Amyotrophic Lateral Sclerosis Mimic Syndrome in a 24-Year-Old Man with Chiari 1 Malformation and Syringomyelia: A Clinical Case
Abstract
:1. Introduction
2. Case Presentation
2.1. Patient History
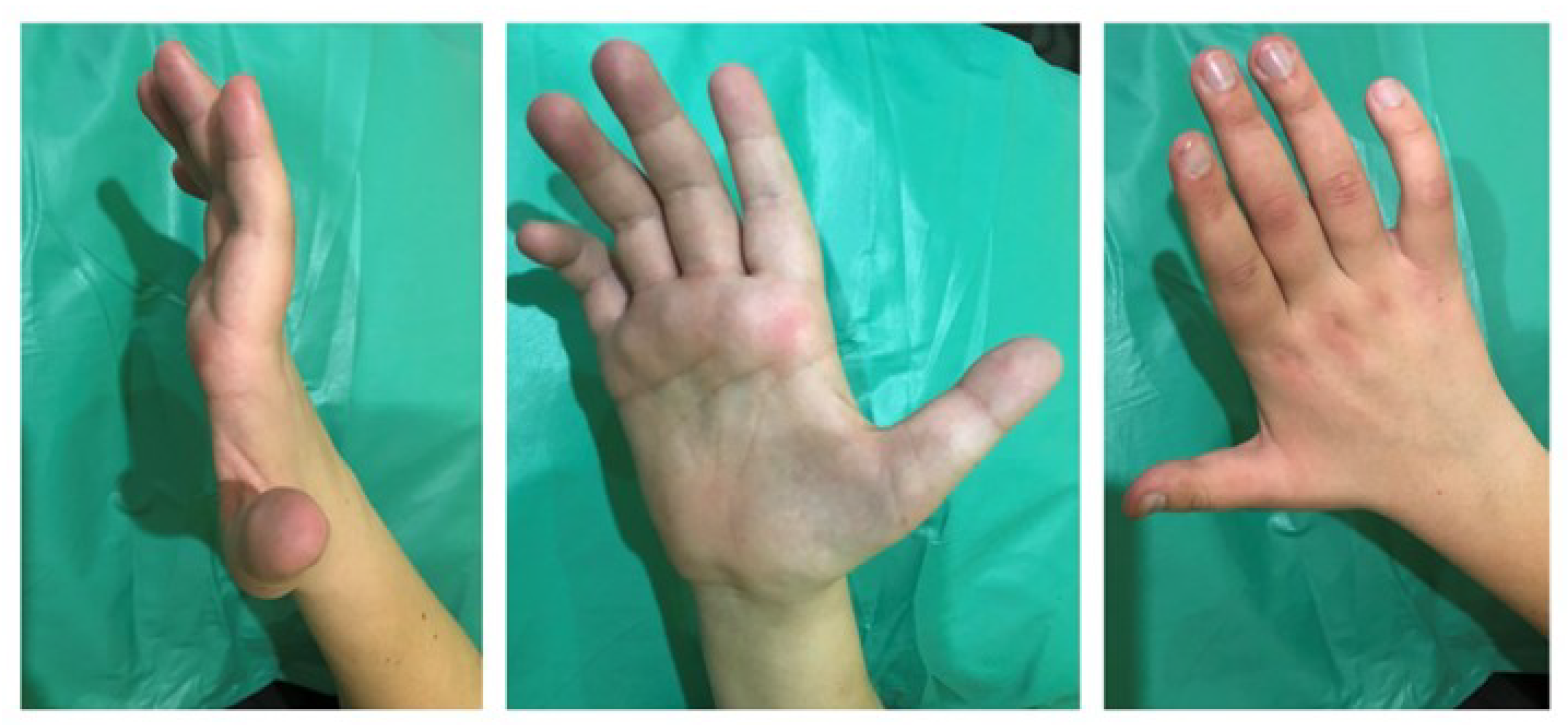
2.2. Physical Examination
2.3. Laboratory Results
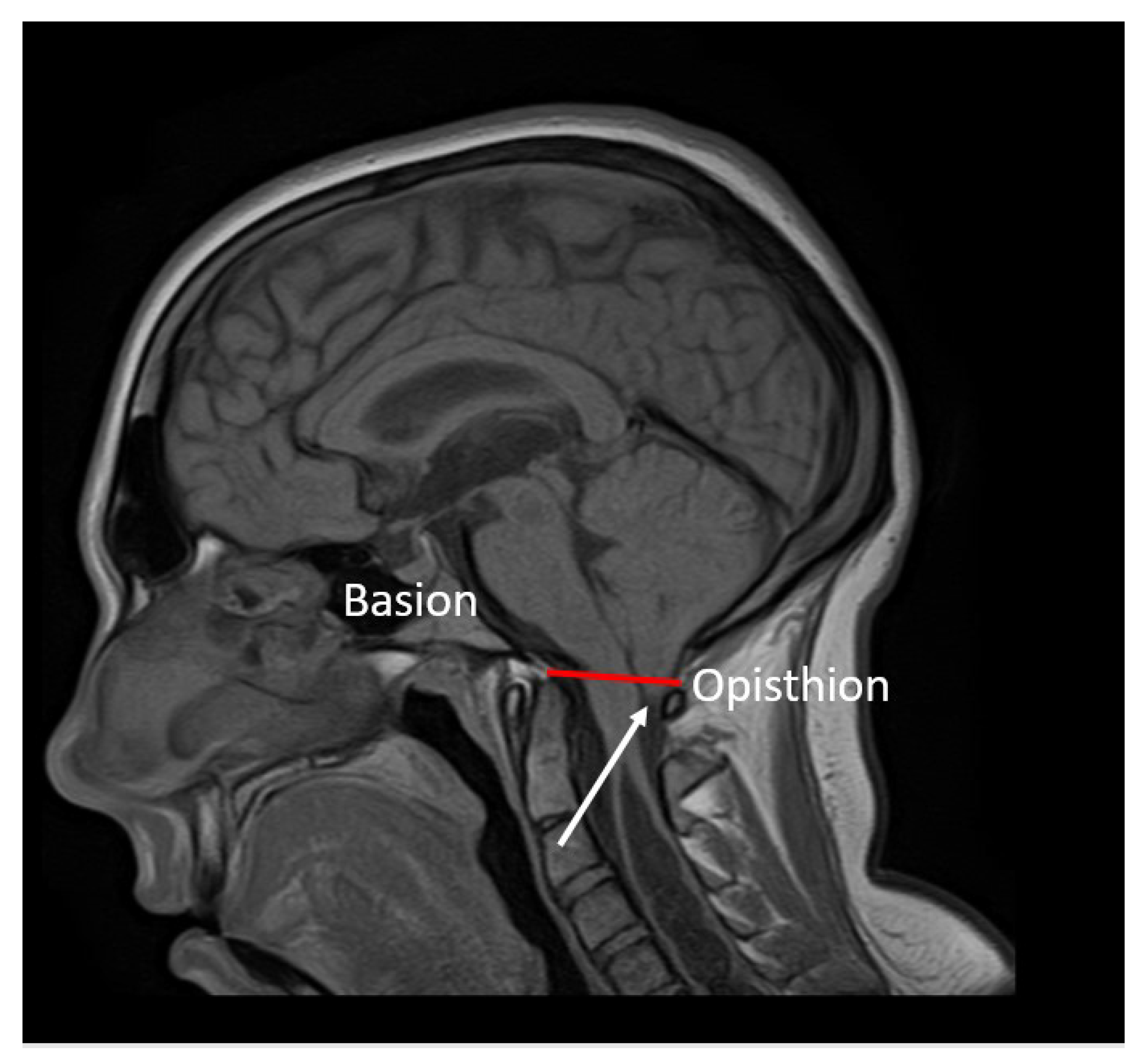
2.4. Neuroimaging
2.5. Electrophysiology
2.5.1. Evoked Electromyography
2.5.2. Quantitative Needle Electromyography
3. Discussion
4. Conclusions
5. Declaration of Patient Consent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kular, S.; Cascella, M. Chiari I Malformation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hidalgo, J.A.; Tork, C.A.; Varacallo, M. Arnold Chiari Malformation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bogdanov, E.I.; Faizutdinova, A.T.; Heiss, J.D. Posterior cranial fossa and cervical spine morphometric abnormalities in symptomatic Chiari type 0 and Chiari type 1 malformation patients with and without syringomyelia. Acta Neurochir 2021, 163, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, E.I.; Mendelevich, E.G.; Khabibrakhmanov, A.N.; Bogdanov, S.E.; Mukhamedzhanova, G.R.; Mukhamedyarov, M.M. Clinical cases of amyotrophic lateral sclerosis concurrent with hydromyelia. Clin Case Rep. 2021, 9, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Massimi, L.; Peretta, P.; Erbetta, A.; Solari, A.; Farinotti, M.; Ciaramitaro, P.; Saletti, V.; Caldarelli, M.; Canheu, A.C.; Celada, C.; et al. International Experts—Jury of the Chiari & Syringomyelia Consensus Conference, “Milan, November 11–13, 2019”. Diagnosis and treatment of Chiari malformation type 1 in children: The International Consensus Document. Neurol Sci. 2022, 43, 1311–1326. [Google Scholar] [CrossRef]
