Differences in Cerebral Oxygenation in Cardiogenic and Respiratory Cardiac Arrest Before, During, and After Cardiopulmonary Resuscitation
Abstract
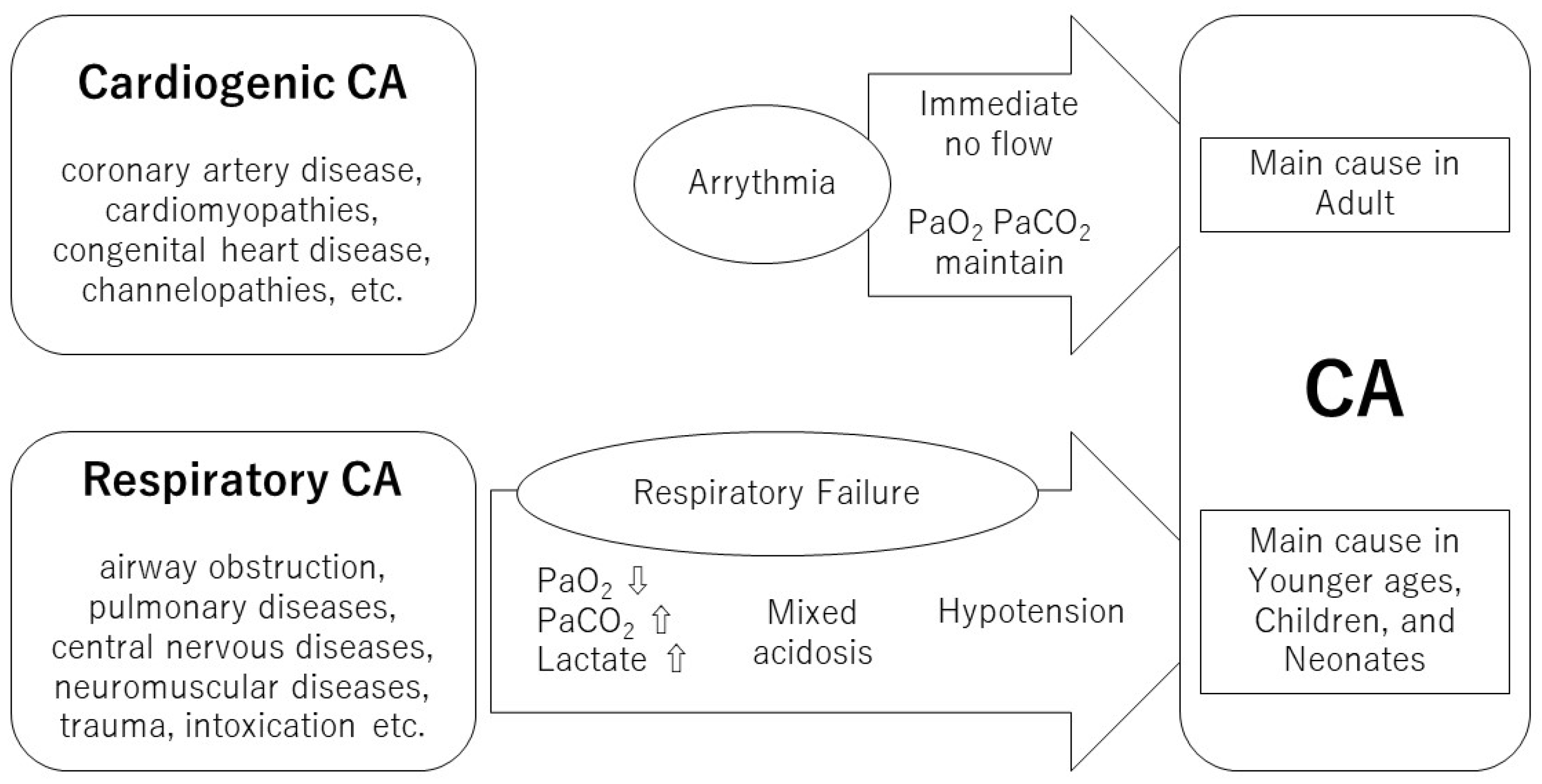
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Cerebral Oxygenation
2.3. Data Collection
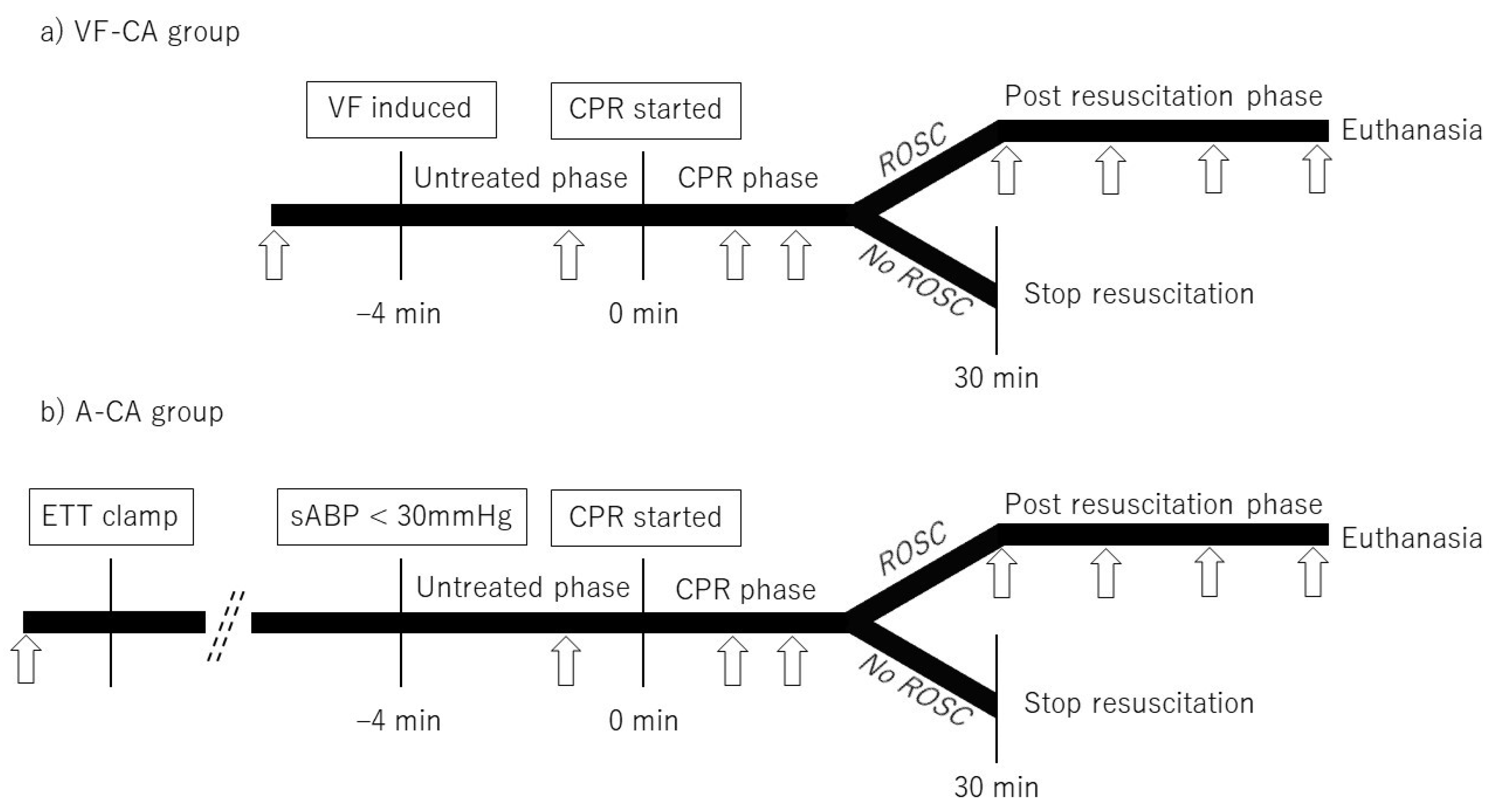
2.4. Study Design
2.5. Statistical Analyses
3. Results
3.1. Baseline
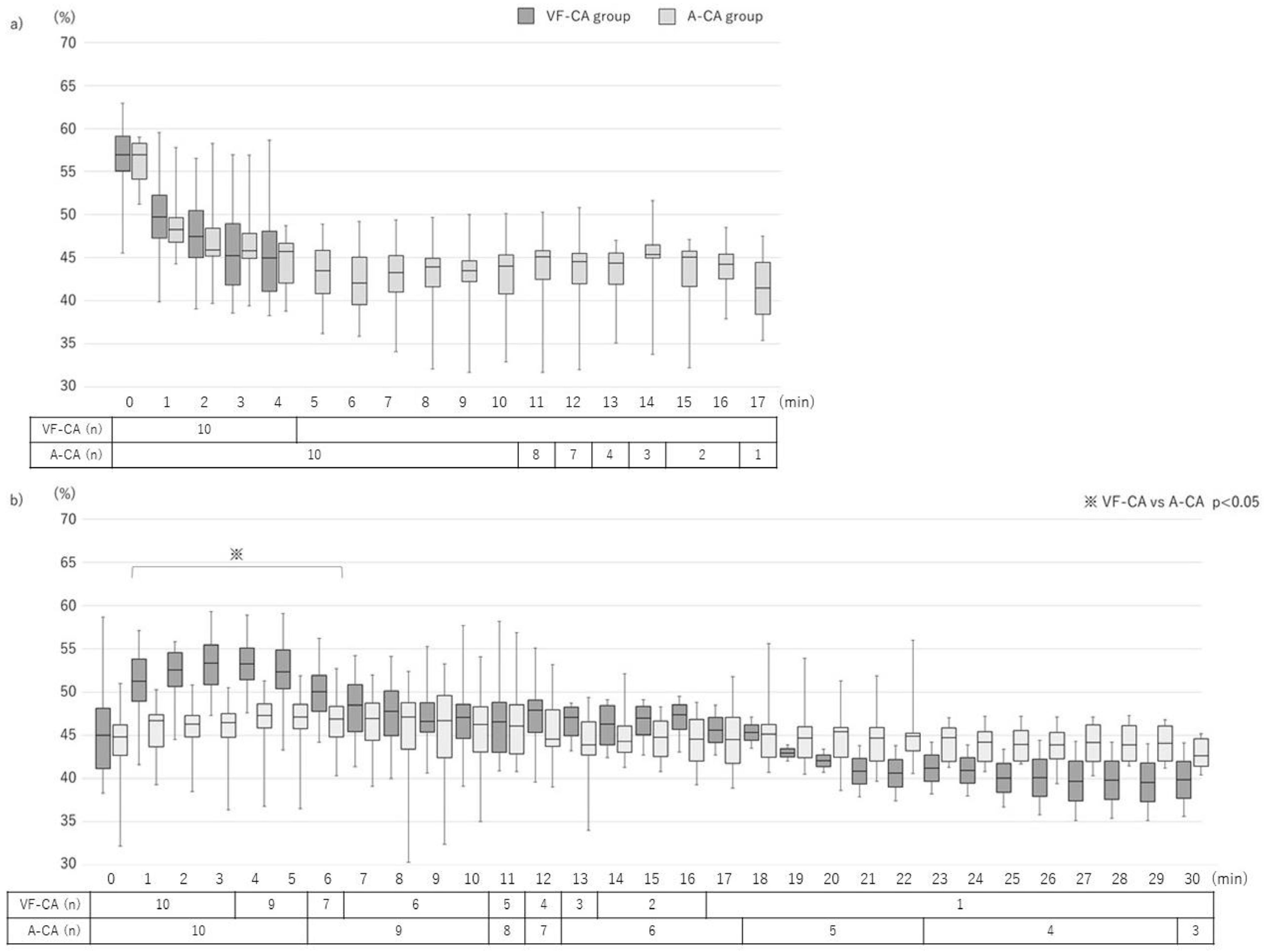
3.2. Pre-CPR Phase
3.3. CPR Phase
3.4. Post-CPR Phase
3.5. Correlation between the TOI and Cerebral Oxygenation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the world: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Handley, A.J.; Koster, R.W.; Castrén, M.; Smyth, M.A.; Olasveengen, T.; Monsieurs, K.G.; Raffay, V.; Gräsner, J.T.; Wenzel, V.; et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015, 95, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ohashi-Fukuda, N.; Matsubara, T.; Doi, K.; Kitsuta, Y.; Nakajima, S.; Yahagi, N. Trends in outcomes for out-of-hospital cardiac arrest by age in Japan: An observational study. Medicine 2015, 94, e2049. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit Care 2017, 21, 90. [Google Scholar] [CrossRef]
- Koyama, Y.; Wada, T.; Lohman, B.D.; Takamatsu, Y.; Matsumoto, J.; Fujitani, S.; Taira, Y. A new method to detect cerebral blood flow waveform in synchrony with chest compression by near-infrared spectroscopy during CPR. Am. J. Emerg. Med. 2013, 31, 1504–1508. [Google Scholar] [CrossRef]
- Schalk, R.; Seeger, F.H.; Mutlak, H.; Schweigkofler, U.; Zacharowski, K.; Peter, N.; Byhahn, C. A feasibility study evaluating the role of cerebral oximetry in predicting return of spontaneous circulation in cardiac arrest. Resuscitation 2012, 83, 982–985. [Google Scholar] [CrossRef]
