulocytes through the increased expression of leukocyte adhesion molecules [45]. The recruited granulocytes, mostly neutrophils, are prone to form neutrophil extracellular traps (NETs) that have been linked to the pathophysiology of plaque erosion and thrombus formation. NETs are constituted by cytokines and tissue factors, and they can entrap circulating platelets, thus promoting atherosclerosis [46]. Finally, impaired endothelium homeostasis has been implied in endothelial to mesenchymal transition, which contributes to plaque erosion and loss of integrity, together with overexpression of MMP-2, transforming growth factor-β (TGF-β), and vascular growth factor (VEGF) [47,48].
3.3. Calcified Nodules
Among less common causes of ACS, there are calcified nodules. Their frequencies range from 4% to 7% as the cause of ACS [49]. These heavily calcified lesions have been found to be more frequent among the elderly [50] and in those with chronic kidney disease [51,52]. From a pathological point of view, they are defined by the presence of a fracture in the calcified sheet mixed with fibrin and a disrupted fibrous cap with an overlying thrombus [53]; a histology study has shown that the break in the calcium sheet into the lumen might be the trigger. Negative remodeling has been observed in calcified nodule lesions more frequently than in lesions with ruptures of erosion [24].
3.4. Spontaneous Coronary Artery Dissection
Spontaneous coronary artery dissection (SCAD) is a condition characterized by an intimal tear leading to the creation of a false lumen in the coronary wall in absence of clear or known mechanical causes, including traumatic events or wire manipulation. The subsequent compression of the vessel lumen leads to ischemia in the post-dissection myocardial area [18]. Epidemiological data show an overall low prevalence of this condition, less than 5%, even if a higher incidence has been displayed in specific subgroup of patients, including pregnant or postpartum women [54]. The optimal treatment of SCAD has still not been determined. Routine percutaneous coronary intervention performance is associated with a particularly elevated rate of technical failure, due to wiring the false lumen or extension of the dissection [55].
3.5. Coronary Spasm
A still poorly understood cause of ACS without plaque rupture is represented by coronary artery spasm. Some hypotheses involve reduced nitric oxide availability due to endothelial dysfunction, leading to increased smooth muscle cell reactivity [56]. A proportion of about 25% of patients undergoing coronary angiography has shown coronary spasm [57]. This should be expected, particularly if coronary angiography provides negligible findings for significant stenosis [58]. The occurrence is higher among older men and postmenopausal women [57], and racial differences have been detected even if without a definite explanation, to date [59]. A previous study has inquired about coronary spasms induced by acetylcholine among patients with stable angina. The authors found a worse prognosis at 28 months among patients with a high degree of coronary spasm [60]. The lack of a systematic approach to diagnose coronary spasm hampers the availability of robust treatment guidelines, even if some authors suggest a routine use of acetylcholine to unhide coronary spasm in ACS patients with coronary angiography negative for clear culprit lesions [61].
4. Recent Known Advances on Immunity
4.1. NETs and NLRP3
Several studies have claimed a central role of neutrophils in CAD progression, including the abrupt presentation of an ACS [62]. The activation of NETs above atherosclerotic plaque together with nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) are known and accepted as key pathogenetic steps [63].
Neutrophil action in promoting atherosclerosis involves monocyte entry into atherosclerotic lesions and macrophage activation, while the released myeloperoxidase accelerates LDL oxidation and then foam cell formation [64]. The formation of NET is a relatively recent element related to neutrophils that worsen atherosclerosis [65]. The network of extracellular fibers composed by the DNA of neutrophils is activated by numerous stimuli involving ROS, factor crystallizable and complement receptors, TLR-2 and -4 signaling [66], with a consequent impact on platelet aggregation and macrophage release of IL-1β [67].
The NLRP3 inflammasome is a key mediator of inflammatory diseases, including atherosclerosis and other vascular diseases. Recent evidence has suggested that IL-1β-mediated inflammation drives atherothrombotic events, indicating that NLRP3 inflammasome is a major contributor to atherosclerosis.
NLRP3 is known to be activated by a wide range of diverse stimuli, most of which have not been shown to directly interact with it (Figure 2). Thus, it is theorized that NLRP3 can sense cellular events that are triggered by activating stimuli. Common upstream events proposed for NLRP3 inflammasome activation include K+ efflux (decrease in intracellular K+), the generation of ROS derived from mitochondria, and cathepsin release from the lysosome. Mitochondria-derived ROS production has been demonstrated to be important for NLRP3 inflammasome activation, with studies suggesting that oxidized mitochondrial DNA released in response to NLRP3 activators can drive the activation. The role of NADPH oxidase in NLRP3 inflammasome activation is controversial, although it has been suggested that NADPH oxidase-4 may play a role in certain conditions. Furthermore, the release of cathepsin from damaged lysosomes is another cellular event that can trigger NLRP3 inflammasome activation. This is commonly caused by the presence of particulate matter such as monosodium urate and cholesterol crystals. These particles are taken up by macrophages, but their digestion in lysosomes is inadequate, thus leading to lysosomal damage and the leakage of cathepsins into the cytoplasm and, consequently, to NLRP3 inflammasome activation [68].
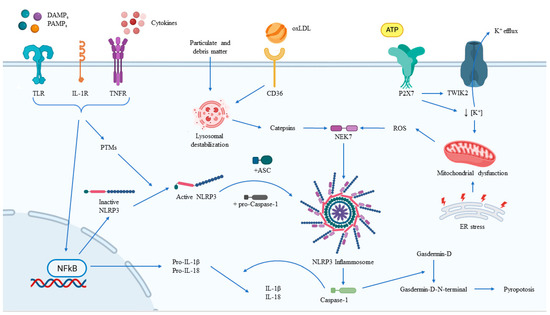
Figure 2.
Regulation of NLRP3 inflammasome activation. The main external and intracellular factors involved in the activation and modulation of the NLRP3 inflammasome, together with its main effects. ASC, adaptor apoptosis-associated speck-like protein containing a caspase-recruitment domain; ATP, adenosine triphosphate; DAMPs, damage/danger-associated molecular patterns; ER, endoplasmic reticulum; IL-1R, interleukin-1 receptor; NFkB, nuclear factor-kB; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; oxLDL, oxide low-density lipoprotein; PAMPs, pathogen-associated molecular patterns; PTMs, post-translational modification; TLR, toll-like receptor; TNFR, tumor-necrosis factor receptor.
Studies have demonstrated that the expression levels of NLRP3 inflammasome components are upregulated in atherosclerosis, which are potential links to the severity of the disease [68]. Furthermore, studies using bone marrow-transplanted mice, lentivirus-mediated NLRP3 gene silencing or the selective NLRP3 inhibitor MCC950 in apoE−/− mice have provided direct evidence that the NLRP3 inflammasome contributes to the progression of atherosclerosis [69]. However, there are conflicting data showing no significant differences in plaque size or macrophage infiltration between apoE−/− mice and apoE−/− mice deficient in NLRP3 or caspase-1 [70]. Very recently, Chen et al. reported that a lack of NLRP3 in bone marrow cells in LDL receptor−/− mice attenuated atherosclerotic lesion formation in female mice but not in male mice [71]. Further research is needed to explore the potential role of the NLRP3 inflammasome in the development of atherosclerosis, also considering sex differences.
4.2. Neutrophils’ Healing Potentiality
Despite this evidence, almost all therapeutic strategies aiming to contrast neutrophils have thus far failed to show clinical benefits [72]. Experimental studies have reported that long-term depletion of neutrophils in MI models did not improve but rather impaired the inflammation process, leading to negative remodeling and reduced cardiac function [73].
The emerging role of neutrophils in the reparative process and healing after an injury has been reported in the last few years. Neutrophils contribute to the biosynthesis of growth factors, including VEGF, and factors favoring the inflammation switch off, such as lipoxins, resolvins, and protectins [74,75]. Therefore, general anti-neutrophil approaches can limit acute post-ischemic tissue injury, but this potential benefit is counterbalanced by the blockage of the subsequent healing response. In a murine model, Horckmans et al found that neutrophil-depleted mice subjected to MI had worsened cardiac function, increased fibrosis, and progressively developed heart failure, enlightening the crucial role of neutrophils into macrophage polarization as a healing phenotype [76]. Similarly, long-term inhibition of the S100 A8/A9 heterodimer has led to the deterioration of cardiac function over time [77].
The promising results observed in the CANTOS trial [78] and COLCOT trial [79] should drive future intervention and research in inflammatory regulation among ACS patients (Table 1). These two large trials inquired about the effect of canakinumab and colchicine in patients with recent ACS, finding benefits in terms of reductions in major cardiovascular adverse events, mainly driven by reductions in ischemic events. The inhibition of the IL-1β pathway has shown positive prognostic impacts after MI, even if some contrasting results have been reported by other drugs used to target the same pathways, such as in the MRC-ILA Heart Study, which failed to demonstrate a benefit of treatment with anakinra, a IL-1 receptor inhibitor, in NSTEMI patients [80]. Advances in understanding neutrophil biology imply a tailored approach to target not only the mechanisms and actors involved, but also the time and duration of the treatment, in order to act without damage [81].

Table 1.
Main clinical study and evidence in humans of the discussed targets.
4.3. Adaptive Immunity
Alongside the role of innate immunity, T and B lymphocytes operate in an elaborate web of signaling and subtype differentiation in the atherosclerosis context [94]. The adaptive immune response is mediated toward lipoprotein components trapped in the artery wall and toward proteins coming from dysfunctional endothelial cells [95].
In the early stages of atherosclerosis, the recruitment of CD4+ T cells with a regulatory phenotype (Treg) rather than an effector phenotype (Teff) is predominant [96]. In an animal model of hypercholesterolemia, a rise in Treg in atherosclerotic plaque has been observed with a concomitant lower number of Teff [97]; moreover, a depletion of CD4+ T cells may accelerate atherosclerosis in mice [98], promoting the concept of an initial atheroprotective role played by CD4+ T cells. However, the determinants of the switch of T lymphocytes from a protective role to atherogenic action are not completely defined. A combination of increased inflammatory cytokines, recruitment chemokines, reduced coinhibitory molecules and anti-inflammatory mediators may be responsible for the variation in the T cell subset proportions in the atherosclerosis milieu [99]. CD4+ T cell subpopulations can differentially impact atherosclerosis progression and ACS events [100]. Recent evidence has suggested T cell plasticity in addition to their heterogeneity. A specific CD4+ T cell subtype can acquire the transcriptomic and phenotypic properties of another subtype [96,101]. A persistent atherogenic condition can influence Treg by modulating the forkhead box P3 (FoxP3) gene, essential for anti-atherogenic actions. Treg might progressively loose the FoxP3 expression in favor of an effector and pro-atherothrombotic phenotype [102].
The role of B lymphocytes is less well characterized, but their contribution in atherosclerosis has been depicted [103]. A primary protective role has been hypothesized, even if the recent definition of B1 and B2 subtypes has provided controversies regarding their role. In particular, the B1 subtype was confirmed to be anti-atherogenic by producing immunoglobulin M antibodies to recognize apoptotic cells and to oxidize LDL [104]. The contribution of B2 lymphocytes still needs more clarification. Their antibody production is mainly derived from germinative centers, and has displayed the capacity to promote atherosclerosis [105]. B2 cells-derived immunoglobulin G antibodies with pathogenic role have been described as recognizing heat shock proteins 60 and 65, released from a damaged endothelium and enhancing inflammation [95].