- Sadler, B.; Kuensting, T.; Strahle, J.; Park, T.S.; Smyth, M.; Limbrick, D.D.; Dobbs, M.B.; Haller, G.; Gurnett, C.A. Prevalence and Impact of Underlying Diagnosis and Comorbidities on Chiari 1 Malformation. Pediatr Neurol. 2020, 106, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Treiber, J.M.; Bauer, D.F. Chiari 1 and Hydrocephalus—A Review. Neurol India. 2021, 69, S362–S366. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, J.S.; Maggio, D.; Pang, Y.; Nazari, M.A.; Gonzales, M.K.; Lechan, R.M.; Smirniotopoulos, J.G.; Zhuang, Z.; Pacak, K.; Heiss, J.D. Chiari Malformation Type 1 in EPAS1-Associated Syndrome. Int. J. Mol. Sci. 2019, 20, 2819. [Google Scholar] [CrossRef]
- Noureldine, M.H.A.; Shimony, N.; Jallo, G.I.; Groves, M.L. Scoliosis in patients with Chiari malformation type I. Childs Nerv. Syst. 2019, 35, 1853–1862. [Google Scholar] [CrossRef]
- Rafay, M.; Gulzar, F.; Jafri, H.M.; Sharif, S. Delayed Presentation in Chiari Malformation. Asian J. Neurosurg. 2021, 16, 701–705. [Google Scholar] [CrossRef]
- Valentini, L.G.; Saletti, V.; Erbetta, A.; Chiapparini, L.; Furlanetto, M. Chiari 1 malformation and untreated sagittal synostosis: A new subset of complex Chiari? Childs Nerv. Syst. 2019, 35, 1741–1753. [Google Scholar] [CrossRef]
- Urbizu, A.; Garrett, M.E.; Soldano, K.; Drechsel, O.; Loth, D.; Marcé-Grau, A.; Mestres, I.; Soler, O.; Poca, M.A.; Ossowski, S.; et al. Rare functional genetic variants in COL7A1, COL6A5, COL1A2 and COL5A2 frequently occur in Chiari Malformation Type 1. PLoS ONE 2021, 16, e0251289. [Google Scholar] [CrossRef]
