The Sympathetic Nervous System in Dental Implantology
Abstract
1. Introduction
2. Methods
- −
- What influence does the SNS have on bone metabolism?
- −
- How does the SNS influence the osseointegration of dental implants?
- −
- What are the underlying mechanisms?
- −
- What is the role of drugs whose target is the SNS?
3. Results
4. Discussion
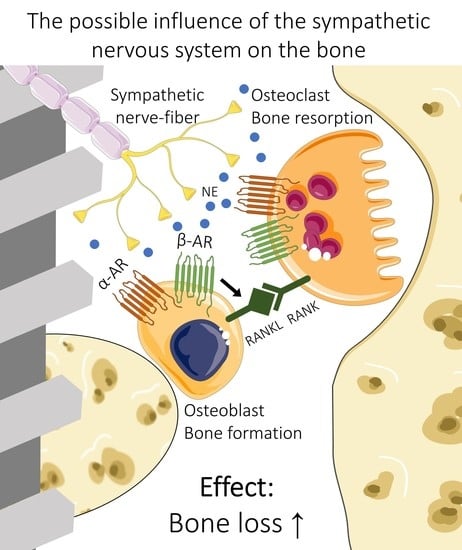
4.1. What Influence Does the SNA Have on Bone Metabolism?
4.2. Included Studies
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennardo, F.; Barone, S.; Vocaturo, C.; Nucci, L.; Antonelli, A.; Giudice, A. Usefulness of Magnetic Mallet in Oral Surgery and Implantology: A Systematic Review. J. Pers. Med. 2022, 12, 108. [Google Scholar] [CrossRef]
- Bennardo, F.; Barone, S.; Buffone, C.; Colangeli, W.; Antonelli, A.; Giudice, A. Removal of dental implants displaced into the maxillary sinus: A retrospective single-center study. Head Face Med. 2022, 18, 34. [Google Scholar] [CrossRef]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Hebel, K.; Gajjar, R.; Hofstede, T. Single-tooth replacement: Bridge vs. implant-supported restoration. J. Can. Dent. Assoc. 2000, 66, 435–438. [Google Scholar] [PubMed]
- Sharma, A.J.; Nagrath, R.; Lahori, M. A comparative evaluation of chewing efficiency, masticatory bite force, and patient satisfaction between conventional denture and implant-supported mandibular overdenture: An in vivo study. J. Indian Prosthodont. Soc. 2017, 17, 361–372. [Google Scholar] [CrossRef]
- Wittneben, J.-G.; Wismeijer, D.; Brägger, U.; Joda, T.; Abou-Ayash, S. Patient-reported outcome measures focusing on aesthetics of implant- and tooth-supported fixed dental prostheses: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 224–240. [Google Scholar] [CrossRef]
- Emami, E.; Heydecke, G.; Rompré, P.H.; de Grandmont, P.; Feine, J.S. Impact of implant support for mandibular dentures on satisfaction, oral and general health-related quality of life: A meta-analysis of randomized-controlled trials. Clin. Oral Implant. Res. 2009, 20, 533–544. [Google Scholar] [CrossRef]
- Cardoso, R.G.; de Melo, L.A.; Barbosa, G.A.S.; Calderon, P.D.S.; Germano, A.R.; Mestriner, W.J.; da Fonte Porto Carreiro, A. Impact of mandibular conventional denture and overdenture on quality of life and masticatory efficiency. Braz. Oral Res. 2016, 30, e102. [Google Scholar] [CrossRef]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef]
- Albrektsson, T.; Albrektsson, B. Osseointegration of bone implants. A review of an alternative mode of fixation. Acta Orthop. Scand. 1987, 58, 567–577. [Google Scholar] [CrossRef]
- Schenk, R.K.; Buser, D. Osseointegration: A reality. Periodontology 2000 1998, 17, 22–35. [Google Scholar] [CrossRef]
- Larsson, C.; Thomsen, P.; Aronsson, B.-O.; Rodahl, M.; Lausmaa, J.; Kasemo, B.; Ericson, L.E. Bone response to surface-modified titanium implants: Studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials 1996, 17, 605–616. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Flores-Fraile, J.; López-Valverde, A. The Unknown Process Osseointegration. Biology 2020, 9, 168. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implant Dent. Relat. Res. 2016, 18, 618–634. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.-L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implant. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration--communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Junker, R.; Dimakis, A.; Thoneick, M.; Jansen, J.A. Effects of implant surface coatings and composition on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 185–206. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. A Review of the Impact of Implant Biomaterials on Osteocytes. J. Dent. Res. 2018, 97, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Donath, K.; Laass, M.; Günzl, H.J. The histopathology of different foreign-body reactions in oral soft tissue and bone tissue. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Beiter, R.; Nash, R.; McCrady, M.; Rhoades, D.; Linscomb, M.; Clarahan, M.; Sammut, S. The prevalence and correlates of depression, anxiety, and stress in a sample of college students. J. Affect. Disord. 2015, 173, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Renouard, F.; Amalberti, R.; Renouard, E. Are “Human Factors” the Primary Cause of Complications in the Field of Implant Dentistry? Int. J. Oral Maxillofac. Implant. 2017, 32, e55–e61. [Google Scholar] [CrossRef]
- Jakovljevic, K.; Kober, K.M.; Block, A.; Cooper, B.A.; Paul, S.M.; Hammer, M.J.; Cartwright, F.; Conley, Y.P.; Wright, F.; Dunn, L.B.; et al. Higher Levels of Stress Are Associated With a Significant Symptom Burden in Oncology Outpatients Receiving Chemotherapy. J. Pain Symptom Manag. 2021, 61, 24–31.e4. [Google Scholar] [CrossRef]
- Brown, J. The impact of stress on acute wound healing. Br. J. Community Nurs. 2016, 21, S16–S22. [Google Scholar] [CrossRef]