Increased attention is warranted regarding perivascular adipose tissue (PVAT) as an active paracrine modulator of inflammation and adaptive immunity. A number of different lymphocyte subtypes have been found in the adipose tissue located on the external surface of arteries, including the coronaries [106,107]. The interplay between PVAT and atherosclerosis is mediated by adiponectin, adipokines and other cytokines [108]. Initially, PVAT exerts a protective action against endothelial dysfunction and conditions promoting atherosclerosis by the production of vasoactive immunoregulatory substances [109]. Impaired PVAT homeostasis, such as in obesity or systemic inflammation, leads to the loss of brown adipocyte components, with consequent atherosclerotic plaque progression [110]: the following infiltration of immune cells, including CD4+ T cells with effector polarization aggravates lesion severity and the stability of plaque [111,112].
5. Metabolic and Lipid-Related Actors in the Atherothrombosis Process
5.1. Adenosine Pathways
The first evidence of adenosine involvement in the cardiovascular system was reported by Berne [113], who described its contribution in coronary vasodilation. Alongside the electrophysiological properties in the diagnosis and treatment of wide QRS tachycardia and supraventricular tachycardia, adenosine has been found to be involved in several pathways related to coronary blood flow and atherothrombotic processes. Effects of adenosine are mediated by four different G protein-coupled receptors, but the major cardiovascular role is played by the A2a type, which is widely expressed in smooth muscle and the endothelial cells of vessels. The protective role in atherosclerosis is constituted by the inhibition of the pro-inflammatory cytokine response [114], together with the stimulation of endothelial cell proliferation during angiogenesis [115]. The promotion of collateral circulation is crucial against ischemia-induced damage. Hypoxia has been reported to increase A2a receptor levels [116]. Upregulation of the A2a receptor counterbalances the inflammatory response mediated by NF-kB and hypoxia-induced factor (HIF)-1α pathways [117].
Another key role of adenosine and the A2a receptor is the regulation of platelet aggregation. In an animal model, mice knockout for the A2a receptor displayed higher platelet aggregation: the effect is mediated by an increase in platelet intracellular cAMP concentration with a consequent reduction in internal calcium mobilization [118]. The magnitude of adenosine impact on platelet aggregation is not negligible: for example, the direct P2Y12 antagonist ticagrelor inhibits equilibrate nucleoside transport 1, with a consequent increase in plasmatic adenosine levels [119]; moreover, genetic polymorphism of the A2a receptor has shown significantly different platelet reactivities among patients on ticagrelor [120]. Similarly, the procedural use of adenosine during the assessment of FFR deserves careful attention in relation to dosage of intracoronary adenosine [121] and genetic variants that may affect the final results [122].
5.2. Lp(a)
Lp(a), an apolipoprotein B (apoB)-containing lipoprotein similar to LDL, is bound to apolipoprotein(a) (apo(a)) on its surface. Apo(a) is a protein encoded by the lipoprotein(a) gene and possesses significant homology with plasminogen [123]. Lp(a) has various physiological and pathological roles, including modulation of coagulation, immune cell interaction, and proliferation of vascular smooth muscle and adhesion molecules. It is also a major carrier of oxidized phospholipids in human plasma, which can have pro-atherogenic and pro-inflammatory effects [123].
In terms of disease, Lp(a) contributes to the formation and progression of atherosclerotic plaques, potentiates thrombus formation to cause MI or ischemic stroke, and promotes inflammation and calcification [124].
The population distribution of circulating Lp(a) concentration is positively skewed, strongly supporting a relationship between raised Lp(a) and the risk of CAD and acute events [125]. Mendelian randomization studies indicate a causal relationship between the lifelong elevation of Lp(a) and MI, with the doubling of Lp(a) plasma levels resulting in a 22% increased risk of MI [126].
The cholesteryl ester transfer protein (CETP) inhibitors anacetrapib and evacetrapib reduced Lp(a) by 34 % and 40%, respectively, in two different phase II trials [82,84]. These drugs did not demonstrate an overall benefit for CVD risk reduction but, importantly, were evaluated in trials that did not enroll patients on the basis of elevated Lp(a). Even when a large study, the REVEAL trial, found a lower incidence of major coronary events in patients treated with anacetrapib than with the use of placebo [83], the overall benefits and cost–benefit ratio hampered to proceed for the introduction in daily practice [127].
Lipoprotein apheresis offers an effective means of lowering apoB-containing lipoproteins, including LDL and Lp(a). It can reduce both Lp(a) and LDL-C from baseline by about 70% even if the reduction in Lp(a) is inconstant and time-sensitive [85].
5.3. Vitamin D and Calcium Homeostasis
Vitamin D is a lipophilic vitamin primarily involved in the homeostasis of calcium, phosphorus and bone tissue, but it also has a wide range of systemic hormonal effects and organ targets, including the cardiovascular system [128]. Cholecalciferol is the compound named vitamin D3, and it is synthesized in the skin upon ultraviolet waves or is derived from food as ergocalciferol. It undergoes first modification in liver cells, receiving a hydroxyl group on C25 and becoming 25(OH) vitamin D; the active form requires further hydroxylation on C1, occurring mainly in the kidneys and other extrarenal sites, with the formation of 1,25(OH)2 vitamin D, named calcitriol [129]. The vitamin D receptor binds vitamin D in the form of 1,25(OH)2 and is expressed widely in the cardiovascular system [130], promoting the signaling cascade and its effects. The cardiovascular protective role of vitamin D has been shown in several studies, which have all elucidated that vitamin D counteracts inflammation and oxidative stress and is a cornerstone of atherosclerosis [131,132,133].
Moreover, among ACS patients, calcitriol deficiency was found in about 9% of patients, even among those with normal 25(OH) vitamin D. A significant inverse relationship of calcitriol with inflammatory and metabolic biomarkers has been reported, suggesting a potential accurate role of calcitriol to predict cardiovascular risk [134]. Another interesting finding is the inverse relationship between vitamin D level and pro-atherogenic lipid profile and the burden of lesion calcification in patients admitted for STEMI [135].
This evidence is in line with and supports the concept of the bone–vascular axis [136,137,138]. Prior studies have found that the increase in bone resorption occurring in elderly and post-menopausal women leads to calcium mobilization and subsequent abnormal deposition in vessels walls [139]. Several mediators including fibroblast growth factor, osteopontin, osteoprotegerin, and matrix proteins have shown a connection between bone homeostasis and vascular calcification [140]. Subsequent modification in vessel components affects smooth muscle cells. When they are exposed to extracellular vesicles from bone with impaired instead of normal metabolism, vascular smooth muscle cells might undergo osteogenic transition with consequent vessel calcification [141]. This so-called calcification paradox was also found in settings different from primary bone affection, such as osteoporosis. In chronic kidney disease, osteogenic transition has been observed [139,142], claiming a role in increased serum phosphorus levels or decreased hydroxylation of 25(OH) vitamin D. Whether calcium deposition in coronary vessels should be considered a risk factor or protective for acute events is still under discussion [143]. These crucial advances in preclinical studies deserve a transition in the clinical setting.
Clinical trials have explored the cardiovascular effects of vitamin D supplementation, reporting contrasting results. Administration of cholecalciferol compared to placebo in patients with hypovitaminosis D has shown no changes in various cholesterol and lipoproteins levels [86]. Moreover, two large trials, ViDA [87] and VITAL [88], enrolled 5110 and 25,871 patients, respectively, to assess the effect of the supplementation of cholecalciferol in healthy adults. No benefit was found in terms of the reduction of major cardiovascular adverse events, including cardiovascular death, over a follow-up period of 3.3 and 5.3 years. Meta-analyses of randomized trials in CAD patients have confirmed these inconclusive results, portraying the need for further investigation [88].
5.4. Lipoprotein-Associated Phospholipase A2
The term phospholipase A2 (PLA2) refers to a superfamily of phospholipase enzymes that hydrolyze the ester bond at the sn-2 position of phospholipids. After hydrolyzation, PLA2 releases free fatty acids. A proportion of them includes arachidonic acid and oleic acid, crucial compounds for energy production and inflammation signaling pathways [144].
The PLA2 superfamily comprises 16 groups identified according to the differences in the chronology of their discovery [145]. Group VII includes lipoprotein-associated PLA2 (Lp-PLA2). It is secreted by macrophages, monocytes, mast cells and T-lymphocytes, and it circulates in the blood in the form of a complex with LDL and high-density lipoprotein [146]. Lp-PLA2 was originally called platelet-activating factor acetyl-hydrolase, because of its hydrolytic action on platelet-activating factor [147].
These effects produce an acceleration of atherosclerosis and plaque development, including the formation of a necrotic core. Increased levels of Lp-PLA2 are found in thin-cap.
Several studies have reported that high plasma Lp-PLA2 levels are independently associated with an increased risk of CAD and stroke [148,149,150]. Moreover, in a murine model, the downregulation of the group VII of PLA2 genes improves inflammation and reduces the burden of atherosclerosis [151], suggesting Lp-PLA2 as a potential target for therapeutics. However, despite the pro-atherogenic effect and prediction of adverse events, the inhibition of Lp-PLA2 with darapladib, the most advanced and most widely studied Lp-PLA2 inhibitor, has failed to provide substantial benefits in patients with stable CAD [152] and admitted for an ACS [89]. In particular, in the SOLID-TIMI 52 trial, more than 13,000 subjects were randomized to receive either once-daily darapladib or placebo within 30 days of hospitalization for an ACS. With a median follow-up of 2.5 years, the primary endpoint, major coronary events, occurred in 16.3% of patients in the darapladib group and in 15.6% in the placebo group (HR = 1.00, 95% CI: 0.91–1.09, p = 0.93) [89].
Some explanations have been proposed to understand the discrepancy between pre-clinical evidence and the lack of clinical benefits. Patients enrolled in the darapladib clinical trials displayed higher percentages of statin therapy, which may reduce the Lp-PLA2 level and limit the magnitude of further Lp-PLA2 reduction through specific inhibitors. Concerns from other authors have involved the absence of stratification of patients according to residual inflammation after an acute event that leads to hospitalization, suggesting the need for a more accurate selection of patients potentially benefiting Lp-PLA2 inhibition [153]. Therefore, Lp-PLA2 may be considered as a reliable biomarker of cardiovascular risk, rather than a causal element of atherosclerosis [154].
6. Genetics: The Different RNAs in ACS
6.1. MicroRNA
MicroRNAs (miRNAs) play an essential role in cardiovascular biology, with dysregulation linked to various pathologies [155]. Several miRNAs have been found to be altered in cardiovascular diseases [156], and cardiac-specific miRNAs have been reported to contribute to the development of CAD, with evidence of differences in miRNA expression between CCS and ACS [157] (Table 2).

Table 2.
Main non-coding RNAs and cardiovascular conditions related to acute coronary syndrome.
From a pathological point of view, miRNAs have been found to be related to alterations involving several key actors of atherosclerosis. A number of miRNAs, including miR-34a, miR-217, and miR-146a, have been associated with regulating mechanisms involved in endothelium senescence [189]. A decrease in sirtulin-1, a gene of longevity, was identified as a main effect of miRNA interaction [190]. On the other hand, some mrRNAs have shown the ability to control inflammation and reduce leukocyte infiltration in the vessel wall and plaques, such as miR-126 [191]. In addition, the homeostasis of smooth muscle has been found to be influenced by miRNAs. miR-143 and miR-145 may promote the differentiation of smooth muscle cells and have been found to be reduced in atherosclerotic vessels [192].