- Nishiyama, K.; Ito, N.; Orita, T.; Hayashida, K.; Arimoto, H.; Abe, M.; Unoki, T.; Endo, T.; Murai, A.; Ishikura, K.; et al. Characteristics of regional cerebral oxygen saturation levels in patients with out-of-hospital cardiac arrest with or without return of spontaneous circulation: A prospective observational multicentre study. Resuscitation 2015, 96, 16–22. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Sulzgruber, P.; Menger, J.; Skhirtladze-Dworschak, K.; Sterz, F.; Dworschak, M. Regional cerebral oxygen saturation during cardiopulmonary resuscitation as a predictor of return of spontaneous circulation and favourable neurological outcome—A review of the current literature. Resuscitation 2018, 125, 39–47. [Google Scholar] [CrossRef]
- Ha, A.C.T.; Doumouras, B.S.; Wang, C.N.; Tranmer, J.; Lee, D.S. Prediction of sudden cardiac arrest in the general population: Review of traditional and emerging risk factors. Can. J. Cardiol. 2022, 38, 465–478. [Google Scholar] [CrossRef]
- Braunwald, E. Cardiomyopathies: An overview. Circ Res 2017, 121, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Khairy, P.; Silka, M.J.; Moore, J.P.; DiNardo, J.A.; Vehmeijer, J.T.; Sheppard, M.N.; van de Bruaene, A.; Chaix, M.A.; Brida, M.; Moore, B.M.; et al. Sudden cardiac death in congenital heart disease. Eur. Heart J. 2022, 43, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Mascia, G.; Bona, R.D.; Ameri, P.; Canepa, M.; Porto, I.; Parati, G.; Crotti, L.; Brignole, M. Brugada syndrome and syncope: A practical approach for diagnosis and treatment. Europace 2021, 23, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Safar, P. Recognition and management of airway obstruction. JAMA 1969, 209, 1722. [Google Scholar] [CrossRef]