- Christian, L.M.; Graham, J.E.; Padgett, D.A.; Glaser, R.; Kiecolt-Glaser, J.K. Stress and wound healing. Neuroimmunomodulation 2006, 13, 337–346. [Google Scholar] [CrossRef]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef]
- Sohn, R.; Rösch, G.; Junker, M.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Adrenergic signalling in osteoarthritis. Cell Signal. 2021, 82, 109948. [Google Scholar] [CrossRef]
- Kupka, J.; Kohler, A.; El Bagdadi, K.; Bostelmann, R.; Brenneis, M.; Fleege, C.; Chan, D.; Zaucke, F.; Meurer, A.; Rickert, M.; et al. Adrenoceptor Expression during Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2020, 21, 2085. [Google Scholar] [CrossRef] [PubMed]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Hamano, S.; Tomokiyo, A.; Hasegawa, D.; Yuda, A.; Sugii, H.; Yoshida, S.; Mitarai, H.; Wada, N.; Maeda, H. Functions of beta2-adrenergic receptor in human periodontal ligament cells. J. Cell. Biochem. 2020, 121, 4798–4808. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Mishra, N.; Alexander, M.; Gupta, S.K. Implant Survival between Endo-osseous Dental Implants in Immediate Loading, Delayed Loading, and Basal Immediate Loading Dental Implants a 3-Year Follow-up. Ann. Maxillofac. Surg. 2017, 7, 237–244. [Google Scholar] [CrossRef]
- Hao, C.-P.; Cao, N.-J.; Zhu, Y.-H.; Wang, W. The osseointegration and stability of dental implants with different surface treatments in animal models: A network meta-analysis. Sci. Rep. 2021, 11, 13849. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Aref, A.; Scharnweber, D.; Bierbaum, S.; Roessler, S.; Sewing, A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin. Oral Implant. Res. 2005, 16, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.-W.; Chien, E.Y.; Chien, H.-H. Dental implant bioactive surface modifications and their effects on osseointegration: A review. Biomark. Res. 2016, 4, 24. [Google Scholar] [CrossRef]
- Redon, J.; Mourad, J.-J.; Schmieder, R.E.; Volpe, M.; Weiss, T.W. Why in 2016 are patients with hypertension not 100% controlled? A call to action. J. Hypertens. 2016, 34, 1480–1488. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Faquih, A.E.; Memon, R.I.; Hafeez, H.; Zeshan, M.; Naveed, S. A Review of Novel Antidepressants: A Guide for Clinicians. Cureus 2019, 11, e4185. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Brisse, B.; Tetsch, P.; Schwill, E.; Bender, F. Comparative study of stress reactions during oral surgery after pindolol and metoprolol. J. Pharmacol. 1983, 14 (Suppl. 2), 21–29. [Google Scholar] [PubMed]
- Brand, H.S.; Gortzak, R.A.; Palmer-Bouva, C.C.; Abraham, R.E.; Abraham-Inpijn, L. Cardiovascular and neuroendocrine responses during acute stress induced by different types of dental treatment. Int. Dent. J. 1995, 45, 45–48. [Google Scholar]
- Miyata, K.; Odanaka, H.; Nitta, Y.; Shimoji, S.; Kanehira, T.; Kawanami, M.; Fujisawa, T. Music before Dental Surgery Suppresses Sympathetic Activity Derived from Preoperative Anxiety: A Randomized Controlled Trial. JDR Clin. Trans. Res. 2016, 1, 153–162. [Google Scholar] [CrossRef]
- Wakana, K.; Kimura, Y.; Nitta, Y.; Fujisawa, T. The Effect of Music on Preoperative Anxiety in an Operating Room: A Single-Blind Randomized Controlled Trial. Anesth. Prog. 2022, 69, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Robles, T.F.; Sharma, R.; Park, K.-S.; Harrell, L.; Yamaguchi, M.; Shetty, V. Utility of a salivary biosensor for objective assessment of surgery-related stress. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2012, 70, 2256–2263. [Google Scholar] [CrossRef]
- Sawaguchi, K.; Matsuura, N.; Ichinohe, T. Comparison of the Effect of Electrical Stimulations on the Chin Skin on Autonomic Nervous Activities During Propofol Sedation With or Without Midazolam. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2016, 74, 1751.e1–1751.e6. [Google Scholar] [CrossRef]
- Wawrzyniak, K.; Kusza, K.; Cywinski, J.B.; Burduk, P.K.; Kazmierczak, W. Premedication with clonidine before TIVA optimizes surgical field visualization and shortens duration of endoscopic sinus surgery—Results of a clinical trial. Rhinology 2013, 51, 259–264. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Zhang, T.; Huang, L.; Peng, W. Comparison in Sedative Effects between Dexmedetomidine and Midazolam in Dental Implantation: A Randomized Clinical Trial. BioMed Res. Int. 2020, 2020, 6130162. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Kang, L.; Wang, Q. Recent Advances in the Clinical Value and Potential of Dexmedetomidine. J. Inflamm. Res. 2021, 14, 7507–7527. [Google Scholar] [CrossRef]
- Davenport, R.E.; Porcelli, R.J.; Iacono, V.J.; Bonura, C.F.; Mallis, G.I.; Baer, P.N. Effects of anesthetics containing epinephrine on catecholamine levels during periodontal surgery. J. Periodontol. 1990, 61, 553–558. [Google Scholar] [CrossRef]
- Cao, H.; Fang, B.; Wang, X.; Zhou, Y. Sympathetic nervous system contributes to orthodontic tooth movement by central neural regulation from hypothalamus. Histol. Histopathol. 2020, 35, 1493–1502. [Google Scholar] [CrossRef]