A number of cardiac- and muscle-specific miRNAs were proposed as biomarkers of acute MI in pivotal studies [193,194]. In particular, mir-208b and mir-499 were found to be significantly effective in predicting MI compared to controls, with an AUC = 0.97 for mir-499, even if no adjunctive impact to troponin diagnostic accuracy was shown [165]. Subsequent studies have confirmed the role of mir-499 in the prediction of acute MI together with mir-133a [169], while mir-208b was suggested to be further investigated [195]. In a study including more than three hundred ACS-suspected patients, cardiac and non-cardiac miRNAs were assessed. The levels of all miRNAs studied were significantly increased in 106 patients diagnosed with ACS, even among those with first high-sensitive troponin that resulted in negative or early symptom onset (i.e., less than 3 h). Cardiac miR-1, miR-499 and non-cardiac miR-21 were found to increase the diagnostic value when added to troponin, independent from patient comorbidities and cardiovascular risk factors. Moreover, the combination of the three miRNAs resulted in a significantly higher AUC than troponin among ACS patients earlier admitted or referred to hospital [159].
In addition, inflammation-related miRNAs have been inquired for their potential relationship with ACS, including miR-181c and miR-362 [196]. The levels of these miRNAs were found to be higher in ACS patients, while other authors reported no increased levels among stable CAD [197]. However, their expression was not found to be related to troponin levels, suggesting a role of these inflammatory miRNAs to be more on plaque vulnerability than on the extent of myocardial injury.
The wide and still unexplored field of miRNAs may be hiding some concerns, and findings must be interpreted with caution. There are several potential biases related to the type of body fluid used for miRNA extraction, sample preparation, platform for analysis, RNA purification, and normalization methods, of which all can affect the concentration and quality of miRNAs [198]. In addition, miRNAs selection that has adopted candidate-driven approaches might lead to biases regarding the magnitude of the effect estimation [199]. Therefore, it is essential to interpret the findings of miRNA studies with caution, as there is an absence of standardization when it comes to selecting miRNAs, normalization methods, and RNA extraction techniques [200].
6.2. Long Non-Coding RNAs
Long non-coding RNAs (lncRNAs) have been shown to play a role in a variety of cellular functions within both the nucleus and cytoplasm and are important for normal development as well as in the progression of disease [201]. RNA-seq studies have been performed to assess lncRNA expression, both in animal models and human tissues [202,203], in the context of cardiovascular disease [204]. For example, a study using RNA-seq to investigate the dynamic regulation of lncRNAs in ischemic heart disease in pigs identified 450 lncRNAs that were dysregulated in cardiac tissue after MI [205].
Additionally, two novel, hypoxia-sensitive human endothelial lncRNAs were discovered using RNA-seq and microarray technologies, which upon further functional characterization through gain- and loss-of-function approaches, were found to have an important role in angiogenesis [206]. Furthermore, the potential value of lncRNAs as diagnostic biomarkers has been explored, and a global transcriptomic analysis of plasma samples from patients with MI led to the identification of 768 lncRNA transcripts that were dysregulated compared to control samples [207]. In particular, the mitochondrial lncRNA uc022bqs.1 (LIPCAR) was found to be downregulated immediately after MI but upregulated during later stages of cardiac damage, suggesting that it could be used to identify patients who are prone to cardiac remodeling following a MI [207].
In a case-control study, HIF-1α antisense RNA 2 (aHIF), KCNQ1 overlapping transcript 1 (KCNQ1OT1), and metastasis-associated lung adenocarcinoma transcript 1 were significantly increased in circulation in patients with acute MI, with further differences in circulating levels according to STEMI or NSTEMI presentation [181].
LncRNA myosin heavy-chain-associated RNA transcripts (MHRT) and other less common lncRNA were proposed as a potential diagnostic tool in the setting of acute MI [208]. Zhang et al., in their study, found increased levels of MHRT among patients referred for ACS compared to healthy volunteers. Moreover, they explored in murine MI models the role of lncRNA MHRT, reporting a protective role against hydrogen-peroxide-induced myocytes apoptosis [182].
Of interest is another lncRNA named urothelial carcinoma-associated 1 (UCA1). It was evaluated by Yan and colleagues in a case-control investigation comparing acute MI patients and healthy control [183]. Their main findings displayed a significant reduction in plasmatic UCA1 levels in the early phase of MI but a significant increase at day 3 after MI, irrespective of hypertension or diabetes diagnosis. UCA1 has been found to play a role in glucose metabolism [209], cell proliferation and inhibition of apoptosis [210], and most cardiac protective actions derive from their interaction with miR-1. Additive value to troponin and other myocardial injury biomarkers has been proposed for UCA1 [183].
6.3. Circular RNAs
Circular RNAs (circRNAs) are a significant class of non-coding RNA molecules that form a covalently closed loop. Spliceosome machinery is responsible for the production of circRNAs through the process of precursor mRNA back-splicing [211]. High-throughput sequencing has identified thousands of circRNAs present in the human body and has demonstrated that a substantial number of these are expressed in a tissue-specific or disease-specific manner [212,213,214]. While the biological functions of the majority of circRNAs are unknown, certain circRNAs have been reported to serve as miRNA sponges [215,216], to interact with RNA-binding proteins, to regulate transcription or to translate into proteins [217,218]. Early research has provided evidence of individual circRNAs playing pivotal regulatory roles in the cardiovascular system [219,220].
Key mechanisms of impact of circRNAs on MI involve oxidation-stress-induced cardiomyocyte apoptosis [221,222], with co-participation of miRNAs such as miR-133a [223], and ischemia-reperfusion-induced damage and cell death. The circRNA MFACR directly promotes mitochondrial fission and ischemia reperfusion apoptosis by interacting with miR-652.
Murine models of MI have been used by Geng et al. to explore the role of circRNA Cdr1as, also named CiRS-7, in infarcted cardiomyocytes. Their main findings were consistent with an increased expression of Cdr1as and cardiac infarct size and cell apoptosis promotion. Its effect was counterbalanced by miR-7a expression, which in turn decreased cell apoptosis and reduced the infarct size [184]. In in vitro models of acute MI, the circRNA circ-tetratricopeptide repeat domain 3 (circ-Ttc3) was found to be significantly upregulated, contributing to the reduction of ATP depletion and cell death. Proposed mechanisms include a potential sponge role of circ-Ttc3 with miR15b-5p, which in turn increases the expression of ADP ribosylation factor-like GTPase 2 (Arl2) protein, the main effector with a beneficial role shown by circ-Ttc3 [186]. A protective role against autophagy has been shown by autophagy-related circular RNA (ACR) [188], while an intriguing role in supporting myocardium healing after MI has been proposed with the co-participation of VEGF and connective tissue growth factor [224,225], as well as its role in the regulation of angiogenesis [185].
Despite the promising findings from pre-clinical studies, the still poor understanding of the biology of circRNA hampers its complete translation into the clinical field, requiring clarification both in their synthesis and activities [226].
7. Example of Genic Therapy
7.1. Olpasiran
Olpasiran is a siRNA drug administered subcutaneously and targeting the liver via its N-acetylgalactosamine moiety. Upon entering the hepatocyte, the antisense strand of olpasiran is loaded into an RNA-induced silencing complex (RISC), which binds to apolipoprotein(a) RNA messenger (mRNA), leading to its degradation. This allows for a prolonged effect, as the RISC can target additional mRNAs [227]. Clinical trials have shown that olpasiran treatment significantly reduces the concentration of Lp(a) in a dose-dependent manner and is safe, with doses reducing the Lp(a) concentration by more than 95% compared with placebo [228]. The pharmacodynamic effects were maintained when the drug was administered every 12 weeks. Although there is a causal role for Lp(a) in atherosclerotic cardiovascular disease [229], large-scale clinical trials are required to demonstrate a clinical benefit of Lp(a) lowering [230,231]. The relationship between Lp(a) and the risk of atherosclerotic cardiovascular disease appears to be broadly continuous and log-linear, making it difficult to identify a clear threshold for patient risk [232].
7.2. Inclisiran
For the treatment of heterozygous familial hypercholesterolemia or CAD requiring additional LDL lowering, inclisiran can be used as an adjunct to diet and maximally tolerated statin therapy [233]. This siRNA inhibitor of proprotein convertase subtilisin/kexin type 9 synthesis is administered via subcutaneous injection every three months in two doses, then every six months thereafter.
Results from the ORION-9, -10, and -11 phase III trials, published in 2020, have demonstrated a placebo-corrected percentage change in LDL from baseline to day 510 of 47.9 percent (95% CI 42.3–53.5) and a time-adjusted reduction of 44.3 percent (95% CI 40.1–48.5) [90,91,92,234]. As such, this may be a viable alternative for those with allergic responses to both evolocumab and alirocumab, as well as for those with difficulty using a pen injector due to arthritis and/or weakness of the hands. However, further clinical trial data are required in order to assess the potential for cardiovascular risk reduction before a broader use of inclisiran is suggested.
The ORION-10 and ORION-11 trials showed that inclisiran significantly reduced LDL levels by approximately 50 percent in patients with cardiovascular disease and at high risk. The pooled analysis results of these trials indicate that the placebo-corrected change in LDL with inclisiran at day 510 was −50.7 percent, with few serious adverse events reported [235].
8. Future Perspectives
The significant advances achieved in recent years have contributed to a finer comprehension of atherosclerosis pathophysiology (Figure 3). The paradigm of ruptured culprit lesions as the only cause of ACS has shifted to a more complex series of mechanisms that probably deserve different approaches from the interventional and non-interventional points of view. On the side of pharmacological strategies, the characterization of the neutrophils’ role could provide attractive targets of treatment, even if a further definition on the best way and time to treat is required to preserve their potential healing properties. Clinical translation of pre-clinical findings in metabolic- and lipid-related factors should be considered and performed, with adequate identification of the subgroups of patients that might take advantage from the treatment. Finally the great advances in genetics have provided a large field of investigation. The amount of data is impressive, but a better definition is needed to understand the potential benefits of all non-coding RNAs that have been investigated.
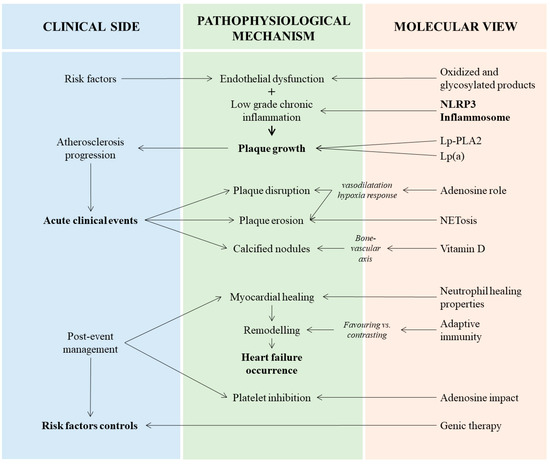
Figure 3.
Interplay between clinical manifestations, pathophysiological mechanisms and main actors related to atherosclerosis in acute coronary syndromes. In the left column are the three main clinical steps of acute coronary syndrome: pre-clinical atherosclerosis progression, acute event occurrence and post-event management. In the center column are the principal signaling pathways and underlying pathological aspects. In the right column are the key elements and substances mediating the pathophysiological process. Lp(a), lipoprotein(a); Lp-PLA2, lipoprotein associated-phospholipase A2; NETosis, neutrophil extracellular traps (NETs) formation; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3.
Author Contributions
Conceptualization, methodology, G.D.L.; writing—original draft preparation, M.N. and N.L.; writing—review and editing, M.V. and D.C.; supervision, G.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because this is a review.
