Paradigm Shifts in Cardiac Pacing: Where Have We Been and What Lies Ahead?
Abstract
1. Introduction
2. Cardiac Pacing
2.1. General Description of Methods of Pacing
2.1.1. Endocardial Pacing
2.1.2. Epicardial Pacing
2.1.3. Leadless Pacing
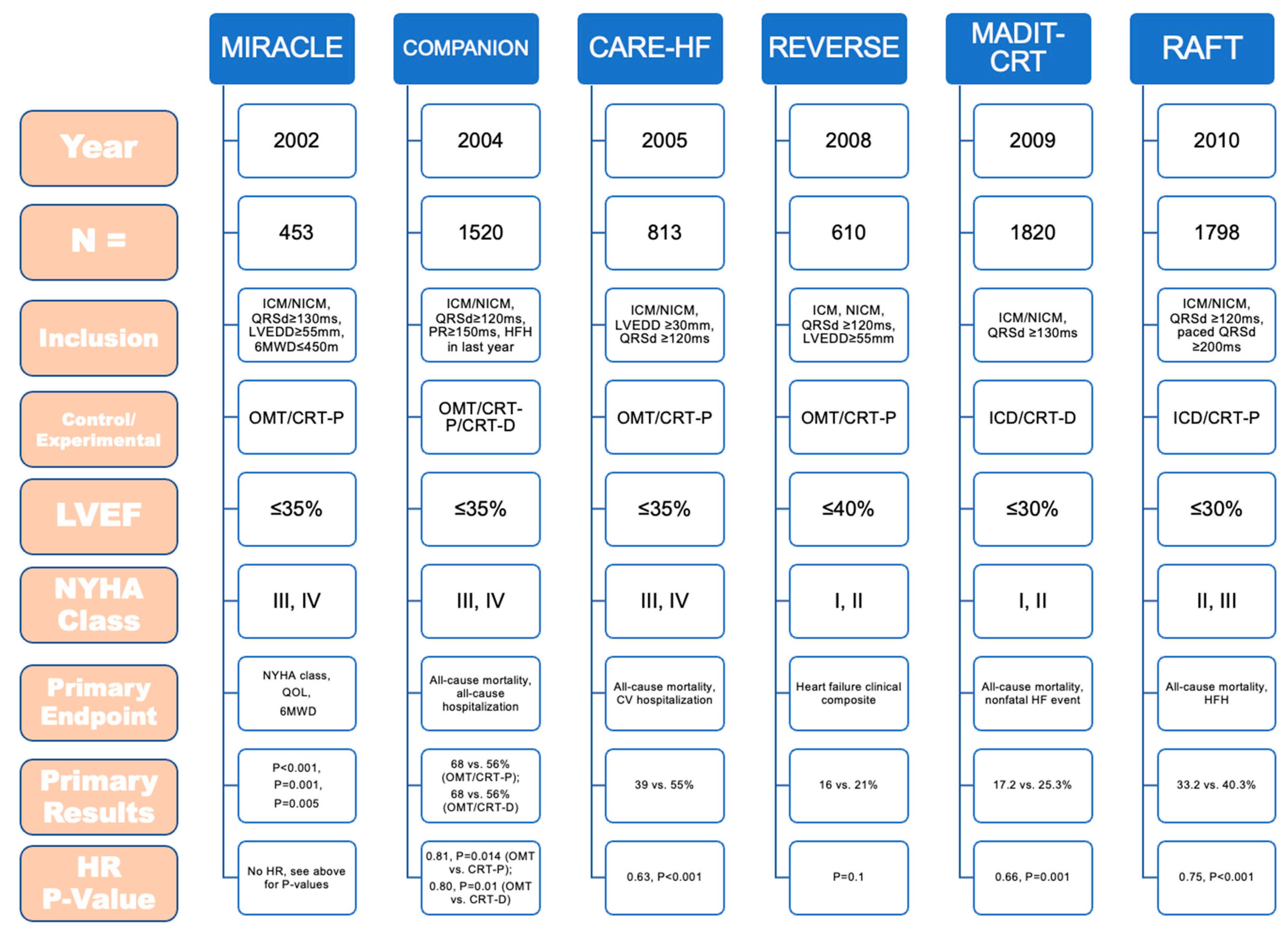
2.2. Introduction to Landmark Trials of Cardiac Pacing
2.3. Trends in Cardiac Device Implantation
| Trial | Year | Clinical Question | Intervention/Control | Population (N=) | Primary Outcome | Results | p-Value |
|---|---|---|---|---|---|---|---|
| CTOPP [20] | 2000 | What is the optimal pacing strategy for symptomatic bradycardia? | DDD/VVI | 1474 | Stroke, CV death | 4.9 vs. 5.5% * | p = 0.33 |
| MOST [21] | 2002 | What is the optimal pacing strategy for SND? | DDD/VVI | 2010 | All-cause mortality or non-fatal stroke | 21.5 vs. 23% † | p = 0.48 |
| DAVID [22] | 2002 | What is the optimal pacing strategy for patients with standard indications for ICD without indications for pacing? | DDDR-ICD/VVI-ICD | 506 | Time to death or HFH | 83.9 vs. 73.3 ‡ | p < 0.03 |