- Haug, S.R.; Brudvik, P.; Fristad, I.; Heyeraas, K.J. Sympathectomy causes increased root resorption after orthodontic tooth movement in rats: Immunohistochemical study. Cell Tissue Res. 2003, 313, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ladizesky, M.G.; Lama, M.A.; Cutrera, R.A.; Boggio, V.; Giglio, M.J.; Cardinali, D.P. Effect of unilateral superior cervical ganglionectomy on mandibular incisor eruption rate in rats. Auton. Neurosci. 2001, 93, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Feng, L.; He, D.; Gan, Y.; Zhou, Y. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J. Dent. Res. 2014, 93, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.R.; Heyeraas, K.J. Immunoglobulin producing cells in the rat dental pulp after unilateral sympathectomy. Neuroscience 2005, 136, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.; Heyeraas, K. Effects of sympathectomy on experimentally induced pulpal inflammation and periapical lesions in rats. Neuroscience 2003, 120, 827–836. [Google Scholar] [CrossRef]
- Csillag, M.; Berggreen, E.; Fristad, I.; Haug, S.R.; Bletsa, A.; Heyeraas, K.J. Effect of electrical tooth stimulation on blood flow and immunocompetent cells in rat dental pulp after sympathectomy. Acta Odontol. Scand. 2004, 62, 305–312. [Google Scholar] [CrossRef]
- Haug, S.R.; Berggreen, E.; Heyeraas, K.J. The effect of unilateral sympathectomy and cavity preparation on peptidergic nerves and immune cells in rat dental pulp. Exp. Neurol. 2001, 169, 182–190. [Google Scholar] [CrossRef]
- Ball, J.; Darby, I. Mental health and periodontal and peri-implant diseases. Periodontology 2000 2022, 90, 106–124. [Google Scholar] [CrossRef]
- Crassous, P.-A.; Denis, C.; Paris, H.; Sénard, J.M. Interest of alpha2-adrenergic agonists and antagonists in clinical practice: Background, facts and perspectives. Curr. Top. Med. Chem. 2007, 7, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E. Clinical implications of autonomic drugs. J. Oral Maxillofac. Surg. 1992, 50, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Al-Abedalla, K.; Eimar, H.; Arekunnath Madathil, S.; Abi-Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Antihypertensive Medications and the Survival Rate of Osseointegrated Dental Implants: A Cohort Study. Clin. Implant Dent. Relat. Res. 2016, 18, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Hasuike, A.; Iwano, Y.; Hagiwara, Y. Influence of antihypertensive medications on the clinical parameters of anodized dental implants: A retrospective cohort study. Int. J. Implant Dent. 2020, 6, 32. [Google Scholar] [CrossRef]
- Chappuis, V.; Avila-Ortiz, G.; Araújo, M.G.; Monje, A. Medication-related dental implant failure: Systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 55–68. [Google Scholar] [CrossRef]
- Saravi, B.; Vollmer, A.; Lang, G.; Adolphs, N.; Li, Z.; Giers, V.; Stoll, P. Impact of renin-angiotensin system inhibitors and beta-blockers on dental implant stability. Int. J. Implant Dent. 2021, 7, 31. [Google Scholar] [CrossRef]
- Hakam, A.E.; Vila, G.; Duarte, P.M.; Mbadu, M.P.; Ai Angary, D.S.; Shuwaikan, H.; Aukhil, I.; Neiva, R.; da Silva, H.D.P.; Chang, J. Effects of different antidepressant classes on dental implant failure: A retrospective clinical study. J. Periodontol. 2021, 92, 196–204. [Google Scholar] [CrossRef]
- Morinaga, K.; Sasaki, H.; Park, S.; Hokugo, A.; Okawa, H.; Tahara, Y.; Colwell, C.S.; Nishimura, I. Neuronal PAS domain 2 (Npas2) facilitated osseointegration of titanium implant with rough surface through a neuroskeletal mechanism. Biomaterials 2019, 192, 62–74. [Google Scholar] [CrossRef]
- Tavakoli, M.; Farshami, M.J.; Torabinia, N.; Yaghini, J.; Shams, S. Evaluating systemic administration effect of propranolol on osseointegration around titanium implants: A histomorphometric study in dogs. Dent. Res. J. Isfahan 2022, 19, 37. [Google Scholar]
- Talo Yildirim, T.; Dündar, S.; Bozoğlan, A.; Karaman, T.; Tekin, S.; Kahraman, O.E. Evaluation of the Effects of ß-Adrenergic Receptor-Propranolol on Osseointegration of the Titanium Implants. J. Craniofac. Surg. 2021, 32, 783–786. [Google Scholar] [CrossRef]
- Tekin, S.; Dundar, S.; Demirci, F.; Bozoglan, A.; Yildirim, T.T.; Karaman, T.; Gul, M. Biomechanical and Biochemical Analyses of the Effects of Propranolol on the Osseointegration of Implants. J. Craniofac. Surg. 2021, 32, 1174–1176. [Google Scholar] [CrossRef] [PubMed]
- Gunes, N.; Gül, M.; Dundar, S.; Artas, G.; Kobat, M.A.; Tekin, S.; Bozoglan, A.; Isayev, A. Effects of Systemic Propranolol Application on the New Bone Formation in Periimplant Guided Bone Regeneration. J. Oral Maxillofac. Res. 2021, 12, e2. [Google Scholar] [CrossRef] [PubMed]