- Mancarella, C.; Delfini, R.; Landi, A. Chiari Malformations. Acta Neurochir. Suppl. 2019, 125, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Holly, L.T.; Batzdorf, U. Chiari malformation and syringomyelia. J. Neurosurg. Spine 2019, 31, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Al-Habib, A.F.; Al Abdulsalam, H.; Ahmed, J.; Albadr, F.; Alhothali, W.; Alzahrani, A.; Abojamea, A.; Altowim, A.; Ullah, A.; Alkubeyyer, M. Association between craniovertebral junction abnormalities and syringomyelia in patients with chiari malformation type-1. Neurosciences 2020, 25, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Garbossa, D.; Peretta, P.; Piatelli, G.; Massimi, L.; Valentini, L.; Migliaretti, G.; Baldovino, S.; Roccatello, D.; Kodra, Y.; et al. Interregional Chiari and Syringomyelia Consortium; on behalf of the Interregional Chiari and Syringomyelia Consortium. Syringomyelia and Chiari Syndrome Registry: Advances in epidemiology, clinical phenotypes and natural history based on a North Western Italy cohort. Ann. Ist Super Sanita. 2020, 56, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Jussila, M.P.; Nissilä, J.; Vakkuri, M.; Olsén, P.; Niinimäki, J.; Leinonen, V.; Serlo, W.; Salokorpi, N.; Suo-Palosaari, M. Preoperative measurements on MRI in Chiari 1 patients fail to predict outcome after decompressive surgery. Acta Neurochir. 2021, 163, 2005–2014. [Google Scholar] [CrossRef]
- Q07.00-Arnold-Chiari syndrome without spina bifida or hydrocephalus. In ICD-10-CM, 10th ed.; Centers for Medicare and Medicaid Services and the National Center for Health Statistics, 2018; Available online: https://www.unboundmedicine.com/icd/view/ICD-10-CM/886972/all/Q07_00___Arnold_Chiari_syndrome_without_spina_bifida_or_hydrocephalus (accessed on 23 December 2021).