Informed Consent Statement
Patient consent was waived because this is a review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Cassetti, E.; Piccolo, R.; Galasso, G.; Marino, P.; Sinigaglia, F.; De Luca, G. Benefits from New ADP Antagonists as Compared with Clopidogrel in Patients with Stable Angina or Acute Coronary Syndrome Undergoing Invasive Management: A Meta-Analysis of Randomized Trials. J. Cardiovasc. Pharmacol. 2014, 63, 339–350. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Tcheng, J.E.; Neumann, F.-J.; Bonizzoni, E.; Topol, E.J.; Chiariello, M. Relationship between Patient’s Risk Profile and Benefits in Mortality from Adjunctive Abciximab to Mechanical Revascularization for ST-Segment Elevation Myocardial Infarction: A Meta-Regression Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2006, 47, 685–686. [Google Scholar] [CrossRef]
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’Ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very High-Pressure Dilatation for Undilatable Coronary Lesions: Indications and Results with a New Dedicated Balloon. EuroIntervention 2016, 12, 359–365. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Smits, P.; Hofma, S.H.; Di Lorenzo, E.; Vlachojannis, G.J.; Van’t Hof, A.W.J.; van Boven, A.J.; Kedhi, E.; Stone, G.W.; Suryapranata, H.; et al. Everolimus Eluting Stent vs First Generation Drug-Eluting Stent in Primary Angioplasty: A Pooled Patient-Level Meta-Analysis of Randomized Trials. Int. J. Cardiol. 2017, 244, 121–127. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Navarese, E.P.; Suryapranata, H. A Meta-Analytic Overview of Thrombectomy during Primary Angioplasty. Int. J. Cardiol. 2013, 166, 606–612. [Google Scholar] [CrossRef]
- De Luca, G.; Schaffer, A.; Wirianta, J.; Suryapranata, H. Comprehensive Meta-Analysis of Radial vs Femoral Approach in Primary Angioplasty for STEMI. Int. J. Cardiol. 2013, 168, 2070–2081. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef]
- GBD 2017 Causes of Death Collaborators Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef]
- Bangalore, S.; Maron, D.J.; Stone, G.W.; Hochman, J.S. Routine Revascularization Versus Initial Medical Therapy for Stable Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Circulation 2020, 142, 841–857. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Verdoia, M.; Cassetti, E.; Schaffer, A.; Cavallino, C.; Bolzani, V.; Marino, P. Novara Atherosclerosis Study Group (NAS) High Fibrinogen Level Is an Independent Predictor of Presence and Extent of Coronary Artery Disease among Italian Population. J. Thromb. Thrombolysis 2011, 31, 458–463. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Santagostino, M.; Secco, G.G.; Cassetti, E.; Giuliani, L.; Franchi, E.; Coppo, L.; Iorio, S.; Venegoni, L.; Rondano, E.; et al. Mean Platelet Volume and the Extent of Coronary Artery Disease: Results from a Large Prospective Study. Atherosclerosis 2009, 206, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.; Verdoia, M.; Cassetti, E.; Marino, P.; Suryapranata, H.; De Luca, G.; Novara Atherosclerosis Study Group (NAS). Relationship between Homocysteine and Coronary Artery Disease. Results from a Large Prospective Cohort Study. Thromb. Res. 2014, 134, 288–293. [Google Scholar] [CrossRef]
- Barbato, E.; Piscione, F.; Bartunek, J.; Galasso, G.; Cirillo, P.; De Luca, G.; Iaccarino, G.; De Bruyne, B.; Chiariello, M.; Wijns, W. Role of Beta2 Adrenergic Receptors in Human Atherosclerotic Coronary Arteries. Circulation 2005, 111, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.A.; Tyler, J.M.; Henry, T.D.; Garberich, R.; Sharkey, S.W.; Schmidt, C.W.; Henry, J.T.; Eckman, P.; Megaly, M.; Brilakis, E.S.; et al. Clinical Characteristics and Outcomes of STEMI Patients with Cardiogenic Shock and Cardiac Arrest. JACC Cardiovasc. Interv. 2020, 13, 1211–1219. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Shmilovich, H.; Cheng, V.Y.; Tamarappoo, B.K.; Dey, D.; Nakazato, R.; Gransar, H.; Thomson, L.E.J.; Hayes, S.W.; Friedman, J.D.; Germano, G.; et al. Vulnerable Plaque Features on Coronary CT Angiography as Markers of Inducible Regional Myocardial Hypoperfusion from Severe Coronary Artery Stenoses. Atherosclerosis 2011, 219, 588–595. [Google Scholar] [CrossRef]
- Vancraeynest, D.; Pasquet, A.; Roelants, V.; Gerber, B.L.; Vanoverschelde, J.-L.J. Imaging the Vulnerable Plaque. J. Am. Coll. Cardiol. 2011, 57, 1961–1979. [Google Scholar] [CrossRef]
- Goldstein, J.A.; Maini, B.; Dixon, S.R.; Brilakis, E.S.; Grines, C.L.; Rizik, D.G.; Powers, E.R.; Steinberg, D.H.; Shunk, K.A.; Weisz, G.; et al. Detection of Lipid-Core Plaques by Intracoronary near-Infrared Spectroscopy Identifies High Risk of Periprocedural Myocardial Infarction. Circ. Cardiovasc. Interv. 2011, 4, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; IJsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; Camaro, C.; et al. Thin-Cap Fibroatheroma Rather Than Any Lipid Plaques Increases the Risk of Cardiovascular Events in Diabetic Patients: Insights from the COMBINE OCT-FFR Trial. Circ. Cardiovasc. Interv. 2022, 15, e011728. [Google Scholar] [CrossRef] [PubMed]
- Kedhi, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Fabris, E.; IJsselmuiden, A.J.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; et al. Thin-Cap Fibroatheroma Predicts Clinical Events in Diabetic Patients with Normal Fractional Flow Reserve: The COMBINE OCT-FFR Trial. Eur. Heart J. 2021, 42, 4671–4679. [Google Scholar] [CrossRef] [PubMed]
- Higuma, T.; Soeda, T.; Abe, N.; Yamada, M.; Yokoyama, H.; Shibutani, S.; Vergallo, R.; Minami, Y.; Ong, D.S.; Lee, H.; et al. A Combined Optical Coherence Tomography and Intravascular Ultrasound Study on Plaque Rupture, Plaque Erosion, and Calcified Nodule in Patients With ST-Segment Elevation Myocardial Infarction: Incidence, Morphologic Characteristics, and Outcomes After Perc. JACC Cardiovasc. Interv. 2015, 8, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Uemura, S.; Souteyrand, G.; Virmani, R.; Motreff, P.; Di Vito, L.; Biondi-Zoccai, G.; Halperin, J.; Fuster, V.; Ozaki, Y.; et al. OCT-Based Diagnosis and Management of STEMI Associated with Intact Fibrous Cap. JACC Cardiovasc. Imaging 2013, 6, 283–287. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Mach, F.; Schönbeck, U.; Bonnefoy, J.Y.; Pober, J.S.; Libby, P. Activation of Monocyte/Macrophage Functions Related to Acute Atheroma Complication by Ligation of CD40: Induction of Collagenase, Stromelysin, and Tissue Factor. Circulation 1997, 96, 396–399. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef]
- Badimon, L.; Badimon, J.J.; Turitto, V.T.; Fuster, V. Role of von Willebrand Factor in Mediating Platelet-Vessel Wall Interaction at Low Shear Rate; the Importance of Perfusion Conditions. Blood 1989, 73, 961–967. [Google Scholar] [CrossRef]
- Stefanini, L.; Bergmeier, W. Negative Regulators of Platelet Activation and Adhesion. J. Thromb. Haemost. 2018, 16, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lee, W.S.; Mintz, G.S.; Hong, Y.J.; Lee, S.Y.; Kim, K.S.; Hahn, J.-Y.; Kumar, K.S.; Won, H.; Hyeon, S.H.; et al. Multimodality Intravascular Imaging Assessment of Plaque Erosion versus Plaque Rupture in Patients with Acute Coronary Syndrome. Korean Circ. J. 2016, 46, 499–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arbustini, E.; Dal Bello, B.; Morbini, P.; Burke, A.P.; Bocciarelli, M.; Specchia, G.; Virmani, R. Plaque Erosion Is a Major Substrate for Coronary Thrombosis in Acute Myocardial Infarction. Heart 1999, 82, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hatakeyama, K.; Yamashita, A.; Marutsuka, K.; Sumiyoshi, A.; Asada, Y. Proportion of Fibrin and Platelets Differs in Thrombi on Ruptured and Eroded Coronary Atherosclerotic Plaques in Humans. Heart 2005, 91, 526–530. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients with Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Yamamoto, E.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; Takano, M.; Niccoli, G.; Higuma, T.; et al. Clinical and Laboratory Predictors for Plaque Erosion in Patients With Acute Coronary Syndromes. J. Am. Heart Assoc. 2019, 8, e012322. [Google Scholar] [CrossRef]
- Niccoli, G.; Montone, R.A.; Cataneo, L.; Cosentino, N.; Gramegna, M.; Refaat, H.; Porto, I.; Burzotta, F.; Trani, C.; Leone, A.M.; et al. Morphological-Biohumoral Correlations in Acute Coronary Syndromes: Pathogenetic Implications. Int. J. Cardiol. 2014, 171, 463–466. [Google Scholar] [CrossRef]
- Kim, H.O.; Kim, C.-J.; Kurihara, O.; Thondapu, V.; Russo, M.; Yamamoto, E.; Sugiyama, T.; Fracassi, F.; Lee, H.; Yonetsu, T.; et al. Angiographic Features of Patients with Coronary Plaque Erosion. Int. J. Cardiol. 2019, 288, 12–16. [Google Scholar] [CrossRef]
- Yamamoto, E.; Thondapu, V.; Poon, E.; Sugiyama, T.; Fracassi, F.; Dijkstra, J.; Lee, H.; Ooi, A.; Barlis, P.; Jang, I.-K. Endothelial Shear Stress and Plaque Erosion: A Computational Fluid Dynamics and Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2019, 12, 374–375. [Google Scholar] [CrossRef]
- Vergallo, R.; Papafaklis, M.I.; Yonetsu, T.; Bourantas, C.V.; Andreou, I.; Wang, Z.; Fujimoto, J.G.; McNulty, I.; Lee, H.; Biasucci, L.M.; et al. Endothelial Shear Stress and Coronary Plaque Characteristics in Humans: Combined Frequency-Domain Optical Coherence Tomography and Computational Fluid Dynamics Study. Circ. Cardiovasc. Imaging 2014, 7, 905–911. [Google Scholar] [CrossRef]
- Fahed, A.C.; Jang, I.-K. Plaque Erosion and Acute Coronary Syndromes: Phenotype, Molecular Characteristics and Future Directions. Nat. Rev. Cardiol. 2021, 18, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.-K. Reassessing the Mechanisms of Acute Coronary Syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Rajavashisth, T.B.; Liao, J.K.; Galis, Z.S.; Tripathi, S.; Laufs, U.; Tripathi, J.; Chai, N.N.; Xu, X.P.; Jovinge, S.; Shah, P.K.; et al. Inflammatory Cytokines and Oxidized Low Density Lipoproteins Increase Endothelial Cell Expression of Membrane Type 1-Matrix Metalloproteinase. J. Biol. Chem. 1999, 274, 11924–11929. [Google Scholar] [CrossRef] [PubMed]