- Al-Subaie, A.E.; Laurenti, M.; Abdallah, M.-N.; Tamimi, I.; Yaghoubi, F.; Eimar, H.; Makhoul, N.; Tamimi, F. Propranolol enhances bone healing and implant osseointegration in rats tibiae. J. Clin. Periodontol. 2016, 43, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; He, F.; Liu, B.; Wei, S. Nerve electrical stimulation enhances osseointegration of implants in the beagle. Sci. Rep. 2019, 9, 4916. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zeng, Y.; Feng, Y.; Wu, H.; Liang, H.; Gong, P. Chemical Sympathectomy Impairs Peri-implant Osseointegration in Mice: Role of the Sympathetic Nervous System in Osseointegration. Int. J. Oral Maxillofac. Implant. 2019, 34, 91–98. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr.; Diaz, K.T.; Aranda, L.; Gabel, F.; Listl, S.; Alarcón, M.A. The risk of bias of animal experiments in implant dentistry: A methodological study. Clin. Oral Implant. Res. 2017, 28, e39–e45. [Google Scholar] [CrossRef]
- Cong, F.; Cheung, A.K.; Huang, S.-M.A. Chemical genetics-based target identification in drug discovery. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 57–78. [Google Scholar] [CrossRef]
- Nagatsu, T.; Levitt, M.; Udenfriend, S. Tyrosine Hydroxylase. The Initial Step in Norepinephrine Biosynthesis. J. Biol. Chem. 1964, 239, 2910–2917. [Google Scholar] [CrossRef]
- Vonend, O.; Okonek, A.; Stegbauer, J.; Habbel, S.; Quack, I.; Rump, L.C. Renovascular effects of sympathetic cotransmitters ATP and NPY are age-dependent in spontaneously hypertensive rats. Cardiovasc. Res. 2005, 66, 345–352. [Google Scholar] [CrossRef]
- Elefteriou, F. Impact of the Autonomic Nervous System on the Skeleton. Physiol. Rev. 2018, 98, 1083–1112. [Google Scholar] [CrossRef]
- Becker, D.E. Psychotropic drugs: Implications for dental practice. Anesth. Prog. 2008, 55, 89–99. [Google Scholar] [CrossRef]
- Danielson, P.; Alfredson, H.; Forsgren, S. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc. Res. Tech. 2007, 70, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A. Autonomic nervous system function. J. Clin. Neurophysiol. 1993, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Lachowska, K.; Schlaich, M. Role of the Sympathetic Nervous System in Stress-Mediated Cardiovascular Disease. Curr. Hypertens. Rep. 2015, 17, 80. [Google Scholar] [CrossRef]
- Goldstein, D.S. Stress-induced activation of the sympathetic nervous system. Baillieres Clin. Endocrinol. Metab. 1987, 1, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Thoma, M.V.; Kirschbaum, C.; Wolf, J.M.; Rohleder, N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol. Psychol. 2012, 91, 342–348. [Google Scholar] [CrossRef]
- Jänig, W. Sympathetic nervous system and inflammation: A conceptual view. Auton. Neurosci. 2014, 182, 4–14. [Google Scholar] [CrossRef]
- Pacifici, R. T cells, osteoblasts, and osteocytes: Interacting lineages key for the bone anabolic and catabolic activities of parathyroid hormone. Ann. N. Y. Acad. Sci. 2016, 1364, 11–24. [Google Scholar] [CrossRef]
- Serre, C.; Farlay, D.; Delmas, P.; Chenu, C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 1999, 25, 623–629. [Google Scholar] [CrossRef]
- Asmus, S.E.; Parsons, S.; Landis, S.C. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J. Neurosci. 2000, 20, 1495–1504. [Google Scholar] [CrossRef]
- Ahmed, M.; Bjurholm, A.; Kreicbergs, A.; Schultzberg, M. Neuropeptide Y, Tyrosine Hydroxylase and Vasoactive Intestinal Polypeptide-Immunoreactive Nerve Fibers in the Vertebral Bodies, Discs, Dura Mater, and Spinal Ligaments of the Rat Lumbar Spine. Spine 1976 1993, 18, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L.; Elde, R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991, 264, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, E.L.; Elde, R.P.; Rysavy, J.A.; Einzig, S.; Gebhard, R.L. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 1986, 232, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Niu, L.-N.; Li, Q.; Ren, G.; Zhao, C.; Liu, Y.; Tay, F.R.; Wang, M. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci. Rep. 2015, 5, 12593. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Grässel, S.; Pongratz, G.; Kees, F.; Miosge, N.; Angele, P.; Straub, R.H. Norepinephrine inhibition of mesenchymal stem cell and chondrogenic progenitor cell chondrogenesis and acceleration of chondrogenic hypertrophy. Arthritis Rheumatol. 2014, 66, 2472–2481. [Google Scholar] [CrossRef]
- Johnson, E.N.; Druey, K.M. Heterotrimeric G protein signaling: Role in asthma and allergic inflammation. J. Allergy Clin. Immunol. 2002, 109, 592–602. [Google Scholar] [CrossRef]
- Docherty, J.R. Subtypes of functional α1-adrenoceptor. Cell. Mol. Life Sci. 2010, 67, 405–417. [Google Scholar] [CrossRef]
- Huang, H.H.; Brennan, T.C.; Muir, M.M.; Mason, R.S. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J. Cell. Physiol. 2009, 220, 267–275. [Google Scholar] [CrossRef]
- Nishiura, T.; Abe, K. Alpha1-adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor kappaB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch. Oral Biol. 2007, 52, 778–785. [Google Scholar] [CrossRef]
- Arai, M.; Nagasawa, T.; Koshihara, Y.; Yamamoto, S.; Togari, A. Effects of β-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim. Biophys. Acta BBA Mol. Cell Res. 2003, 1640, 137–142. [Google Scholar] [CrossRef]
- Docherty, J.R. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur. J. Pharmacol. 1998, 361, 1–15. [Google Scholar] [CrossRef]