- Hickey, R.W.; Painter, M.J. Brain injury from cardiac arrest in children. Neurol. Clin. 2006, 24, 147–158. [Google Scholar] [CrossRef]
- Varvarousis, D.; Varvarousi, G.; Iacovidou, N.; D’Aloja, E.; Gulati, A.; Xanthos, T. The pathophysiologies of asphyxial vs dysrhythmic cardiac arrest: Implications for resuscitation and post-event management. Am. J. Emerg. Med. 2015, 33, 1297–1304. [Google Scholar] [CrossRef]
- Johnson, N.J.; Caldwell, E.; Carlbom, D.J.; Gaieski, D.F.; Prekker, M.E.; Rea, T.D.; Sayre, M.; Hough, C.L. The acute respiratory distress syndrome after out-of-hospital cardiac arrest: Incidence, risk factors, and outcomes. Resuscitation 2019, 135, 37–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.S.; Wu, C.J.; Yang, J.; Hang, C.C. Comparison of cerebral metabolism between pig ventricular fibrillation and asphyxial cardiac arrest models. Chin. Med. J. 2015, 128, 1643–1648. [Google Scholar] [CrossRef]
- Wu, C.J.; Li, C.S.; Zhang, Y.; Yang, J. Application of positron emission tomography in the detection of myocardial metabolism in pig ventricular fibrillation and asphyxiation cardiac arrest models after resuscitation. Biomed. Environ. Sci. 2014, 27, 531–536. [Google Scholar] [CrossRef]
- Lurie, K.G.; Zielinski, T.; McKnite, S.; Aufderheide, T.; Voelckel, W. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation 2002, 105, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, A.; Sakuramoto, H.; Unoki, T.; Yoshino, Y.; Hosino, H.; Koyama, Y.; Enomoto, Y.; Shimojo, N.; Mizutani, T.; Inoue, Y. Effects of manual rib cage compressions