- Franck, G.; Mawson, T.; Sausen, G.; Salinas, M.; Masson, G.S.; Cole, A.; Beltrami-Moreira, M.; Chatzizisis, Y.; Quillard, T.; Tesmenitsky, Y.; et al. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ. Res. 2017, 121, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Quillard, T.; Araújo, H.A.; Franck, G.; Shvartz, E.; Sukhova, G.; Libby, P. TLR2 and Neutrophils Potentiate Endothelial Stress, Apoptosis and Detachment: Implications for Superficial Erosion. Eur. Heart J. 2015, 36, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1α and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef]
- Cooley, B.C.; Nevado, J.; Mellad, J.; Yang, D.; St Hilaire, C.; Negro, A.; Fang, F.; Chen, G.; San, H.; Walts, A.D.; et al. TGF-β Signaling Mediates Endothelial-to-Mesenchymal Transition (EndMT) during Vein Graft Remodeling. Sci. Transl. Med. 2014, 6, 227ra34. [Google Scholar] [CrossRef]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.-R.; D’Escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to Mesenchymal Transition Is Common in Atherosclerotic Lesions and Is Associated with Plaque Instability. Nat. Commun. 2016, 7, 11853. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Xu, Y.; Mintz, G.S.; Tam, A.; McPherson, J.A.; Iñiguez, A.; Fajadet, J.; Fahy, M.; Weisz, G.; De Bruyne, B.; Serruys, P.W.; et al. Prevalence, Distribution, Predictors, and Outcomes of Patients with Calcified Nodules in Native Coronary Arteries: A 3-Vessel Intravascular Ultrasound Analysis from Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPE. Circulation 2012, 126, 537–545. [Google Scholar] [CrossRef]
- Budoff, M.J.; Rader, D.J.; Reilly, M.P.; Mohler, E.R.; Lash, J.; Yang, W.; Rosen, L.; Glenn, M.; Teal, V.; Feldman, H.I.; et al. Relationship of Estimated GFR and Coronary Artery Calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2011, 58, 519–526. [Google Scholar] [CrossRef]
- Koukoulaki, M.; Papachristou, E.; Kalogeropoulou, C.; Papathanasiou, M.; Zampakis, P.; Vardoulaki, M.; Alexopoulos, D.; Goumenos, D.S. Increased Prevalence and Severity of Coronary Artery Calcification in Patients with Chronic Kidney Disease Stage III and IV. Nephron Extra 2012, 2, 192–204. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the Vulnerable Plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Tanaka, A.; Ozaki, Y.; Taruya, A.; Fukuda, S.; Taguchi, H.; Iwaguro, T.; Ueno, S.; Okumoto, Y.; Akasaka, T. Prevalence of Spontaneous Coronary Artery Dissection in Patients with Acute Coronary Syndrome. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.M.; Rihal, C.S.; et al. Clinical Features, Management, and Prognosis of Spontaneous Coronary Artery Dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Kurachi, Y. A New Insight into the Pathogenesis of Coronary Vasospasm. Circ. Res. 2006, 98, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-J.; Hu, P.; Hung, M.-Y. Coronary Artery Spasm: Review and Update. Int. J. Med. Sci. 2014, 11, 1161–1171. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Vokshi, I.; Bastiaenen, R.; Kubik, S.; Hill, S.; Schäufele, T.; Mahrholdt, H.; Kaski, J.C.; et al. Clinical Usefulness, Angiographic Characteristics, and Safety Evaluation of Intracoronary Acetylcholine Provocation Testing among 921 Consecutive White Patients with Unobstructed Coronary Arteries. Circulation 2014, 129, 1723–1730. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Sasayama, S.; Maseri, A. Racial Heterogeneity in Coronary Artery Vasomotor Reactivity: Differences between Japanese and Caucasian Patients. J. Am. Coll. Cardiol. 1999, 33, 1442–1452. [Google Scholar] [CrossRef]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A. Long-Term Follow-up of Patients with Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef]
- Radico, F.; Cicchitti, V.; Zimarino, M.; De Caterina, R. Angina Pectoris and Myocardial Ischemia in the Absence of Obstructive Coronary Artery Disease: Practical Considerations for Diagnostic Tests. JACC Cardiovasc. Interv. 2014, 7, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Dentali, F.; Castiglioni, L.; Maroni, L.; Marino, F.; Squizzato, A.; Ageno, W.; Gianni, M.; Gaudio, G.; Grandi, A.M.; et al. Neutrophils and Clinical Outcomes in Patients with Acute Coronary Syndromes and/or Cardiac Revascularisation. A Systematic Review on More than 34,000 Subjects. Thromb. Haemost. 2011, 106, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Hally, K.E.; Parker, O.M.; Brunton-O’Sullivan, M.M.; Harding, S.A.; Larsen, P.D. Linking Neutrophil Extracellular Traps and Platelet Activation: A Composite Biomarker Score for Predicting Outcomes after Acute Myocardial Infarction. Thromb. Haemost. 2021, 121, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Boudjeltia, K.Z.; Noyon, C.; Furtmüller, P.G.; Nuyens, V.; Slomianny, M.-C.; Madhoun, P.; Desmet, J.-M.; Raynal, P.; Dufour, D.; et al. Impact of Myeloperoxidase-LDL Interactions on Enzyme Activity and Subsequent Posttranslational Oxidative Modifications of ApoB-100. J. Lipid Res. 2014, 55, 747–757. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Takahashi, M. NLRP3 Inflammasome as a Key Driver of Vascular Disease. Cardiovasc. Res. 2022, 118, 372–385. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, D.; Sun, Y.; Suo, Y.; Yu, Q.; Zeng, M.; Gao, Q.; Yu, B.; Jiang, X.; Wang, Y. The Selective NLRP3 Inhibitor MCC950 Hinders Atherosclerosis Development by Attenuating Inflammation and Pyroptosis in Macrophages. Sci. Rep. 2021, 11, 19305. [Google Scholar] [CrossRef]
- Menu, P.; Pellegrin, M.; Aubert, J.-F.; Bouzourene, K.; Tardivel, A.; Mazzolai, L.; Tschopp, J. Atherosclerosis in ApoE-Deficient Mice Progresses Independently of the NLRP3 Inflammasome. Cell Death Dis. 2011, 2, e137. [Google Scholar] [CrossRef]
- Chen, S.; Markman, J.L.; Shimada, K.; Crother, T.R.; Lane, M.; Abolhesn, A.; Shah, P.K.; Arditi, M. Sex-Specific Effects of the Nlrp3 Inflammasome on Atherogenesis in LDL Receptor-Deficient Mice. JACC Basic Transl. Sci. 2020, 5, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J. Involvement of Neutrophils in the Pathogenesis of Lethal Myocardial Reperfusion Injury. Cardiovasc. Res. 2004, 61, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Fournier, B.M.; Parkos, C.A. The Role of Neutrophils during Intestinal Inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, C.J. The Role of Neutrophil Apoptosis in Influencing Tissue Repair. J. Wound Care 2003, 12, 13–16. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Horckmans, M.; Ring, L.; Duchene, J.; Santovito, D.; Schloss, M.J.; Drechsler, M.; Weber, C.; Soehnlein, O.; Steffens, S. Neutrophils Orchestrate Post-Myocardial Infarction Healing by Polarizing Macrophages towards a Reparative Phenotype. Eur. Heart J. 2017, 38, 187–197. [Google Scholar] [CrossRef]
- Marinković, G.; Koenis, D.S.; de Camp, L.; Jablonowski, R.; Graber, N.; de Waard, V.; de Vries, C.J.; Goncalves, I.; Nilsson, J.; Jovinge, S.; et al. S100A9 Links Inflammation and Repair in Myocardial Infarction. Circ. Res. 2020, 127, 664–676. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Morton, A.C.; Rothman, A.M.K.; Greenwood, J.P.; Gunn, J.; Chase, A.; Clarke, B.; Hall, A.S.; Fox, K.; Foley, C.; Banya, W.; et al. The Effect of Interleukin-1 Receptor Antagonist Therapy on Markers of Inflammation in Non-ST Elevation Acute Coronary Syndromes: The MRC-ILA Heart Study. Eur. Heart J. 2015, 36, 377–384. [Google Scholar] [CrossRef]
- Sreejit, G.; Johnson, J.; Jaggers, R.M.; Dahdah, A.; Murphy, A.J.; Hanssen, N.M.J.; Nagareddy, P.R. Neutrophils in Cardiovascular Disease: Warmongers, Peacemakers, or Both? Cardiovasc. Res. 2022, 118, 2596–2609. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Zhou, H.; Karmally, W.; Ramakrishnan, R.; Holleran, S.; Liu, Y.; Jumes, P.; Wagner, J.A.; Hubbard, B.; Previs, S.F.; et al. CETP (Cholesteryl Ester Transfer Protein) Inhibition with Anacetrapib Decreases Production of Lipoprotein(a) in Mildly Hypercholesterolemic Subjects. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- HPS3/TIMI55–REVEAL Collaborative Group. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ruotolo, G.; Brewer, H.B.; Wang, M.-D.; Liu, L.; Willey, M.B.; Deeg, M.A.; Krueger, K.A.; Nissen, S.E. Evacetrapib Alone or in Combination with Statins Lowers Lipoprotein(a) and Total and Small LDL Particle Concentrations in Mildly Hypercholesterolemic Patients. J. Clin. Lipidol. 2016, 10, 519–527.e4. [Google Scholar] [CrossRef] [PubMed]
- Leebmann, J.; Roeseler, E.; Julius, U.; Heigl, F.; Spitthoever, R.; Heutling, D.; Breitenberger, P.; Maerz, W.; Lehmacher, W.; Heibges, A.; et al. Lipoprotein Apheresis in Patients with Maximally Tolerated Lipid-Lowering Therapy, Lipoprotein(a)-Hyperlipoproteinemia, and Progressive Cardiovascular Disease: Prospective Observational Multicenter Study. Circulation 2013, 128, 2567–2576. [Google Scholar] [CrossRef]
- Ponda, M.P.; Dowd, K.; Finkielstein, D.; Holt, P.R.; Breslow, J.L. The Short-Term Effects of Vitamin D Repletion on Cholesterol: A Randomized, Placebo-Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2510–2515. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Braunwald, E.; White, H.D.; Lukas, M.A.; Tarka, E.; Steg, P.G.; Hochman, J.S.; Bode, C.; Maggioni, A.P.; Im, K.; et al. Effect of Darapladib on Major Coronary Events after an Acute Coronary Syndrome: The SOLID-TIMI 52 Randomized Clinical Trial. JAMA 2014, 312, 1006–1015. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Qian, S.; Gong, Y.; Ren, J.; Zhao, L.; Wang, D.; Wang, X.; Zhang, Y.; Wang, Z.; Zhang, Q. Deep Sequencing of the T Cell Receptor β Repertoire Reveals Signature Patterns and Clonal Drift in Atherosclerotic Plaques and Patients. Oncotarget 2017, 8, 99312–99322. [Google Scholar] [CrossRef]
- Wick, G.; Jakic, B.; Buszko, M.; Wick, M.C.; Grundtman, C. The Role of Heat Shock Proteins in Atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 516–529. [Google Scholar] [CrossRef]
- Li, J.; McArdle, S.; Gholami, A.; Kimura, T.; Wolf, D.; Gerhardt, T.; Miller, J.; Weber, C.; Ley, K. CCR5+T-Bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ. Res. 2016, 118, 1540–1552. [Google Scholar] [CrossRef]
- Maganto-García, E.; Tarrio, M.L.; Grabie, N.; Bu, D.; Lichtman, A.H. Dynamic Changes in Regulatory T Cells Are Linked to Levels of Diet-Induced Hypercholesterolemia. Circulation 2011, 124, 185–195. [Google Scholar] [CrossRef]