- Fonseca, T.L.; Jorgetti, V.; Costa, C.C.; Capelo, L.P.; Covarrubias, A.E.; Moulatlet, A.C.; Teixeira, M.B.; Hesse, E.; Morethson, P.; Beber, E.H.; et al. Double disruption of α2A- and α2C-adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J. Bone Miner. Res. 2011, 26, 591–603. [Google Scholar] [CrossRef]
- Mlakar, V.; Jurkovic Mlakar, S.; Zupan, J.; Komadina, R.; Prezelj, J.; Marc, J. ADRA2A is involved in neuro-endocrine regulation of bone resorption. J. Cell. Mol. Med. 2015, 19, 1520–1529. [Google Scholar] [CrossRef]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Esp. Cardiol. Engl. Ed. 2019, 72, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Abosamak, N.R.; Shahin, M.H. Beta 2 Receptor Agonists/Antagonists; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Meyers, D.S.; Skwish, S.; Dickinson, K.E.; Kienzle, B.; Arbeeny, C.M. Beta 3-adrenergic receptor-mediated lipolysis and oxygen consumption in brown adipocytes from cynomolgus monkeys. J. Clin. Endocrinol. Metab. 1997, 82, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Rasmussen, S.G.F.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Togari, A.; Arai, M.; Mizutani, S.; Mizutani, S.; Koshihara, Y.; Nagatsu, T. Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 1997, 233, 125–128. [Google Scholar] [CrossRef]
- Moore, R.E.; Smith, C.K.; Bailey, C.S.; Voelkel, E.F.; Tashjian, A.H. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993, 23, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Nyman, J.S.; Tao, H.; Moss, H.H.; Yang, X.; Elefteriou, F. β2-Adrenergic Receptor Signaling in Osteoblasts Contributes to the Catabolic Effect of Glucocorticoids on Bone. Endocrinology 2011, 152, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. Beta2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys. 2009, 482, 96–103. [Google Scholar] [CrossRef]
- Bonnet, N.; Benhamou, C.L.; Brunet-Imbault, B.; Arlettaz, A.; Horcajada, M.N.; Richard, O.; Vico, L.; Collomp, K.; Courteix, D. Severe bone alterations under β2 agonist treatments: Bone mass, microarchitecture and strength analyses in female rats. Bone 2005, 37, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Pierroz, D.D.; Bonnet, N.; Bianchi, E.N.; Bouxsein, M.L.; Baldock, P.A.; Rizzoli, R.; Ferrari, S.L. Deletion of β-adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J. Bone Miner. Res. 2012, 27, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Hannan, R.; Harbell, J.W.; Coecke, S.; Balls, M.; Bowe, G.; Cervinka, M.; Clothier, R.; Hermann, F.; Klahm, L.K.; de Lange, J.; et al. The principles of good laboratory practice: Application to in vitro toxicology studies. Altern. Lab. Anim. 1999, 27, 539–577. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; McCarville, K.; Morinaga, K.; Mengatto, C.M.; Langfelder, P.; Hokugo, A.; Tahara, Y.; Colwell, C.S.; Nishimura, I. Titanium biomaterials with complex surfaces induced aberrant peripheral circadian rhythms in bone marrow mesenchymal stromal cells. PLoS ONE 2017, 12, e0183359. [Google Scholar] [CrossRef]
- Hedderich, J.; El Bagdadi, K.; Angele, P.; Grässel, S.; Meurer, A.; Straub, R.H.; Zaucke, F.; Jenei-Lanzl, Z. Norepinephrine Inhibits the Proliferation of Human Bone Marrow-Derived Mesenchymal Stem Cells via β2-Adrenoceptor-Mediated ERK1/2 and PKA Phosphorylation. Int. J. Mol. Sci. 2020, 21, 3924. [Google Scholar] [CrossRef] [PubMed]
- van den Dolder, J.; de Ruijter, A.J.; Spauwen, P.H.; Jansen, J.A. Observations on the effect of BMP-2 on rat bone marrow cells cultured on titanium substrates of different roughness. Biomaterials 2003, 24, 1853–1860. [Google Scholar] [CrossRef]
- Dard, M. Methods and interpretation of performance studies for dental implants. In Biocompatibility and Performance of Medical Devices; Boutrand, J.-P., Ed.; Woodhead Pub: Philadelphia, PA, USA, 2012; pp. 308–344. ISBN 9780857090706. [Google Scholar]
- Staubli, N.; Schmidt, J.C.; Rinne, C.A.; Signer-Buset, S.L.; Rodriguez, F.R.; Walter, C. Animal Experiments in Periodontal and Peri-Implant Research: Are There Any Changes? Dent. J. 2019, 7, 46. [Google Scholar] [CrossRef]
- Jividen, G.; Misch, C.E. Reverse Torque Testing and Early Loading Failures: Help or Hindrance? J. Oral Implantol. 2000, 26, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive boen loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar]
- Renz, H. Praktische Labordiagnostik: Lehrbuch zur Laboratoriumsmedizin, klinischen Chemie und Hämatologie, 3rd ed.; Aktualisierte und Erweiterte Auflage; De Gruyter: Berlin, Germany, 2018. [Google Scholar]
- Kazimierczak, P.; Przekora, A. Osteoconductive and Osteoinductive Surface Modifications of Biomaterials for Bone Regeneration: A Concise Review. Coatings 2020, 10, 971. [Google Scholar] [CrossRef]
- Thorey, F.; Menzel, H.; Lorenz, C.; Gross, G.; Hoffmann, A.; Windhagen, H. Osseointegration by bone morphogenetic protein-2 and transforming growth factor beta2 coated titanium implants in femora of New Zealand white rabbits. Indian J. Orthop. 2011, 45, 57–62. [Google Scholar] [CrossRef]