on mucus clearance in mechanically ventilated pigs. Respir. Care 2020, 65, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Terakado, T.; Marushima, A.; Koyama, Y.; Tsuruta, W.; Takigawa, T.; Ito, Y.; Hino, T.; Sato, M.; Hayakawa, M.; Ishikawa, E.; et al. Effectiveness of near-infrared spectroscopy (NIRO-200NX, pulse mode) for risk management in carotid artery stenting. World Neurosurg. 2019, 131, e425–e432. [Google Scholar] [CrossRef] [PubMed]
- Zaramella, P.; Saraceni, E.; Freato, F.; Falcon, E.; Suppiej, A.; Milan, A.; Laverda, A.M.; Chiandetti, L. Can tissue oxygenation index (TOI) and cotside neurophysiological variables predict outcome in depressed/asphyxiated newborn infants? Early Hum. Dev. 2007, 83, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Bein, B.; Cavus, E.; Stadlbauer, K.H.; Tonner, P.H.; Steinfath, M.; Scholz, J.; Dörges, V. Monitoring of cerebral oxygenation with near infrared spectroscopy and tissue oxygen partial pressure during cardiopulmonary resuscitation in pigs. Eur. J. Anaesthesiol. 2006, 23, 501–509. [Google Scholar] [CrossRef]
- Genbrugge, C.; De Deyne, C.; Eertmans, W.; Anseeuw, K.; Voet, D.; Mertens, I.; Sabbe, M.; Stroobants, J.; Bruckers, L.; Mesotten, D.; et al. Cerebral saturation in cardiac arrest patients measured with near-infrared technology during pre-hospital advanced life support. Results from Copernicus I cohort study. Resuscitation 2018, 129, 107–113. [Google Scholar] [CrossRef]