- Sage, A.P.; Nus, M.; Bagchi Chakraborty, J.; Tsiantoulas, D.; Newland, S.A.; Finigan, A.J.; Masters, L.; Binder, C.J.; Mallat, Z. X-Box Binding Protein-1 Dependent Plasma Cell Responses Limit the Development of Atherosclerosis. Circ. Res. 2017, 121, 270–281. [Google Scholar] [CrossRef]
- Mallat, Z.; Binder, C.J. The Why and How of Adaptive Immune Responses in Ischemic Cardiovascular Disease. Nat. Cardiovasc. Res. 2022, 1, 431–444. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T Cell Subsets and Functions in Atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Butcher, M.J.; Filipowicz, A.R.; Waseem, T.C.; McGary, C.M.; Crow, K.J.; Magilnick, N.; Boldin, M.; Lundberg, P.S.; Galkina, E.V. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNγ+ Th1/Tregs. Circ. Res. 2016, 119, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Gaddis, D.E.; Padgett, L.E.; Wu, R.; McSkimming, C.; Romines, V.; Taylor, A.M.; McNamara, C.A.; Kronenberg, M.; Crotty, S.; Thomas, M.J.; et al. Apolipoprotein AI Prevents Regulatory to Follicular Helper T Cell Switching during Atherosclerosis. Nat. Commun. 2018, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, H.; Liu, J.; Pang, Y.; Liu, Q. Advances in Immunotherapy Modalities for Atherosclerosis. Front. Pharmacol. 2023, 13, 5531. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Diehl, C.J.; Witztum, J.L.; Binder, C.J. B Cells and Humoral Immunity in Atherosclerosis. Circ. Res. 2014, 114, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.; Liu, Y.-H.; Kanellakis, P.; Kallies, A.; Li, Y.; Cao, A.; Hosseini, H.; Tipping, P.; Toh, B.-H.; Bobik, A.; et al. Follicular B Cells Promote Atherosclerosis via T Cell-Mediated Differentiation Into Plasma Cells and Secreting Pathogenic Immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e71–e84. [Google Scholar] [CrossRef]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernández-Alfonso, M.S. Regional Differences in Perivascular Adipose Tissue Impacting Vascular Homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Withers, S.B.; Forman, R.; Meza-Perez, S.; Sorobetea, D.; Sitnik, K.; Hopwood, T.; Lawrence, C.B.; Agace, W.W.; Else, K.J.; Heagerty, A.M.; et al. Eosinophils Are Key Regulators of Perivascular Adipose Tissue and Vascular Functionality. Sci. Rep. 2017, 7, 44571. [Google Scholar] [CrossRef]
- Szasz, T.; Webb, R.C. Perivascular Adipose Tissue: More than Just Structural Support. Clin. Sci. 2012, 122, 1–12. [Google Scholar] [CrossRef]
- Kauser, K.; da Cunha, V.; Fitch, R.; Mallari, C.; Rubanyi, G.M. Role of Endogenous Nitric Oxide in Progression of Atherosclerosis in Apolipoprotein E-Deficient Mice. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1679–H1685. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, X.; Villacorta, L.; Rom, O.; Garcia-Barrio, M.T.; Guo, Y.; Fan, Y.; Zhu, T.; Zhang, J.; Zeng, R.; et al. Brown Adipocyte-Specific PPARγ (Peroxisome Proliferator-Activated Receptor γ) Deletion Impairs Perivascular Adipose Tissue Development and Enhances Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1738–1747. [Google Scholar] [CrossRef]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular Adipose Tissue (PVAT) in Atherosclerosis: A Double-Edged Sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alfonso, M.S.; Gil-Ortega, M.; García-Prieto, C.F.; Aranguez, I.; Ruiz-Gayo, M.; Somoza, B. Mechanisms of Perivascular Adipose Tissue Dysfunction in Obesity. Int. J. Endocrinol. 2013, 2013, 402053. [Google Scholar] [CrossRef] [PubMed]
- BERNE, R.M. Cardiac Nucleotides in Hypoxia: Possible Role in Regulation of Coronary Blood Flow. Am. J. Physiol. 1963, 204, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sands, W.A.; Palmer, T.M. Adenosine Receptors and the Control of Endothelial Cell Function in Inflammatory Disease. Immunol. Lett. 2005, 101, 1–11. [Google Scholar] [CrossRef]
- Burnstock, G.; Pelleg, A. Cardiac Purinergic Signalling in Health and Disease. Purinergic Signal. 2015, 11, 1–46. [Google Scholar] [CrossRef]
- By, Y.; Jacquin, L.; Franceschi, F.; Durand-Gorde, J.-M.; Condo, J.; Michelet, P.; Guieu, R.; Ruf, J. Fall in Oxygen Tension of Culture Medium Stimulates the Adenosinergic Signalling of a Human T Cell Line. Purinergic Signal. 2012, 8, 661–667. [Google Scholar] [CrossRef]
- Bruzzese, L.; Fromonot, J.; By, Y.; Durand-Gorde, J.-M.; Condo, J.; Kipson, N.; Guieu, R.; Fenouillet, E.; Ruf, J. NF-ΚB Enhances Hypoxia-Driven T-Cell Immunosuppression via Upregulation of Adenosine A(2A) Receptors. Cell. Signal. 2014, 26, 1060–1067. [Google Scholar] [CrossRef]
- Paul, S.; Feoktistov, I.; Hollister, A.S.; Robertson, D.; Biaggioni, I. Adenosine Inhibits the Rise in Intracellular Calcium and Platelet Aggregation Produced by Thrombin: Evidence That Both Effects Are Coupled to Adenylate Cyclase. Mol. Pharmacol. 1990, 37, 870–875. [Google Scholar]
- Armstrong, D.; Summers, C.; Ewart, L.; Nylander, S.; Sidaway, J.E.; van Giezen, J.J.J. Characterization of the Adenosine Pharmacology of Ticagrelor Reveals Therapeutically Relevant Inhibition of Equilibrative Nucleoside Transporter 1. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 209–219. [Google Scholar] [CrossRef]
- Nardin, M.; Verdoia, M.; Pergolini, P.; Rolla, R.; Barbieri, L.; Marino, P.; Bellomo, G.; Kedhi, E.; Suryapranata, H.; Carriero, A.; et al. Impact of Adenosine A2a Receptor Polymorphism Rs5751876 on Platelet Reactivity in Ticagrelor Treated Patients. Pharmacol. Res. 2018, 129, 27–33. [Google Scholar] [CrossRef]
- De Luca, G.; Venegoni, L.; Iorio, S.; Giuliani, L.; Marino, P. Effects of Increasing Doses of Intracoronary Adenosine on the Assessment of Fractional Flow Reserve. JACC Cardiovasc. Interv. 2011, 4, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Nardin, M.; Verdoia, M.; Negro, F.; Suryapranata, H.; Khedi, E.; De Luca, G. Relationship between Adenosine A2a Receptor Polymorphism Rs5751876 and Fractional Flow Reserve during Percutaneous Coronary Intervention. Heart Vessel. 2020, 35, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Maranhão, R.C.; Carvalho, P.O.; Strunz, C.C.; Pileggi, F. Lipoprotein(a): Structure, Pathophysiology and Clinical Implications. Arq. Bras. Cardiol. 2014, 103, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Takamura, M.; Kawashiri, M.-A. Lipoprotein(a) as an Old and New Causal Risk Factor of Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2019, 26, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Çaku, A.; McQueen, M.; Anand, S.S.; Enas, E.; Clarke, R.; Boffa, M.B.; Koschinsky, M.; Wang, X.; Yusuf, S.; et al. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation 2019, 139, 1472–1482. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjaerg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef]
- Swerdlow, D.I.; Rider, D.A.; Yavari, A.; Wikström Lindholm, M.; Campion, G.V.; Nissen, S.E. Treatment and Prevention of Lipoprotein(a)-Mediated Cardiovascular Disease: The Emerging Potential of RNA Interference Therapeutics. Cardiovasc. Res. 2022, 118, 1218–1231. [Google Scholar] [CrossRef]
- Verdoia, M.; Gioscia, R.; Nardin, M.; Rognoni, A.; De Luca, G. Low Levels of Vitamin D and Coronary Artery Disease: Is It Time for Therapy? Kardiol. Pol. 2022, 80, 409–416. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E. The Activating Enzymes of Vitamin D Metabolism (25- and 1α-Hydroxylases). In Vitamin D; Elsevier: Amsterdam, The Netherlands, 2011; pp. 23–42. [Google Scholar]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Sartori, C.; Nardin, M.; Schaffer, A.; Barbieri, L.; Daffara, V.; Marino, P.; Bellomo, G.; et al. Vitamin D Levels and High-Residual Platelet Reactivity in Patients Receiving Dual Antiplatelet Therapy with Clopidogrel or Ticagrelor. Platelets 2016, 27, 576–582. [Google Scholar] [CrossRef]
- Verdoia, M.; Nardin, M.; Rolla, R.; Negro, F.; Gioscia, R.; Saghir Afifeh, A.M.; Viglione, F.; Suryapranata, H.; Marcolongo, M.; De Luca, G.; et al. Cholecalciferol Levels, Inflammation and Leukocytes Parameters: Results from a Large Single-Centre Cohort of Patients. Clin. Nutr. 2021, 40, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and Cardiovascular Health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Saghir Afifeh, A.M.; Verdoia, M.; Nardin, M.; Negro, F.; Viglione, F.; Rolla, R.; De Luca, G. Novara Atherosclerosis Study Group (NAS) Determinants of Vitamin D Activation in Patients with Acute Coronary Syndromes and Its Correlation with Inflammatory Markers. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Viglione, F.; Boggio, A.; Stefani, D.; Panarotto, N.; Malabaila, A.; Rolla, R.; Soldà, P.L.; Stecco, A.; Carriero, A.; et al. Relationship between Vitamin D and Cholesterol Levels in STEMI Patients Undergoing Primary Percutaneous Coronary Intervention. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 957–964. [Google Scholar] [CrossRef]
- De Maré, A.; Maudsley, S.; Azmi, A.; Hendrickx, J.O.; Opdebeeck, B.; Neven, E.; D’Haese, P.C.; Verhulst, A. Sclerostin as Regulatory Molecule in Vascular Media Calcification and the Bone-Vascular Axis. Toxins 2019, 11, 428. [Google Scholar] [CrossRef]
- Fadini, G.P.; Rattazzi, M.; Matsumoto, T.; Asahara, T.; Khosla, S. Emerging Role of Circulating Calcifying Cells in the Bone-Vascular Axis. Circulation 2012, 125, 2772–2781. [Google Scholar] [CrossRef]
- Thompson, B.; Towler, D.A. Arterial Calcification and Bone Physiology: Role of the Bone-Vascular Axis. Nat. Rev. Endocrinol. 2012, 8, 529–543. [Google Scholar] [CrossRef]
- Jing, L.; Li, L.; Sun, Z.; Bao, Z.; Shao, C.; Yan, J.; Pang, Q.; Geng, Y.; Zhang, L.; Wang, X.; et al. Role of Matrix Vesicles in Bone-Vascular Cross-Talk. J. Cardiovasc. Pharmacol. 2019, 74, 372–378. [Google Scholar] [CrossRef]
- Vassalle, C.; Mazzone, A. Bone Loss and Vascular Calcification: A Bi-Directional Interplay? Vascul. Pharmacol. 2016, 86, 77–86. [Google Scholar] [CrossRef]