- Gutwald, R.; Haberstroh, J.; Stricker, A.; Rüther, E.; Otto, F.; Xavier, S.P.; Oshima, T.; Marukawa, E.; Seto, I.; Enomoto, S.; et al. Influence of rhBMP-2 on bone formation and osseointegration in different implant systems after sinus-floor elevation. An in vivo study on sheep. J. Craniomaxillofac. Surg. 2010, 38, 571–579. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz Fernandes, J.; de Lima, V.N.; Bonardi, J.P.; Filho, O.M.; Queiroz, S.B.F. Bone regeneration with recombinant human bone morphogenetic protein 2: A systematic review. J. Maxillofac. Oral Surg. 2018, 17, 13–18. [Google Scholar] [CrossRef]
- de Freitas, R.M.; Spin-Neto, R.; Marcantonio Junior, E.; Pereira, L.A.V.D.; Wikesjö, U.M.E.; Susin, C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: A systematic review. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. 1), e192–e201. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.; Feroze, A.H.; Nistal, D.; Robinson, E.; Saigal, R. Impact of surgeon rhBMP-2 cost awareness on complication rates and health system costs for spinal arthrodesis. Neurosurg. Focus 2021, 50, E5. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors 2014, 40, 459–481. [Google Scholar] [CrossRef]
- Skovrlj, B.; Koehler, S.M.; Anderson, P.A.; Qureshi, S.A.; Hecht, A.C.; Iatridis, J.C.; Cho, S.K. Association Between BMP-2 and Carcinogenicity. Spine 1976 2015, 40, 1862–1871. [Google Scholar] [CrossRef]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.; Takahashi, T.; Kurokawa, Y.; Kobayashi, T.; Saito, T.; Ishida, T.; Serada, S.; Fujimoto, M.; Naka, T.; Wada, N.; et al. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric Cancer 2021, 24, 1037–1049. [Google Scholar] [CrossRef]
- Phadke, S.; Clamon, G. Beta blockade as adjunctive breast cancer therapy: A review. Crit. Rev. Oncol. Hematol. 2019, 138, 173–177. [Google Scholar] [CrossRef]
- Rodrigues, W.F.; Madeira, M.F.M.; da Silva, T.A.; Clemente-Napimoga, J.T.; Miguel, C.B.; Dias-da-Silva, V.J.; Barbosa-Neto, O.; Lopes, A.H.; Napimoga, M.H. Low dose of propranolol down-modulates bone resorption by inhibiting inflammation and osteoclast differentiation. Br. J. Pharmacol. 2012, 165, 2140–2151. [Google Scholar] [CrossRef]
- Berg, R.A.; Moss, J.; Baum, B.J.; Crystal, R.G. Regulation of collagen production by the beta-adrenergic system. J. Clin. Investig. 1981, 67, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Pierroz, D.D.; Ferrari, S.L. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J. Musculoskelet. Neuronal Interact. 2008, 8, 94–104. [Google Scholar] [PubMed]
- Aguirre, J.I.; Altman, M.K.; Vanegas, S.M.; Franz, S.E.; Bassit, A.C.F.; Wronski, T.J. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010, 16, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Molinoff, P.B. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs 1984, 28 (Suppl. 2), 1–15. [Google Scholar] [CrossRef]
- Gille, E.; Lemoine, H.; Ehle, B.; Kaumann, A.J. The affinity of (−)-propranolol for β1- and β2-adrenoceptors of human heart. Differential antagonism of the positive inotropic effects and adenylate cyclase stimulation by (−)-noradrenaline and (−)-adrenaline. Naunyn Schmiedebergs. Arch. Pharmacol. 1985, 331, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Dergin, G.; Akta, M.; Gürsoy, B.; Devecioglu, Y.; Kürkçü, M.; Benlidayi, E. Direct current electric stimulation in implant osseointegration: An experimental animal study with sheep. J. Oral Implantol. 2013, 39, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Cho, T.H.; Pan, H.; Song, Y.M.; Kim, I.S.; Lee, T.H.; Hwang, S.J.; Kim, S.J. An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics 2009, 30, 374–384. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Y.; Zheng, X.-F.; Jiang, S.-D.; Chen, X.-D.; Jiang, L.-S. Sympathetic neuron can promote osteoblast differentiation through BMP signaling pathway. Cell. Signal. 2013, 25, 1372–1378. [Google Scholar] [CrossRef]
- Thoenen, H.; Tranzer, J.P. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-Hydroxydopamine. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1968, 261, 271–288. [Google Scholar] [CrossRef]
- Kondo, M.; Kondo, H.; Miyazawa, K.; Goto, S.; Togari, A. Experimental tooth movement-induced osteoclast activation is regulated by sympathetic signaling. Bone 2013, 52, 39–47. [Google Scholar] [CrossRef]
- Cherruau, M.; Facchinetti, P.; Baroukh, B.; Saffar, J. Chemical sympathectomy impairs bone resorption in rats: A role for the sympathetic system on bone metabolism. Bone 1999, 25, 545–551. [Google Scholar] [CrossRef]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]
- Haimov, H.; Yosupov, N.; Pinchasov, G.; Juodzbalys, G. Bone Morphogenetic Protein Coating on Titanium Implant Surface: A Systematic Review. J. Oral Maxillofac. Res. 2017, 8, e1. [Google Scholar] [CrossRef]