- Klekamp, J. How Should Syringomyelia be Defined and Diagnosed? World Neurosurg. 2018, 111, e729–e745. [Google Scholar] [CrossRef]
- Brickell, K.L.; Anderson, N.E.; Charleston, A.J.; Hope, J.K.; Bok, A.P.; Barber, P.A. Ethnic differences in syringomyelia in New Zealand. J. Neurol. Neurosurg. Psychiatry 2006, 77, 989–991. [Google Scholar] [CrossRef]
- Goncharova, P.; Davidova, T.; Shnayder, N.; Novitsky, M.; Nasyrova, R. Epidemiology of amyotrophic lateral sclerosis. Pers. Psychiatry Neurol. 2022, 2, 57–66. [Google Scholar] [CrossRef]
- Goncharova, P.S.; Davydova, T.K.; Popova, T.E.; Novitsky, M.A.; Petrova, M.M.; Gavrilyuk, O.A.; Al-Zamil, M.; Zhukova, N.G.; Nasyrova, R.F.; Shnayder, N.A. Nutrient effects on motor neurons and the risk of amyotrophic lateral sclerosis. Nutrients 2021, 13, 3804. [Google Scholar] [CrossRef]
- Sakushima, K.; Tsuboi, S.; Yabe, I.; Hida, K.; Terae, S.; Uehara, R.; Nakano, I.; Sasaki, H. Nationwide survey on the epidemiology of syringomyelia in Japan. J. Neurol. Sci. 2012, 313, 147–152. [Google Scholar] [CrossRef]
- Holste, K.G.; Muraszko, K.M.; Maher, C.O. Epidemiology of Chiari I malformation and syringomyelia. Neurosurg. Clin. N. Am. 2023, 34, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.S.; Sampath, R. Syringomyelia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bogdanov, E.I.; Faizutdinova, A.T.; Mendelevich, E.G.; Sozinov, A.S.; Heiss, J.D. Epidemiology of Symptomatic Chiari Malformation in Tatarstan: Regional and Ethnic Differences in Prevalence. Neurosurgery 2019, 84, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Guan, J.; Du, Y.; Fang, Z.; Wang, X.; Yao, Q.; Zhang, C.; Jia, S.; Liu, Z.; Wang, K.; et al. Spinal Obstruction-Related vs. Craniocervical Junction-Related Syringomyelia: A Comparative Study. Front. Neurol. 2022, 13, 900441. [Google Scholar] [CrossRef] [PubMed]
- Trojsi, F.; D’Alvano, G.; Bonavita, S.; Tedeschi, G. Genetics and Sex in the Pathogenesis of Amyotrophic Lateral Sclerosis (ALS): Is There a Link? Int. J. Mol. Sci. 2020, 21, 3647. [Google Scholar] [CrossRef]
- Bogdanov, E.I.; Heiss, J.D.; Mendelevich, E.G.; Mikhaylov, I.M.; Haass, A. Clinical and neuroimaging features of “idiopathic” syringomyelia. Neurology 2004, 62, 791–794. [Google Scholar] [CrossRef]
- Bryukhovetskiy, A.S.; Grivtsova, L.Y.; Sharma, H.S. Is the ALS a motor neuron disease or a hematopoietic stem cell disease? Prog. Brain Res. 2020, 258, 381–396. [Google Scholar] [CrossRef]
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 377, 1688–1700. [Google Scholar] [CrossRef]
- Leclerc, A.; Matveeff, L.; Emery, E. Syringomyelia and hydromyelia: Current understanding and neurosurgical management. Rev. Neurol. 2021, 177, 498–507. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhu, Z.; Wang, B.; Yu, Y. Abnormal spread of junctional acetylcholine receptor of paraspinal muscles in scoliosis associated with syringomyelia. Stud. Health Technol. Inform. 2006, 123, 117–122. [Google Scholar]
- Zhu, Z.Z.; Qiu, Y.; Wang, B.; Yu, Y.; Wu, L.; Qian, B.P.; Ma, W.W. Histochemical changes of muscle fibers and motor end-plates of paravertebral muscles in scoliosis associated with syringomyelia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006, 28, 790–794. (In Chinese) [Google Scholar]
- McCormick, J.R.; Sama, A.J.; Schiller, N.C.; Butler, A.J.; Donnally, C.J., 3rd. Cervical Spondylotic Myelopathy: A Guide to Diagnosis and Management. J. Am. Board Fam. Med. 2020, 33, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Y.; Xu, Q.; Gu, R.; Zhao, J. Cervical spondylotic amyotrophy: A systematic review. Eur. Spine J. 2019, 28, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, Y.; Wu, J.; Luo, S.; Zheng, C.; Sun, C.; Nie, C.; Xia, X.; Ma, X.; Lyu, F.; et al. Update on the Pathogenesis, Clinical Diagnosis, and Treatment of Hirayama Disease. Front. Neurol. 2022, 12, 811943. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Cascella, M. Multifocal Motor Neuropathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yeh, W.Z.; Dyck, P.J.; van den Berg, L.H.; Kiernan, M.C.; Taylor, B.V. Multifocal motor neuropathy: Controversies and priorities. J. Neurol. Neurosurg. Psychiatry 2020, 91, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kassubek, J.; Pagani, M. Imaging in amyotrophic lateral sclerosis: MRI and PET. Curr. Opin. Neurol. 2019, 32, 740–746. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Zhang, S.; Fan, D. Upper Motor Neuron Signs in the Cervical Region of Patients With Flail Arm Syndrome. Front. Neurol. 2021, 12, 610786. [Google Scholar] [CrossRef]