- Persy, V.; D’Haese, P. Vascular Calcification and Bone Disease: The Calcification Paradox. Trends Mol. Med. 2009, 15, 405–416. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamashita, M.; Horimai, C.; Hayashi, M. Smooth Muscle-Selective Nuclear Factor-ΚB Inhibition Reduces Phosphate-Induced Arterial Medial Calcification in Mice With Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e007248. [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.B.; Mayrhofer, T.; Park, J.; Lu, M.T.; Liu, T.; Maurovich-Horvat, P.; Ghemigian, K.; Bittner, D.O.; Fleg, J.L.; Udelson, J.E.; et al. Differences in the Association of Total versus Local Coronary Artery Calcium with Acute Coronary Syndrome and Culprit Lesions in Patients with Acute Chest Pain: The Coronary Calcium Paradox. Atherosclerosis 2018, 274, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kudo, I. Phospholipase A2. J. Biochem. 2002, 131, 285–292. [Google Scholar] [CrossRef]
- Six, D.A.; Dennis, E.A. The Expanding Superfamily of Phospholipase A2 Enzymes: Classification and Characterization. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1488, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tellis, C.C.; Tselepis, A.D. Pathophysiological Role and Clinical Significance of Lipoprotein-Associated Phospholipase A₂ (Lp-PLA2) Bound to LDL and HDL. Curr. Pharm. Des. 2014, 20, 6256–6269. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A 2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J.; O’Reilly, D.S.; Caslake, M.J.; McMahon, A.D.; Ford, I.; Cooney, J.; Macphee, C.H.; Suckling, K.E.; Krishna, M.; Wilkinson, F.E.; et al. Lipoprotein-Associated Phospholipase A2 as an Independent Predictor of Coronary Heart Disease. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 2000, 343, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration. Lipid-Related Markers and Cardiovascular Disease Prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef]
- Lp-PLA(2) Studies Collaboration. Lipoprotein-Associated Phospholipase A(2) and Risk of Coronary Disease, Stroke, and Mortality: Collaborative Analysis of 32 Prospective Studies. Lancet 2010, 375, 1536–1544. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Cao, C.; Zhang, W.; Liu, G.; Zhang, J. Amelioration of Atherosclerosis in Apolipoprotein E-Deficient Mice by Combined RNA Interference of Lipoprotein-Associated Phospholipase A2 and YKL-40. PLoS ONE 2018, 13, e0202797. [Google Scholar] [CrossRef]
- STABILITY Investigators. Darapladib for Preventing Ischemic Events in Stable Coronary Heart Disease. N. Engl. J. Med. 2014, 370, 1702–1711. [Google Scholar] [CrossRef]
- Huang, F.; Wang, K.; Shen, J. Lipoprotein-associated Phospholipase A2: The Story Continues. Med. Res. Rev. 2020, 40, 79–134. [Google Scholar] [CrossRef]
- Wallentin, L.; Held, C.; Armstrong, P.W.; Cannon, C.P.; Davies, R.Y.; Granger, C.B.; Hagström, E.; Harrington, R.A.; Hochman, J.S.; Koenig, W.; et al. Lipoprotein-Associated Phospholipase A2 Activity Is a Marker of Risk But Not a Useful Target for Treatment in Patients With Stable Coronary Heart Disease. J. Am. Heart Assoc. 2016, 5, e003407. [Google Scholar] [CrossRef] [PubMed]
- Navickas, R.; Gal, D.; Laucevičius, A.; Taparauskaitė, A.; Zdanytė, M.; Holvoet, P. Identifying Circulating MicroRNAs as Biomarkers of Cardiovascular Disease: A Systematic Review. Cardiovasc. Res. 2016, 111, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Karakas, M.; Zeller, T. MicroRNAs in Cardiovascular Disease—Clinical Application. Clin. Chem. Lab. Med. 2017, 55, 687–704. [Google Scholar] [CrossRef]
- Ahlin, F.; Arfvidsson, J.; Vargas, K.G.; Stojkovic, S.; Huber, K.; Wojta, J. MicroRNAs as Circulating Biomarkers in Acute Coronary Syndromes: A Review. Vascul. Pharmacol. 2016, 81, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, T.; Liu, L.; Zou, J.; Zhang, X.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. Increased Expression of MicroRNA-146a Decreases Myocardial Ischaemia/Reperfusion Injury. Cardiovasc. Res. 2013, 97, 432–442. [Google Scholar] [CrossRef]
- Oerlemans, M.I.F.J.; Mosterd, A.; Dekker, M.S.; de Vrey, E.A.; van Mil, A.; Pasterkamp, G.; Doevendans, P.A.; Hoes, A.W.; Sluijter, J.P.G. Early Assessment of Acute Coronary Syndromes in the Emergency Department: The Potential Diagnostic Value of Circulating MicroRNAs. EMBO Mol. Med. 2012, 4, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Bei, Y.; Kong, X.; Liu, X.; Lei, Z.; Xu, T.; Wang, H.; Xuan, Q.; Chen, P.; Xu, J.; et al. MiR-17-3p Contributes to Exercise-Induced Cardiac Growth and Protects against Myocardial Ischemia-Reperfusion Injury. Theranostics 2017, 7, 664–676. [Google Scholar] [CrossRef]
- Pankratz, F.; Hohnloser, C.; Bemtgen, X.; Jaenich, C.; Kreuzaler, S.; Hoefer, I.; Pasterkamp, G.; Mastroianni, J.; Zeiser, R.; Smolka, C.; et al. MicroRNA-100 Suppresses Chronic Vascular Inflammation by Stimulation of Endothelial Autophagy. Circ. Res. 2018, 122, 417–432. [Google Scholar] [CrossRef]
- Di Gregoli, K.; Mohamad Anuar, N.N.; Bianco, R.; White, S.J.; Newby, A.C.; George, S.J.; Johnson, J.L. MicroRNA-181b Controls Atherosclerosis and Aneurysms Through Regulation of TIMP-3 and Elastin. Circ. Res. 2017, 120, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yao, K.; Wang, Q.; Guo, J.; Shi, H.; Ma, L.; Liu, H.; Gao, W.; Zou, Y.; Ge, J. Circulating MiR-181a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. Cell. Physiol. Biochem. 2016, 40, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Keller, A.; Frese, K.S.; Kloos, W.; Kayvanpour, E.; Sedaghat-Hamedani, F.; Hassel, S.; Marquart, S.; Beier, M.; Giannitis, E.; et al. Refining Diagnostic MicroRNA Signatures by Whole-MiRNome Kinetic Analysis in Acute Myocardial Infarction. Clin. Chem. 2013, 59, 410–418. [Google Scholar] [CrossRef]
- Devaux, Y.; Mueller, M.; Haaf, P.; Goretti, E.; Twerenbold, R.; Zangrando, J.; Vausort, M.; Reichlin, T.; Wildi, K.; Moehring, B.; et al. Diagnostic and Prognostic Value of Circulating MicroRNAs in Patients with Acute Chest Pain. J. Intern. Med. 2015, 277, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fang, Z.; Jiang, T.; Zhang, Q.; Liu, C.; Zhang, C.; Xiang, Y. Serum MicroRNAs Profile from Genome-Wide Serves as a Fingerprint for Diagnosis of Acute Myocardial Infarction and Angina Pectoris. BMC Med. Genomics 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, J.; Li, R.; Shi, J.; Sun, J.; Gu, R.; Ding, L.; Wang, L.; Xu, B. Overexpression of MicroRNA-99a Attenuates Heart Remodelling and Improves Cardiac Performance after Myocardial Infarction. J. Cell. Mol. Med. 2014, 18, 919–928. [Google Scholar] [CrossRef]
- Lesizza, P.; Prosdocimo, G.; Martinelli, V.; Sinagra, G.; Zacchigna, S.; Giacca, M. Single-Dose Intracardiac Injection of Pro-Regenerative MicroRNAs Improves Cardiac Function After Myocardial Infarction. Circ. Res. 2017, 120, 1298–1304. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Zhang, M.-F.; Wen, H.-Y.; Hu, C.-L.; Liu, R.; Wei, H.-Y.; Ai, C.-M.; Wang, G.; Liao, X.-X.; Li, X. Comparing the Diagnostic Values of Circulating MicroRNAs and Cardiac Troponin T in Patients with Acute Myocardial Infarction. Clinics 2013, 68, 75–80. [Google Scholar] [CrossRef]
- Martinez, E.C.; Lilyanna, S.; Wang, P.; Vardy, L.A.; Jiang, X.; Armugam, A.; Jeyaseelan, K.; Richards, A.M. MicroRNA-31 Promotes Adverse Cardiac Remodeling and Dysfunction in Ischemic Heart Disease. J. Mol. Cell. Cardiol. 2017, 112, 27–39. [Google Scholar] [CrossRef]
- Tao, L.; Bei, Y.; Chen, P.; Lei, Z.; Fu, S.; Zhang, H.; Xu, J.; Che, L.; Chen, X.; Sluijter, J.P.; et al. Crucial Role of MiR-433 in Regulating Cardiac Fibrosis. Theranostics 2016, 6, 2068–2083. [Google Scholar] [CrossRef]
- Ganesan, J.; Ramanujam, D.; Sassi, Y.; Ahles, A.; Jentzsch, C.; Werfel, S.; Leierseder, S.; Loyer, X.; Giacca, M.; Zentilin, L.; et al. MiR-378 Controls Cardiac Hypertrophy by Combined Repression of Mitogen-Activated Protein Kinase Pathway Factors. Circulation 2013, 127, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 Suppresses Myocardial Fibrosis through a Paracrine Mechanism at the Early Stage of Cardiac Hypertrophy Following Mechanical Stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Huang, K. Inhibition of the LncRNA Mirt1 Attenuates Acute Myocardial Infarction by Suppressing NF-ΚB Activation. Cell. Physiol. Biochem. 2017, 42, 1153–1164. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Xu, Y.; Liu, Y.; Li, Z.; Xue, X.; Wan, S.; Wang, H. Long Noncoding RNA Upregulated in Hypothermia Treated Cardiomyocytes Protects against Myocardial Infarction through Improving Mitochondrial Function. Int. J. Cardiol. 2018, 266, 213–217. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Wang, H.; Li, M.; Huang, S.; Chen, G.; Jing, Y.; Wang, S.; Chen, Y.; Liao, W.; et al. Loss of AZIN2 Splice Variant Facilitates Endogenous Cardiac Regeneration. Cardiovasc. Res. 2018, 114, 1642–1655. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, Z.-A.; Liu, J.; Hao, K.; Yu, Y.; Han, X.; Li, J.; Wang, Y.; Lei, W.; Dong, N.; et al. Long Noncoding RNA Meg3 Regulates Cardiomyocyte Apoptosis in Myocardial Infarction. Gene Ther. 2018, 25, 511–523. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Guo, S.; Yao, R.; Wu, L.; Xiao, L.; Wang, Z.; Liu, Y.; Zhang, Y. Circulating Long Noncoding RNA HOTAIR Is an Essential Mediator of Acute Myocardial Infarction. Cell. Physiol. Biochem. 2017, 44, 1497–1508. [Google Scholar] [CrossRef]
- Woo, C.J.; Kingston, R.E. HOTAIR Lifts Noncoding RNAs to New Levels. Cell 2007, 129, 1257–1259. [Google Scholar] [CrossRef]
- Vausort, M.; Wagner, D.R.; Devaux, Y. Long Noncoding RNAs in Patients with Acute Myocardial Infarction. Circ. Res. 2014, 115, 668–677. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, C.; Meng, M.; Tang, H. Long Noncoding RNA MHRT Protects Cardiomyocytes against H2O2-Induced Apoptosis. Biomol. Ther. 2016, 24, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, B.; Liu, N.; Qi, C.; Xiao, Y.; Tian, X.; Li, T.; Liu, B. Circulating Long Noncoding RNA UCA1 as a Novel Biomarker of Acute Myocardial Infarction. BioMed Res. Int. 2016, 2016, 8079372. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.-H.; Li, R.; Su, Y.-M.; Xiao, J.; Pan, M.; Cai, X.-X.; Ji, X.-P. The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of MiR-7a on Its Target Genes Expression. PLoS ONE 2016, 11, e0151753. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated CircRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Qi, B.; Wu, X.; Peng, S.; Zhou, G.; Wei, Y.; Xu, J.; Chen, S.; Liu, S. Circular RNA Ttc3 Regulates Cardiac Function after Myocardial Infarction by Sponging MiR-15b. J. Mol. Cell. Cardiol. 2019, 130, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, T.-Y.; Li, N.; Liu, C.-Y.; Zhou, L.-Y.; Gao, J.-N.; Chen, C.; Yan, K.-W.; Ponnusamy, M.; Zhang, Y.-H.; et al. Circular RNA Mediates Cardiomyocyte Death via MiRNA-Dependent Upregulation of MTP18 Expression. Cell Death Differ. 2017, 24, 1111–1120. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Zhai, M.; Huang, Y.; Xu, S.; An, T.; Wang, Y.-H.; Zhang, R.-C.; Liu, C.-Y.; Dong, Y.-H.; Wang, M.; et al. The Circular RNA ACR Attenuates Myocardial Ischemia/Reperfusion Injury by Suppressing Autophagy via Modulation of the Pink1/ FAM65B Pathway. Cell Death Differ. 2019, 26, 1299–1315. [Google Scholar] [CrossRef]