- Jakkula, P.; Hästbacka, J.; Reinikainen, M.; Pettilä, V.; Loisa, P.; Tiainen, M.; Wilkman, E.; Bendel, S.; Birkelund, T.; Pulkkinen, A.; et al. Near-infrared spectroscopy after out-of-hospital cardiac arrest. Crit. Care 2019, 23, 171. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Reis, C.; Akyol, O.; Araujo, C.; Huang, L.; Enkhjargal, B.; Malaguit, J.; Gospodarev, V.; Zhang, J.H. Pathophysiology and the monitoring methods for cardiac arrest associated brain injury. Int. J. Mol. Sci. 2017, 18, 129. [Google Scholar] [CrossRef]
- Cavus, E.; Bein, B.; Dörges, V.; Stadlbauer, K.H.; Wenzel, V.; Steinfath, M.; Hanss, R.; Scholz, J. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation 2006, 71, 97–106. [Google Scholar] [CrossRef]
- Mavroudis, C.D.; Ko, T.S.; Morgan, R.W.; Volk, L.E.; Landis, W.P.; Smood, B.; Xiao, R.; Hefti, M.; Boorady, T.W.; Marquez, A.; et al. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit. Care 2020, 24, 583. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Xanthos, T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail. Rev. 2012, 17, 117–128. [Google Scholar] [CrossRef]
- Mayr, V.D.; Wenzel, V.; Voelckel, W.G.; Krismer, A.C.; Mueller, T.; Lurie, K.G.; Lindner, K.H. Developing a vasopressor combination in a pig model of adult asphyxial cardiac arrest. Circulation 2001, 104, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Cournoyer, A.; Iseppon, M.; Chauny, J.M.; Denault, A.; Cossette, S.; Notebaert, E. Near-infrared spectroscopy monitoring during cardiac arrest: A systematic review and meta-analysis. Acad. Emerg. Med. 2016, 23, 851–862. [Google Scholar] [CrossRef] [PubMed]

| VF-CA (N = 10) | A-CA (N = 10) | p-Value | |
|---|---|---|---|
| Age (days) | 67.5 (60.3–71.0) | 76.0 (61.5–81.5) | 0.201 |
| Body weight (kg) | 24.0 (23.8–25.2) | 23.3 (22.2–23.8) | 0.055 |
| TOI (%) | 58.2 (55.1–60.1) | 56.3 (55.4–57.4) | 0.069 |
| Vital signs | |||