- Braun, N.; Macklin, E.A.; Sinani, E.; Sherman, A.; Weber, M. Pooled Resource Open-Access ALS Clinical Trials Consortium. The revised El Escorial criteria “clinically probable laboratory supported ALS”-once a promising now a superfluous category? Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 24–28. [Google Scholar] [CrossRef]
- Petit, H.; Rousseaux, M.; Gozet, G.; Mazingue, M. Amyotrophie spinale cervico-thoracique pure par hydromyélie. Stabilisation par dérivation ventriculaire. Isolated cervicothoracic spinal amyotrophy caused by hydromyelia. Stabilization by a ventricular shunt. Rev. Neurol. 1984, 140, 144–147. (In French) [Google Scholar]
- Lim, S.H.; Wong, M.C.; Puvan, K. Syringomyelia with Arnold Chiari I malformation: A report of four cases. Singapore Med. J. 1989, 30, 376–379. [Google Scholar]
- Hamada, K.; Sudoh, K.; Fukaura, H.; Yanagihara, T.; Hamada, T.; Tashiro, K.; Isu, T. An autopsy case of amyotrophic lateral sclerosis associated with cervical syringomyelia. No Shinkei 1990, 42, 527–531. (In Japanese) [Google Scholar] [PubMed]
- Yoshitoshi, M.; Shinohara, Y.; Akiyama, K.; Yoshii, F.; Takeoka, T. A case of syringomyelia with proximal dominant muscle weakness and without superficial sensory disturbance. Rinsho Shinkeigaku 1992, 32, 1143–1145. (In Japanese) [Google Scholar] [PubMed]
- Titlic, M.; Jukic, I.; Tonkic, A.; Buca, A.; Dolic, K. Vertigo associated with Chiari I malformation and syringomyelia. Bratisl. Lek. Listy 2008, 109, 168–170. [Google Scholar] [PubMed]
- Cağan, E.; Sayin, R.; Doğan, M.; Peker, E.; Cağan, H.H.; Caksen, H. Bilateral brachial plexus palsy and right Horner syndrome due to congenital cervicothoracal syringomyelia. Brain Dev. 2010, 32, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Waqar, M.; Vohra, A.H. Dissociated sensory loss and muscle wasting in a young male with headaches: Syringomyelia with type 1 Arnold-Chiari malformation. BMJ Case Rep. 2013, 29, bcr2013201708. [Google Scholar] [CrossRef]
- Kadoya, T.; Takenaka, I.; Kinoshita, Y.; Shiraishi, M.; Uehara, H.; Yamamoto, T.; Joyashiki, T. Monitoring of Somatosensory Evoked Potentials during Foramen Magnum Decompression of Chiari Malformation Type I Complicated with Syringomyelia: A Report of Two Cases. Masui 2015, 64, 313–317. (In Japanese) [Google Scholar]
- Mora, J.R.; Rison, R.A.; Beydoun, S.R. Chiari malformation type I with cervicothoracic syringomyelia masquerading as bibrachial amyotrophy: A case report. J. Med. Case Rep. 2015, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.G.; Richard, S.A.; Liu, J.; You, C. Chiari type I malformation with cervicothoracic syringomyelia subterfuge as flail arm syndrome. Neurol. Int. 2017, 9, 7336. [Google Scholar] [CrossRef]
- Storti, B.; Diamanti, S.; Tremolizzo, L.; Riva, N.; Lunetta, C.; Filippi, M.; Ferrarese, C. Appollonio I: ALS Mimics due to Affection of the Cervical Spine: From Common Compressive Myelopathy to Rare CSF Epidural Collection. Case Rep. Neurol. 2021, 13, 145–156. [Google Scholar] [CrossRef]
- Zheng, Y.-C.; Liu, Y.-T.; Wei, K.-C.; Huang, Y.-C.; Chen, P.-Y.; Hsu, Y.-H.; Lin, C.-L. Outcome predictors and clinical presentation of syringomyelia. Asian J. Surg. 2023, 46, 705–711. [Google Scholar] [CrossRef]
- Lo Coco, D.; Militello, A.; Piccoli, F.; La Bella, V. Bulbar-onset amyotrophic lateral sclerosis in a patient with Chiari I malformation. Acta Neurol. Scand. 2001, 104, 243–245. [Google Scholar] [CrossRef] [PubMed]











| Reflex | Left | Right | Comparison between the Parties |
|---|---|---|---|
| Brow reflex | Normal | Normal | D = S |
| Mandibular reflex | Normal | Normal | D = S |
| Flexion-elbow reflex | Decreased | Decreased | D < S |
| Carporadial reflex | Decreased | Decreased | D < S |
| Bekhterev scapulohumeral reflex | Normal | Normal | D = S |
| Extensor-elbow reflex | Decreased | Decreased | D < S |
| Bekhterev bone-abdominal reflex | Normal | Normal | D = S |
| Deep abdominal reflex | Normal | Normal | D = S |
| Knee reflex | Normal | Normal | D = S |
| Achilles reflex | Normal | Normal | D = S |
| Disorder | Clinical Findings | MRI Findings | Pathogenesis |
|---|---|---|---|