- Bienz, S.P.; Payer, M.; Hjerppe, J.; Hüsler, J.; Jakse, N.; Schmidlin, P.R.; Hämmerle, C.H.F.; Jung, R.E.; Thoma, D.S. Primary bone augmentation leads to equally stable marginal tissue conditions comparing the use of xenograft blocks infused with BMP-2 and autogenous bone blocks: A 3D analysis after 3 years. Clin. Oral Implant. Res. 2021, 32, 1433–1443. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Liu, Y.; Muff, M.; Haegi, T.; Shintani, N.; Lippuner, K. The slow release of BMP-7 at a low dose accelerates dental implant healing in an osteopenic environment. Eur. Cells Mater. 2021, 41, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Calderón, D.M.; Assao, A.; Garcia, N.G.; Coutinho-Camillo, C.M.; Roffé, M.; Germano, J.N.; Oliveira, D.T. Beta adrenergic receptor activation inhibits oral cancer migration and invasiveness. Arch. Oral Biol. 2020, 118, 104865. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Reinicke, K.; Sagheb, K.; König, J.; Al-Nawas, B.; Grötz, K.A. Dental implants in patients with head and neck cancer-A systematic review and meta-analysis of the influence of radiotherapy on implant survival. Clin. Oral Implant. Res. 2022, 33, 967–999. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, J.; Kim, Y.-J.; Jung, S.-Y.; Kim, H.J.; Choi, N.-K.; Park, B.-J. β1 selectivity of β-blockers and reduced risk of fractures in elderly hypertension patients. Bone 2012, 51, 1008–1015. [Google Scholar] [CrossRef]
- Gaetti-Jardim, E.C.; Santiago-Junior, J.F.; Goiato, M.C.; Pellizer, E.P.; Magro-Filho, O.; Jardim Junior, E.G. Dental implants in patients with osteoporosis: A clinical reality? J. Craniofac. Surg. 2011, 22, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef]
- Carnero-Martín de Soto, P.; Tamimi-Mariño, I.; Bautista-Enrique, D.; Bravo-Zurita, M.J.; Cáceres, A.G.; Tamimi, F.; Dawid-Milner, M.S. Use of beta-blockers and risk of aseptic loosening in total hip and knee arthroplasty: A nested case - control study. J. Musculoskelet. Neuronal Interact. 2019, 19, 104–111. [Google Scholar] [PubMed]
- Kupka, J.R.; Sagheb, K.; Al-Nawas, B.; Schiegnitz, E. Surgical safety checklists for dental implant surgeries-a scoping review. Clin. Oral Investig. 2022. [Google Scholar] [CrossRef]
- Toff, N.J. Human factors in anaesthesia: Lessons from aviation. Br. J. Anaesth. 2010, 105, 21–25. [Google Scholar] [CrossRef]
- Catchpole, K.; Cohen, T.; Alfred, M.; Lawton, S.; Kanji, F.; Shouhed, D.; Nemeth, L.; Anger, J. Human Factors Integration in Robotic Surgery. Hum. Factors 2022. [Google Scholar] [CrossRef] [PubMed]
- Carayon, P. Handbook of Human Factors and Ergonomics in Health Care and Patient Safety, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 0429152019. [Google Scholar]
- Pan, L.; Tang, J.; Liu, H.; Cheng, B. Sympathetic nerves: How do they affect angiogenesis, particularly during wound healing of soft tissues? Clin. Hemorheol. Microcirc. 2016, 62, 181–191. [Google Scholar] [CrossRef]
- Eijkelkamp, N.; Engeland, C.G.; Gajendrareddy, P.K.; Marucha, P.T. Restraint stress impairs early wound healing in mice via alpha-adrenergic but not beta-adrenergic receptors. Brain Behav. Immun. 2007, 21, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Kantowitz, B.H. Selecting measures for human factors research. Hum. Factors 1992, 34, 387–398. [Google Scholar] [CrossRef]
- Chackartchi, T.; Romanos, G.E.; Sculean, A. Soft tissue-related complications and management around dental implants. Periodontology 2000 2019, 81, 124–138. [Google Scholar] [CrossRef]
- Edwall, B.; Gazelius, B.; Fazekas, A.; Theodorsson-Norheim, E.; Lundberg, J.M. Neuropeptide Y (NPY) and sympathetic control of blood flow in oral mucosa and dental pulp in the cat. Acta Physiol. Scand. 1985, 125, 253–264. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Da Sasso, G.R.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]


| Autonomous Nervous System | Dental Implantology | |
|---|---|---|
| Adrenergic * OR Adrenoceptor * OR Norepinephrin * OR sympathetic nerv * OR autonomic nervous system OR parasympathetic nerv * | AND | maxillofacial surge * OR oral surge * OR dental surge * OR operative dentist * OR oral implant * OR dental implant * OR peri implant * OR periimplant * |
| Test-Subject | Implant (Type and Localization) | Intervention | Outcome-Parameter | |
|---|---|---|---|---|
| Morinaga et al. (2019) [68] | Mouse primary BMSCs | The cells were cultured on a polystyrene plate, sandblasted and acid-etched titanium discs or machined titanium discs | Chemical genetics study Identification of relevant signaling pathways. Under the following citation, a detailed description of the process of chemical genetics analysis is given [77] Expression of ARs RTPCR, mRNA levels of α1a, α1b, α1d, α2a, α2b, α2c, β1, β2 and β3 adrenergic receptors | |
| Tavakoli et al. (2022) [69] | Four nondomestic male street dogs | Bone level implants (SNUCON, Korea), 4 mm in diameter and 10 mm in length Second, third, and fourth premolar in the left mandible | Extraction of three teeth Test: propranolol oral tablet 0.2 mg/kg, Control: saline Three implants after 2 months, submerged healing After 4 and 9 weeks, dental implants and the peripheral bone were removed using a 6-mm trephine drill | Histological analysis Bone implant contact (BIC) |