| UKPACE [23] | 2005 | What is the optimal pacing strategy for patients with high grade AVB? | DDD/VVI | 2021 | All-cause mortality | 7.4 vs. 7.2% ¶ | p = 0.56 |
| DANPACE [24] | 2011 | What is the optimal pacing strategy for SND? | DDDR/AAIR | 1415 | All-cause mortality | 27.3 vs. 29.6% § | p = 0.53 |
2.4. Cardiac Implantable Electronic Device Complication Rates
3. New Developments in Cardiac Pacing
3.1. Cardiac Resynchronization Therapy
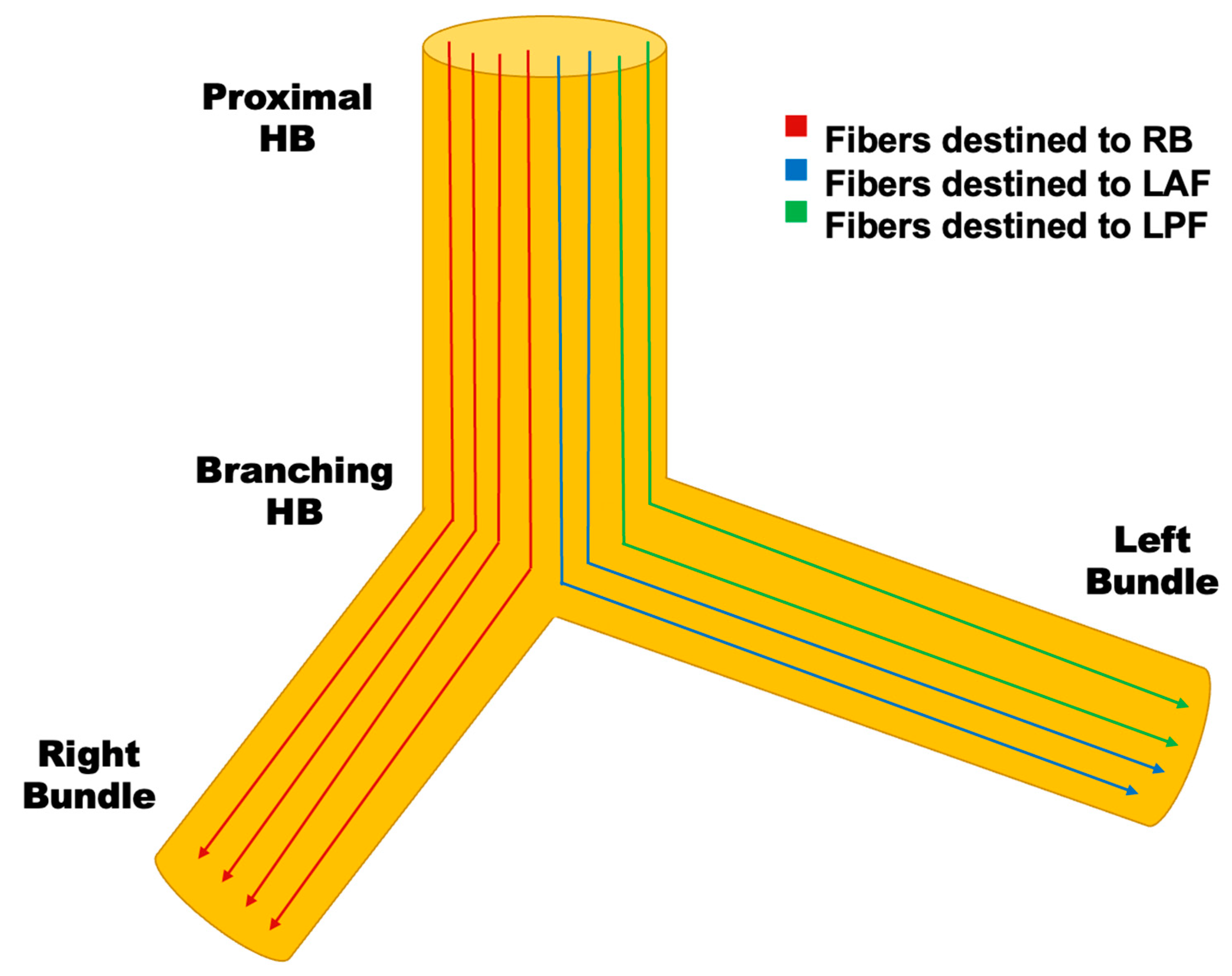
3.2. Conduction System Pacing
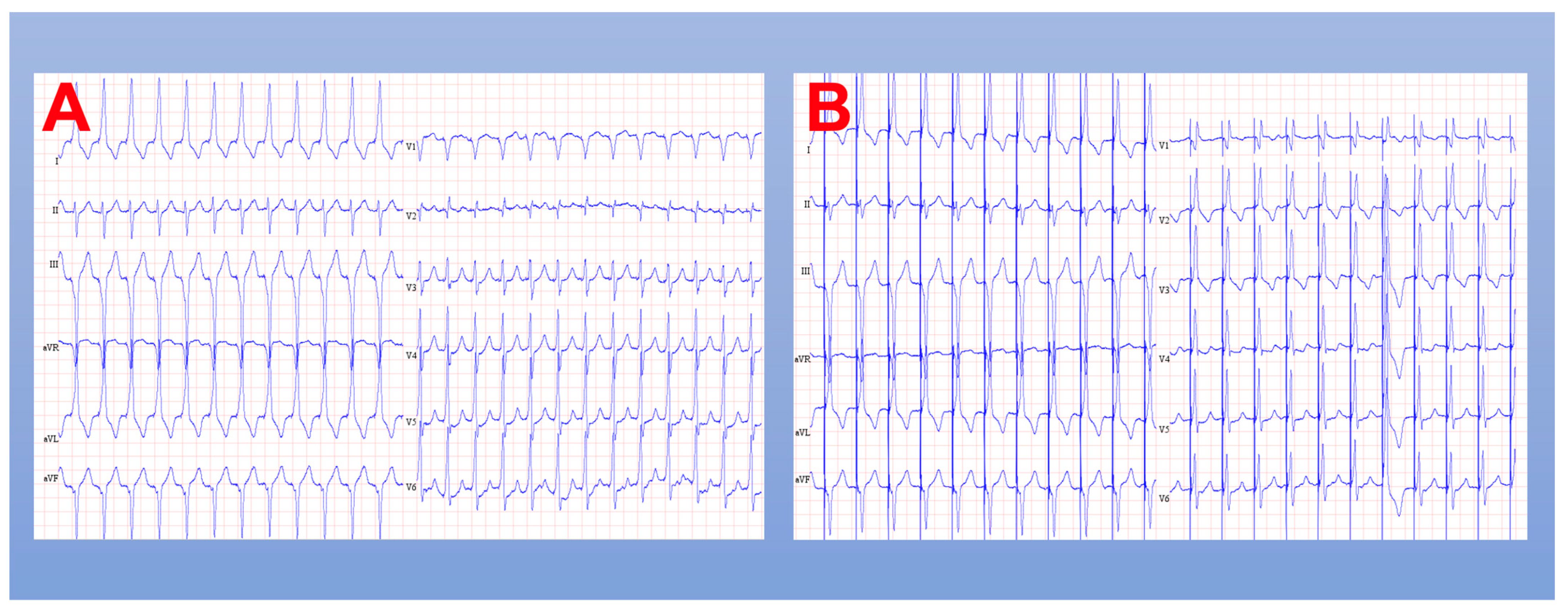
3.2.1. His Bundle Pacing
| Study | His-SYNC [88] | His-Alternative [89] | LEVEL-AT [90] | LBBP-RESYNC [91] |
|---|---|---|---|---|
| Year of Publication | 2019 | 2021 | 2022 | 2022 |
| Type of CSP | HBP | HBP | HBP, LBBP | LBBP |
| Number of Patients | 41 | 50 | 70 | 40 |
| Age | 64 ± 13 | 63.8 ± 9 | 65.7 ± 9 | 63.7 ± 11 |
| Mean LVEF (%) | 28 | 30 ± 6 | 27 ± 7 | 28.3 ± 5 |
| Follow-up (mon) | 6.2 | 6.0 | 6.0 | 6.0 |
| LBBB (%) | 85 | 100 | 60 | 100 |