| mABP (mmHg) | 103.5 (92.0–113.5) | 80.0 (69.3–94.8) | 0.134 |
| EtCO2 (mmHg) | 42.5 (40.5–46.0) | 43.5 (40.5–46.5) | 0.573 |
| Arterial blood gas | |||
| pH | 7.43 (7.41–7.47) | 7.43 (7.41–7.46) | 0.573 |
| PaO2 (mmHg) | 93 (76–118) | 104 (90–119) | 0.416 |
| PaCO2 (mmHg) | 40.9 (38.1–44.3) | 41.6 (37.7–43.7) | 0.564 |
| Lactate (mmol/L) | 2.70 (1.65–3.58) | 2.54 (1.82–4.38) | 0.535 |
| Pre-CPR Phase | CPR Phase | Post-CPR Phase | ||||
|---|---|---|---|---|---|---|
| VF-CA | A-CA | VF-CA | A-CA | VF-CA | A-CA | |
| TOI (%) | ||||||
| Initial | 58.2 (55.1–60.1) | 56.3 * (55.4–57.4) | 45.0 (41.2–48.2) | 44.9 (42.7–46.2) | 50.1 (46.2–54.6) | 49.2 (47.2–51.2) |
| Maximum | 58.8 (55.4–60.5) | 56.9 (55.6–57.8) | 56.8 (52.6–59.4) | 49.0 * (47.9–50.6) | 59.0 (56.4–61.5) | 53.1 * (51.3–56.5) |
| Minimum | 41.8 (40.6–46.9) | 39.3 * (35.6–41.4) | 41.9 (39.2–43.4) | 42.5 (38.4–44.3) | 45.6 (41.9–47.8) | 45.1 (42.9–46.8) |
| Median | 48.5 (45.1–50.2) | 45.1 * (43.2–45.4) | 49.3 (46.3–53.4) | 46.3 * (44.6–48.8) | 52.0 (49.9–54.3) | 49.6 (47.8–52.0) |
| Value at the singular point | – | – | 50.1 (48.7–52.5) | 47.2 * (44.9–48.2) | – | – |
| Vital signs (mmHg) | ||||||
| mABP initial | – | – | 69.5 (60.0–88.0) | 30.5 * (17.8–39.0) | 143.5 (113.8–167.8) | 108.5 (45.3–127.5) |
| mABP median | 30.0 (23.0–32.8) | 26.0 (15.5–28.8) | 66.3 (58.1–78.5) | 50.0 * (44.0–57.3) | 62.0 (57.0–84.0) | 56.0 (51.0–63.3) |
| EtCO2 median | – | – | 22.0 (17.5–31.0) | 22.5 (11.8–34.6) | 38.0 (35.0–41.0) | 37.0 (25.8–40.8) |
| Arterialblood gas | ||||||
| pH | 7.42 (7.41–7.45) | 7.01 * (6.95–7.03) | 7.299 (7.212–7.458) | 7.141 * (7.074–7.219) | 7.171 (7.125–7.221) | 6.980 (6.926–7.107) |