| Cervical myelopathy [35] | Neck pain, hand numbness and paresthesia, loss of fine dexterity, amyotrophy and weakness of both the upper and the lower limbs, gait difficulties, Lhermitte’s phenomenon, and sphincter incontinence. | Intramedullary signal abnormalities due to edema and structural changes with a hyperintense signal on T2-weighted MRI (Figure 12) | Compression of the spinal cord by spondylotic changes. |
| Cervical spondylotic amyotrophy [36] | Slowly progressive amyotrophy with weakness of the proximal extremity of one or both upper limbs, often preceded by a transient period of shoulder pain without sensory loss | Narrowed intervertebral space between the vertebras of the cervical level. Sagittal T2-weighted magnetic resonance image showing root compression and impingement against prolapsed cervical discs | Isolated intradural compression of multiple motor roots, without damage to either the spinal cord or the sensory roots. |
| Hirayama disease [37] | Predominantly unilateral upper extremity weakness and atrophy, cold paresis, and no sensory or pyramidal tract involvement. It is also characterized by muscle weakness and atrophy in the hand and forearm with sparing of the brachioradialis, giving the characteristic appearance of oblique amyotrophy that affects the C7, C8 and T1 myotomes. The amyotrophy is unilateral in most patients, asymmetrically bilateral in some and rarely symmetric. | Abnormal T2-weighted signal of the spinal cord at the site of maximum forward shift without an obvious cause neutral position: abnormal T2-weighted signal of the spinal cord at the site of maximum forward shift without an obvious cause. | Spinal cord compression by the posterior dural sac during neck flexion |
| Multifocal motor neuropathy [38,39] | The pattern of weakness may be related to specific nerves. Due to the development of paresis as a result of conduction block, a high degree of weakness does not correlate with moderate muscle atrophy. Sensory disturbances could not be detected. Often recognized in patients with a short disease duration. In EMG complex unstable MUPs without spontaneous activity at rest. Rapid recruitment pattern disproportionate to muscular atrophy. Fasciculation potentials may be recoded. | No specific changes on MRI | Chronic progressive immune-mediated motor neuropathy, which leads to progressive asymmetric weakness and partial motor conduction block in EMG. Anti-GM1 antibodies are identified in at least 40% of patients. |
| Idiopathic ALS [40,41] | Progression of lower motor neuron signs (including EMG features in clinically unaffected muscles) and upper motor neuron signs, muscle atrophy, paralysis, frontotemporal dementia with absence of sensory signs. EMG is characterized by fasciculations in one or more regions, neurogenic changes, normal motor and sensory nerve conduction and absence of conduction blocks. | Bilateral symmetric T2 and FLAIR hyperintensities of the corticospinal tract. Decrease of cerebral volume in the gray matter of the frontal and temporal lobes. Gliosis and axonal degeneration of the anterolateral spinal cord, manifested by T1 hyperintensity and T2 hyperintensity. | The pathogenesis of ALS is still unknown. Mitochondrial dysfunction in cells leads to serious disorders associated with pathological changes in ALS, such as disturbances in the calcium buffer period, excessive formation of free radicals, which leads to an increase in mitochondrial membrane permeability and oxidative stress. |
| The flail arm syndrome [42] | Slow progressive, predominantly proximal weakness, and atrophy of the upper limbs. Symptoms may be limited to the cervical region without functional involvement of the lower extremities, pectorals, or bulbar muscles for at least 12 months. Five patients out of 10 develop signs of the UMN in the upper extremities and 7 patients out of 10 in the lower extremities. | Same as ALS | Involvement of the corticospinal tract in Flail arm syndrome is identical to that in the patients with ‘classic’ ALS. Flail arm syndrome had a pattern of microstructural alterations in corticofugal tracts identical to those seen in ALS. Because of that there is a hypothesis that flail arm syndrome is such a phenotype of ALS. |
| Author | Year | Sex | M/Age | Cl/ Age | Upper Extremities | Lower Extremities | EMG | Initial Side | Asymmetry | MNI |