- Deng, S.; Wang, H.; Jia, C.; Zhu, S.; Chu, X.; Ma, Q.; Wei, J.; Chen, E.; Zhu, W.; Macon, C.J.; et al. MicroRNA-146a Induces Lineage-Negative Bone Marrow Cell Apoptosis and Senescence by Targeting Polo-Like Kinase 2 Expression. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 280–290. [Google Scholar] [CrossRef]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Vasa-Nicotera, M.; Ippoliti, A.; Novelli, G.; Melino, G.; et al. MicroRNA 217 Modulates Endothelial Cell Senescence via Silent Information Regulator 1. Circulation 2009, 120, 1524–1532. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of Flow-Sensitive MicroRNAs in Endothelial Dysfunction and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef]
- Holmberg, J.; Bhattachariya, A.; Alajbegovic, A.; Rippe, C.; Ekman, M.; Dahan, D.; Hien, T.T.; Boettger, T.; Braun, T.; Swärd, K.; et al. Loss of Vascular Myogenic Tone in MiR-143/145 Knockout Mice Is Associated With Hypertension-Induced Vascular Lesions in Small Mesenteric Arteries. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Zeiher, A.M. Circulating MicroRNAs: Novel Biomarkers for Cardiovascular Diseases? Eur. Heart J. 2010, 31, 2705–2707. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-K.; Zhu, J.-Q.; Zhang, J.-T.; Li, Q.; Li, Y.; He, J.; Qin, Y.-W.; Jing, Q. Circulating MicroRNA: A Novel Potential Biomarker for Early Diagnosis of Acute Myocardial Infarction in Humans. Eur. Heart J. 2010, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, Q.; You, W.; Chen, M.; Xia, J. MiRNAs as Biomarkers of Myocardial Infarction: A Meta-Analysis. PLoS ONE 2014, 9, e88566. [Google Scholar] [CrossRef]
- Barraclough, J.Y.; Joglekar, M.V.; Januszewski, A.S.; Martínez, G.; Celermajer, D.S.; Keech, A.C.; Hardikar, A.A.; Patel, S. A MicroRNA Signature in Acute Coronary Syndrome Patients and Modulation by Colchicine. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 444–455. [Google Scholar] [CrossRef]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Röxe, T.; Müller-Ardogan, M.; et al. Circulating MicroRNAs in Patients With Coronary Artery Disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef]
- Farina, N.H.; Wood, M.E.; Perrapato, S.D.; Francklyn, C.S.; Stein, G.S.; Stein, J.L.; Lian, J.B. Standardizing Analysis of Circulating MicroRNA: Clinical and Biological Relevance. J. Cell. Biochem. 2014, 115, 805–811. [Google Scholar] [CrossRef]
- Kaur, A.; Mackin, S.T.; Schlosser, K.; Wong, F.L.; Elharram, M.; Delles, C.; Stewart, D.J.; Dayan, N.; Landry, T.; Pilote, L. Systematic Review of MicroRNA Biomarkers in Acute Coronary Syndrome and Stable Coronary Artery Disease. Cardiovasc. Res. 2020, 116, 1113–1124. [Google Scholar] [CrossRef]
- Ouyang, T.; Liu, Z.; Han, Z.; Ge, Q. MicroRNA Detection Specificity: Recent Advances and Future Perspective. Anal. Chem. 2019, 91, 3179–3186. [Google Scholar] [CrossRef]
- Bär, C.; Chatterjee, S.; Thum, T. Long Noncoding RNAs in Cardiovascular Pathology, Diagnosis, and Therapy. Circulation 2016, 134, 1484–1499. [Google Scholar] [CrossRef]
- Yang, K.-C.; Yamada, K.A.; Patel, A.Y.; Topkara, V.K.; George, I.; Cheema, F.H.; Ewald, G.A.; Mann, D.L.; Nerbonne, J.M. Deep RNA Sequencing Reveals Dynamic Regulation of Myocardial Noncoding RNAs in Failing Human Heart and Remodeling with Mechanical Circulatory Support. Circulation 2014, 129, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Ounzain, S.; Micheletti, R.; Beckmann, T.; Schroen, B.; Alexanian, M.; Pezzuto, I.; Crippa, S.; Nemir, M.; Sarre, A.; Johnson, R.; et al. Genome-Wide Profiling of the Cardiac Transcriptome after Myocardial Infarction Identifies Novel Heart-Specific Long Non-Coding RNAs. Eur. Heart J. 2015, 36, 353–368a. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Gao, C.; Peng, G.; Greer, C.; Ren, S.; Wang, Y.; Xiao, X. Analysis of Transcriptome Complexity through RNA Sequencing in Normal and Failing Murine Hearts. Circ. Res. 2011, 109, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Halonen, P.; Liu, O.H.-F.; Turunen, T.A.; Pajula, J.; Moreau, P.; Selvarajan, I.; Tuomainen, T.; Aavik, E.; Tavi, P.; et al. Genome-Wide Dynamics of Nascent Noncoding RNA Transcription in Porcine Heart After Myocardial Infarction. Circ. Cardiovasc. Genet. 2017, 10, e001702. [Google Scholar] [CrossRef]
- Fiedler, J.; Breckwoldt, K.; Remmele, C.W.; Hartmann, D.; Dittrich, M.; Pfanne, A.; Just, A.; Xiao, K.; Kunz, M.; Müller, T.; et al. Development of Long Noncoding RNA-Based Strategies to Modulate Tissue Vascularization. J. Am. Coll. Cardiol. 2015, 66, 2005–2015. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients with Heart Failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef]
- Li, L.; Cong, Y.; Gao, X.; Wang, Y.; Lin, P. Differential Expression Profiles of Long Non-Coding RNAs as Potential Biomarkers for the Early Diagnosis of Acute Myocardial Infarction. Oncotarget 2017, 8, 88613–88621. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Wu, S.; Xue, M.; Chen, W. Long Non-Coding RNA UCA1 Promotes Glycolysis by Upregulating Hexokinase 2 through the MTOR-STAT3/MicroRNA143 Pathway. Cancer Sci. 2014, 105, 951–955. [Google Scholar] [CrossRef]
- Sayed, A.S.M.; Xia, K.; Yang, T.-L.; Peng, J. Circulating MicroRNAs: A Potential Role in Diagnosis and Prognosis of Acute Myocardial Infarction. Dis. Markers 2013, 35, 561–566. [Google Scholar] [CrossRef]
- Starke, S.; Jost, I.; Rossbach, O.; Schneider, T.; Schreiner, S.; Hung, L.-H.; Bindereif, A. Exon Circularization Requires Canonical Splice Signals. Cell Rep. 2015, 10, 103–111. [Google Scholar] [CrossRef]
- Aufiero, S.; van den Hoogenhof, M.M.G.; Reckman, Y.J.; Beqqali, A.; van der Made, I.; Kluin, J.; Khan, M.A.F.; Pinto, Y.M.; Creemers, E.E. Cardiac CircRNAs Arise Mainly from Constitutive Exons Rather than Alternatively Spliced Exons. RNA 2018, 24, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs Are Abundant, Conserved, and Associated with ALU Repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient MicroRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Wiklund, E.D.; Bramsen, J.B.; Villadsen, S.B.; Statham, A.L.; Clark, S.J.; Kjems, J. MiRNA-Dependent Gene Silencing Involving Ago2-Mediated Cleavage of a Circular Antisense RNA. EMBO J. 2011, 30, 4414–4422. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-O.; Chen, T.; Xiang, J.-F.; Yin, Q.-F.; Xing, Y.-H.; Zhu, S.; Yang, L.; Chen, L.-L. Circular Intronic Long Noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.-M.; Meitinger, T.; Engelhardt, S. Characterization of Circular RNAs in Human, Mouse and Rat Hearts. J. Mol. Cell. Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef]
- Tan, W.L.W.; Lim, B.T.S.; Anene-Nzelu, C.G.O.; Ackers-Johnson, M.; Dashi, A.; See, K.; Tiang, Z.; Lee, D.P.; Chua, W.W.; Luu, T.D.A.; et al. A Landscape of Circular RNA Expression in the Human Heart. Cardiovasc. Res. 2017, 113, 298–309. [Google Scholar] [CrossRef]
- Zhou, J.; Li, L.; Hu, H.; Wu, J.; Chen, H.; Feng, K.; Ma, L. Circ-HIPK2 Accelerates Cell Apoptosis and Autophagy in Myocardial Oxidative Injury by Sponging MiR-485-5p and Targeting ATG101. J. Cardiovasc. Pharmacol. 2020, 76, 427–436. [Google Scholar] [CrossRef]
- Cui, X.; Dong, Y.; Li, M.; Wang, X.; Jiang, M.; Yang, W.; Liu, G.; Sun, S.; Xu, W. A Circular RNA from NFIX Facilitates Oxidative Stress-Induced H9c2 Cells Apoptosis. In Vitro Cell. Dev. Biol.-Anim. 2020, 56, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ding, W.; Tariq, M.A.; Chang, W.; Zhang, X.; Xu, W.; Hou, L.; Wang, Y.; Wang, J. A Circular Transcript of Ncx1 Gene Mediates Ischemic Myocardial Injury by Targeting MiR-133a-3p. Theranostics 2018, 8, 5855–5869. [Google Scholar] [CrossRef]
- Si, X.; Zheng, H.; Wei, G.; Li, M.; Li, W.; Wang, H.; Guo, H.; Sun, J.; Li, C.; Zhong, S.; et al. CircRNA Hipk3 Induces Cardiac Regeneration after Myocardial Infarction in Mice by Binding to Notch1 and MiR-133a. Mol. Ther.-Nucleic Acids 2020, 21, 636–655. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b Modulates Cardiac Repair after Myocardial Infarction via FUS/VEGF-A Axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-J.; Xin, H.; Wang, Y.-C.; Liu, H.-W.; Gao, Y.-Y.; Zhang, Y.-F. Emerging Roles of CircRNAs in the Pathological Process of Myocardial Infarction. Mol. Ther.-Nucleic Acids 2021, 26, 828–848. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA Interference: Biology, Mechanism, and Applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.-J.; Binder, C.J.; Hörkkö, S.; Krauss, R.M.; Chapman, M.J.; et al. A Novel Function of Lipoprotein [a] as a Preferential Carrier of Oxidized Phospholipids in Human Plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef]
- Que, X.; Hung, M.-Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized Phospholipids Are Proinflammatory and Proatherogenic in Hypercholesterolaemic Mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; et al. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Giugliano, R.P.; Wiviott, S.D.; Atar, D.; Keech, A.; Kuder, J.F.; Im, K.; Murphy, S.A.; Flores-Arredondo, J.H.; López, J.A.G.; et al. Long-Term Evolocumab in Patients With Established Atherosclerotic Cardiovascular Disease. Circulation 2022, 146, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.S.; Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Friedman, A.; et al. Pooled Patient-Level Analysis of Inclisiran Trials in Patients With Familial Hypercholesterolemia or Atherosclerosis. J. Am. Coll. Cardiol. 2021, 77, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).