| Yildirim et al. (2021) [70] | 20 Sprague–Dawley rats | Machined-surfaced titanium implants, 4 mm in length, with a diameter of 2.5 mm (Implance Dental Implant System, AGS Medical, Istanbul, Turkey) Metaphyseal part of each tibia | Insertion of the implant Test: 10 mg/kg propranolol orally on every day for 4 weeks Control: No further treatment | Blood sample analysis Alkaline phosphatase, calcium, phosphorus Histological analysis Bone implant connection (BIC) |
| Tekin et al. (2021) [71] | 24 Sprague–Dawley rats | Machined-surfaced titanium implants, 4 mm in length, with a diameter of 2.5 mm (Implance Dental Implant System, AGS Medical, Istanbul, Turkey) Metaphyseal part of each tibia | Implant insertion Three groups for the 4-week experiment: (1) No further treatment (2) 5 mg/kg propranolol orally 3 days a week (3) 10 mg/kg propranolol orally 3 days a week | Blood sample analysis Alkaline phosphatase, calcium, phosphorus Biomechanical analysis Reverse torque test |
| Gunes et al. (2021) [72] | 24 Sprague–Dawley rats | Resorbable blast material titanium implants, 2.5 mm diameter and 4 mm in length with eight threads (AGS Medical Corporation; Istanbul, Turkey) Metaphyseal part of each tibia | After implant insertion, a three-walled standard defect of 2.5 mm width and 2 mm length was opened, hydroxyapatite bovine bone graft was placed in the defect Three groups for the 8-week experiment: (1) No further treatment (2) 5 mg/kg propranolol orally 3 days a week (3) 10 mg/kg propranolol orally 3 days a week | Blood sample analysis Alkaline phosphatase, calcium, creatinine, phosphorus Histological analysis Newly formed bone area New bone formation rate |
| Al-Subaie et al. (2016) [73] | 24 Sprague–Dawley female rats | Cylindrical cuts of a titanium rod, 1.5 mm in diameter and 2 mm in length Metaphyseal part of each tibia | One side: hole with 1.5 mm in diameter Contralateral: hole with 2.5 mm in diameter Test group: 5 mg/kg propranolol, subcutaneous, daily for 2 weeks Control: saline | Microcomputed tomography Cortical defect volume, bone volume/tissue volume, trabecular thickness, trabecular number, and trabecular separation Histology of the bone defects Osteoclast number per square millimeter of mineralized tissue, mineralized tissue percentage and collagen percentage Histology of the bone implant contact Bone implant contact measurements (total, cortical and medullary), cortical and medullary peri-implant bone volume/tissue volume (BV/TV) |
| Zhou et al. (2019) [74] | Eight female beagles | Pure titanium self-produced machined implants 4.0 mm in diameter 7.0 mm in length Maxillary lateral incisors | Immediate implantation Three double-implant beagles and one single-implant beagle for each group 1 week after implantation: test group electrically stimulated transcutaneously for 45 min each day for 3 weeks Control group: no stimulation | Microelectrodes bilaterally in the infraorbital nerves Electric potential of the sympathetic nerve fibers in the infraorbital nerve ECG monitor Blood oxygen saturation and heart rate Microcomputed tomography analysis Bone volume percentage, trabecular thickness, trabecular number, trabecular separation Histological analysis Morphological analysis |
| Yao et al. (2019) [75] | 40 C57BL/6J mice | Rod-shaped machined titanium implants, 3 mm in length and 1 mm in diameter in the anterior-distal surfaces of both femurs in each mouse | Test group: sympathectomy with 6-hydroxydopamine 5 days before surgery Implant placement One femur from each mouse was harvested at week 2 and 4 after surgery | Microcomputed tomography (micro-CT) Bone volume to total volume ratio, bone surface to total bone volume ratio, mean trabecular number, mean trabecular thickness, mean trabecular separation, percentage of osseointegration Blood sample analysis Osteocalcin and C-terminal collagen I cross-links Histological analysis Mineral apposition rate, bone formation rate per bone surface, bone-to-implant contact, number of osteoclasts per peri-implant surface Biomechanical analysis Push-in test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupka, J.R.; Sagheb, K.; Al-Nawas, B.; Schiegnitz, E. The Sympathetic Nervous System in Dental Implantology. J. Clin. Med. 2023, 12, 2907. https://doi.org/10.3390/jcm12082907
Kupka JR, Sagheb K, Al-Nawas B, Schiegnitz E. The Sympathetic Nervous System in Dental Implantology. Journal of Clinical Medicine. 2023; 12(8):2907. https://doi.org/10.3390/jcm12082907
Chicago/Turabian StyleKupka, Johannes Raphael, Keyvan Sagheb, Bilal Al-Nawas, and Eik Schiegnitz. 2023. "The Sympathetic Nervous System in Dental Implantology" Journal of Clinical Medicine 12, no. 8: 2907. https://doi.org/10.3390/jcm12082907
APA StyleKupka, J. R., Sagheb, K., Al-Nawas, B., & Schiegnitz, E. (2023). The Sympathetic Nervous System in Dental Implantology. Journal of Clinical Medicine, 12(8), 2907. https://doi.org/10.3390/jcm12082907