| Baseline QRS Duration (ms) | 168 ± 18 | 165 ± 14 | 177 ± 21 | 174.6 ± 14 |
| ICM (%) | 65.0 | 20.0 | 31.4 | 0.0 |
| Threshold (V) | 1.7 | 2.4 ± 1.6 | 1.0 ± 0.4 | 0.82 ± 0.20 |
| Pulse Width (ms) | 1.0 | 1.0 | 0.6 ± 0.3 | 0.5 |
| Procedure Time (min) | NR | 137 ± 46 | 125 ± 35 | 129 ± 32 |
| Complications (%) | NR | 0 | 11.4% * | NR |
| Dislodgements (%) | 0 | 0 | 1 | 0 |
| Delta QRS duration (ms) | −28 | −34 | −53 ± 20 | −43 |
| Delta LVEF (%) | +9.1 | +16 ± 7 | +12.2 ± 9 | +5.6 |
| Delta LVESV (mL) | NR | −53 | −37 ± 59 | −25 |
| Delta LVAT (ms) | NR | NR | −28 ± 26 | 79.74 ± 9.94 † |
| Other Comments | No difference in CV hospitalization or death | --- | No difference in mortality or HFH | BNP favoured LBBP |
3.2.2. Left Bundle Branch Pacing
3.2.3. Conduction System Pacing Implantation Techniques
General
His Bundle Pacing
- Selective response: capture of the HB alone, with ventricular capture exclusively through the conduction system;
- Non-selective response: activation of HB and local myocardium. Ventricular capture results from fusion of both wavefronts.