|---|---|---|---|---|---|---|---|---|---|---|
| Petit et al. [44] | 1984 | M | 24 | 25 | Weakness, amyotrophy, fasciculations and areflexia were found in the upper limbs without sensory disorders. | Hyperreflexia in the lower limbs. | Both sides | S = D | UMN LMN | |
| Lim et al. [45] | 1989 | F | 30 | 40 | Progressive weakness and hypotrophy of the upper limbs with hypotonia and areflexia were noted especially in the left side. Strength in the proximal and distal muscles generally was 2/5 points in the right hand and 1/5 points in the left hand. Dissociated sensory loss to pain and temperature was over C4 to T1 dermatomes. | Weakness and spasticity in the lower limbs. Strength in the flexor muscles was 4/5 points. | Both sides | S > D | LMN UMN | |
| Lim et al. [45] | 1989 | F | 37 | 43 | The first symptom was numbness, followed by weakness in the left upper extremity and then in the right upper extremity Subsequently, the numbness progressed to involve the chest anteriorly and posteriorly. The upper limbs were hypotonic and areflexic. Strength in the hands was 1/5 point on the left and 3/5 points on the right. There was diminished sensation of all modalities including the vibration and position senses but especially the pain and temperature from left C5 to L1 dermatomes | Spasticity and weakness in the lower limbs. Strength was 4/5 points. | Left side | S > D | LMN UMN | |
| Lim et al. [45] | 1989 | F | 42 | 62 | Right-sided ptosis was diagnosed at the age of 42 years. At age of 62 years, hypotrophy, weakness, and numbness of the upper extremities first developed in the right hand and then spread to the left with hypotonia and hyporeflexia. Strength in the proximal muscles was 3/5 points and 2/5 points in the distal muscles. | Spasticity and mild weakness in the lower extremities. | Right side | S = D | LMN UMN | |
| Lim et al. [45] | 1989 | M | 26 | 27 | Hypotrophy and weakness of both hands progressively developed. Hypotonia and hyporeflexia were observed only in the right hand. Sensory disorders began with a painless burning feeling over the left forearm. Later, progressive numbness developed in the left side of the neck, left upper limb, right upper limb, and spread to the left side of the trunk down to the middle of the left thigh. Loss of pain and temperature sensation with the preservation of touch; position and vibration senses were found over the left C2 to L2 and over the right C2 to Th7 | Mild spasticity and weakness of the lower extremity. | Both sides | D > S | LMN UMN | |
| Hamada et al. [46] | 1990 | M | 54 | 59 | Weakness and atrophy of the proximal muscles of both the upper extremities at the age of 56 years. Hyperreflexia in the upper extremities. | Weakness of both the lower extremities started at the age of 54 years. Hyperreflexia and respiratory muscle weakness, pathological reflexes on both legs, and tongue fasciculation developed successively within 5 years before death from distress at 59 years of age. | Both sides | S = D | LMN UMN | |
| Yoshitoshi et al. [47] | 1992 | F | 39 | 43 | Muscle weakness and atrophy were prominent in the bilateral deltoid muscles. No hypotrophy and weakness signs were noted in the bilateral forearms and hand muscle. There was no sign of sensory impairment except vibratory sensation. | Normal | Neurogenic disorder of motor unit potential in EMG in the deltoid muscles. | Both sides | S = D | LMN |
| Titlic et al. [48] | 2008 | F | 42 | 44 | Left hand Weakness in 4–5 fingers. Hypoesthesia in C4–C8 segments | Normal | EMG indicates a moderate chronic radicular bilateral lesion of the C7 root and an initial right-side lesion of the C6 and C8 roots | Left side | LMN | |
| Cağan et al. [49] | 2010 | Weakness of the upper extremities with decrease of strength up to 1/5 point bilaterally. | Normal | EMG revealed bilateral brachial plexus palsy. | Both sides | S = D | LMN | |||
| Waqar et al. [50] | 2013 | F | 16 | 21 | Slight weakness in the hands and hypotrophy of the thenar and hypothenar eminences of the left hand. Numbness and pain in the upper limbs and loss of pain and temperature sensation in the C3-T2 dermatomes, bilaterally. | Normal | Left side | S > D | LMN | |
| Kadoya et al. [51] | 2015 | 40 | 40 (2 Months) | Numbness and muscular weakness of the bilateral upper limbs. | Normal | Both sides | S = D | LMN | ||