| PaO2 (mmHg) | 76.8 (56.7–96.6) | 10.2 * (5.8–12.5) | 137.0 (109.0–202.8) | 165.8 (71.8–219.2) | 166.0 (121.0–265.0) | 259.7 (215.1–368.5) |
| PaCO2 (mmHg) | 42.5 (37.7–46.6) | 107.0 * (100.2–131.3) | 50.8 (31.5–54.4) | 48.7 (33.7–56.9) | 53.0 (38.9–56.2) | 46.9 (43.5–57.0) |
| Lactate (mmol/L) | 2.7 (1.8–3.6) | 12.0 * (7.8–14.2) | 8.1 (7.3–8.9) | 14.4 * (13.3–15.5) | 10.1 (9.1–12.9) | 15.2 * (14.2–18.2) |
| VF-CA (n = 10) | A-CA (n = 10) | p-Value | |
|---|---|---|---|
| ROSC | 9 | 6 | 0.303 |
| Survived for 60 min after ROSC | 7 | 3 | 0.180 |
| Recovered movement | 7 | 1 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyama, Y.; Ouchi, A.; Shimojo, N.; Inoue, Y. Differences in Cerebral Oxygenation in Cardiogenic and Respiratory Cardiac Arrest Before, During, and After Cardiopulmonary Resuscitation. J. Clin. Med. 2023, 12, 2923. https://doi.org/10.3390/jcm12082923
Koyama Y, Ouchi A, Shimojo N, Inoue Y. Differences in Cerebral Oxygenation in Cardiogenic and Respiratory Cardiac Arrest Before, During, and After Cardiopulmonary Resuscitation. Journal of Clinical Medicine. 2023; 12(8):2923. https://doi.org/10.3390/jcm12082923
Chicago/Turabian StyleKoyama, Yasuaki, Akira Ouchi, Nobutake Shimojo, and Yoshiaki Inoue. 2023. "Differences in Cerebral Oxygenation in Cardiogenic and Respiratory Cardiac Arrest Before, During, and After Cardiopulmonary Resuscitation" Journal of Clinical Medicine 12, no. 8: 2923. https://doi.org/10.3390/jcm12082923
APA StyleKoyama, Y., Ouchi, A., Shimojo, N., & Inoue, Y. (2023). Differences in Cerebral Oxygenation in Cardiogenic and Respiratory Cardiac Arrest Before, During, and After Cardiopulmonary Resuscitation. Journal of Clinical Medicine, 12(8), 2923. https://doi.org/10.3390/jcm12082923