| Kadoya et al. [51] | 2015 | 32 | 32 (5 Months) | Muscular weakness of the bilateral upper limbs | Numbness and muscular weakness of the lower limbs developed for 2 months. | Both sides | S = D | LMN UMN | ||
| Mora et al. [52] | 2015 | M | 16 | 55 | At the age of 16 years, weakness was observed in the right hand. At 55 years old, the patient was diagnosed with asymmetric focal segmental atrophy of his bilateral forearm flexor and extensor muscle groups with preservation of the bilateral brachioradialis muscles. Strength in the proximal muscles was 4/5 to 5/5 points and 1/5 point in the distal muscles. | Mild weakness. Strength in the left hip extension and flexion was 4/5 points and 4/5 points in the bilateral knee extensors. | Absence of CMAP of his left median, ulnar, and radial nerves. SNAP responses were normal and slightly low in the ulnar and radial nerves. CMAP of the peroneal and tibial nerves and SNAP of sural nerves were normal. SNAPs were normal. EMG of his upper extremities demonstrated evidence of diffuse chronic neurogenic changes in the C5 to T1 innervated muscles as well as evidence of active denervation in his right triceps brachii. In his left lower extremity, chronic neurogenic changes were noted in the gastrocnemius medial head. His left cervical paraspinal muscles were normal on EMG. | Left side | S > D | LMN UMN |
| Lan et al. [53] | 2017 | M | 21 | 44 | Severe weakness: Strength in the proximal muscles was 0/5 points and 0/5 points in the distal muscles. Severe atrophy of the bilateral forearm flexor and extensor muscle groups. | Normal | Absence of CMAP of the median, ulnar, and radial nerves with slightly abnormal SNAP of the median, ulnar, and radial nerves. EMG of all nerves in the lower extremities was normal. All the nerves on the lower limbs have normal CMAPs and normal SNAPs. | Both sides | S = D | LMN |
| Storti et al. [54] | 2021 | M | 20 | 42 | Distal and proximal weakness and hypotrophy in the hands developed and progressed slowly to all four limbs especially on the left side for 20 years. Bilateral areflexia of the upper extremities was noted. | Spasticity, hyperreflexia, pathologic positive Babinski sign bilaterally and clonus in the lower extremities | EMG, performed 3 years later, was consistent with a diagnosis of definite ALS. | Both sides | S > D | LMN UMN |
| Zheng et al. [55] | 2022 | F | 55 | 73 | Mild weakness of the arms. Sensory loss to pain and temperature in the whole body sparing the face. | Mild weakness in the lower extremities | Both sides | S = D | LMN UMN | |
| Zheng et al. [55] | 2022 | M | 18 | 60 | Atrophy and weakness of the shoulder abduction and intrinsic hand muscles more on the left hand than in the right hand. | Normal | EMG studies showed chronic denervation–reinnervation in C5 to C7 muscles bilaterally and in C8 muscles on the left | Left side | S > D | LMN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zamil, M.; Shnayder, N.A.; Davydova, T.K.; Nasyrova, R.F.; Trefilova, V.V.; Narodova, E.A.; Petrova, M.M.; Romanova, I.V.; Chumakova, G.A. Amyotrophic Lateral Sclerosis Mimic Syndrome in a 24-Year-Old Man with Chiari 1 Malformation and Syringomyelia: A Clinical Case. J. Clin. Med. 2023, 12, 2932. https://doi.org/10.3390/jcm12082932
Al-Zamil M, Shnayder NA, Davydova TK, Nasyrova RF, Trefilova VV, Narodova EA, Petrova MM, Romanova IV, Chumakova GA. Amyotrophic Lateral Sclerosis Mimic Syndrome in a 24-Year-Old Man with Chiari 1 Malformation and Syringomyelia: A Clinical Case. Journal of Clinical Medicine. 2023; 12(8):2932. https://doi.org/10.3390/jcm12082932
Chicago/Turabian StyleAl-Zamil, Mustafa, Natalia A. Shnayder, Tatiana K. Davydova, Regina F. Nasyrova, Vera V. Trefilova, Ekaterina A. Narodova, Marina M. Petrova, Irina V. Romanova, and Galina A. Chumakova. 2023. "Amyotrophic Lateral Sclerosis Mimic Syndrome in a 24-Year-Old Man with Chiari 1 Malformation and Syringomyelia: A Clinical Case" Journal of Clinical Medicine 12, no. 8: 2932. https://doi.org/10.3390/jcm12082932
APA StyleAl-Zamil, M., Shnayder, N. A., Davydova, T. K., Nasyrova, R. F., Trefilova, V. V., Narodova, E. A., Petrova, M. M., Romanova, I. V., & Chumakova, G. A. (2023). Amyotrophic Lateral Sclerosis Mimic Syndrome in a 24-Year-Old Man with Chiari 1 Malformation and Syringomyelia: A Clinical Case. Journal of Clinical Medicine, 12(8), 2932. https://doi.org/10.3390/jcm12082932