Left Bundle Branch Pacing
- V1—QS complex with notch in the descending limb near the nadir (“W” complex);
- Tall R-waves in leads II, III (ideally, II > III);
- Discordant QRS in leads aVR (negative) and aVL (positive).
- Gradual deployment while monitoring the paced QRS morphology and impedance;
- Gradual increase in R’ wave in V1 with progression of R wave to the terminal component of the QRS (Qr pattern or rSr’ pattern);
- Gradual increase in impedance before drop of 100–200 Ohms prior to LV subendocardium;
- Myocardium current of injury;
- Rapid deployment with monitoring of PVC morphology;
- PVC morphology changes from wide QRS to narrowed QRS with RBBB morphology (duration < 130 ms).
- RBBB pattern during pacing;
- Presence of LBB potential during pacing (visualized in less than 50% of cases);
- Short and constant LVAT high- (5 V) and low- (1 V) pacing outputs;
- Demonstration of selective and non-selective LBB pacing;
- Evidence for direct LBB capture.
3.3. Leadless Pacing
- Absence of subcutaneous pocket;
- Substantially reduced device surface area;
- Minimal physical handling of device pre-implant;
- Extensive encapsulation of device after implant;
- Turbulent hemodynamic environment with high-velocity blood flow;
- Parylene-coated titanium material that may reduce bacterial adherence.
4. Future Directions
4.1. Expanding Applications for Conduction System Pacing
4.2. Extraction of Conduction System Pacing Leads
4.3. Leadless Pacing Combined with Subcutaneous Implantable Cardioverter Defibrillators
4.4. Optimizing Cardiac Resynchronization
4.5. The WiSE-CRT System
4.6. Future Economic Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furman, S. Early History of Cardiac Pacing and Defibrillation. Indian Pacing Electrophysiol. J. 2002, 2, 2. [Google Scholar] [PubMed]
- Zoll, P.M. Resuscitation of the Heart in Ventricular Standstill by External Electric Stimulation. N. Engl. J. Med. 1952, 247, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Kenny, R. The Natural History of Sick Sinus Syndrome. Pacing Clin. Electrophysiol. 1986, 9, 1110–1114. [Google Scholar] [CrossRef]
- Tung, R.T.; Shen, W.K.; Hayes, D.L.; Hammill, S.C.; Bailey, K.R.; Gersh, B.J. Long-Term Survival after Permanent Pacemaker Implantation for Sick Sinus Syndrome. Am. J. Cardiol. 1994, 74, 1016–1020. [Google Scholar] [CrossRef]
- Alboni, P.; Menozzi, C.; Brignole, M.; Paparella, N.; Gaggioli, G.; Lolli, G.; Cappato, R. Effects of Permanent Pacemaker and Oral Theophylline in Sick Sinus Syndrome the THEOPACE Study: A Randomized Controlled Trial. Circulation 1997, 96, 260–266. [Google Scholar] [CrossRef]
- Fleischmann, K.E.; Orav, E.J.; Lamas, G.A.; Mangione, C.M.; Schron, E.; Lee, K.L.; Goldman, L. Pacemaker Implantation and Quality of Life in the Mode Selection Trial (MOST). Heart Rhythm 2006, 3, 653–659. [Google Scholar] [CrossRef]
- Newman, D.; Lau, C.; Tang, A.S.L.; Irvine, J.; Paquette, M.; Woodend, K.; Dorian, P.; Gent, M.; Kerr, C.; Connolly, S.J. Effect of Pacing Mode on Health-Related Quality of Life in the Canadian Trial of Physiologic Pacing. Am. Heart J. 2003, 145, 430–437. [Google Scholar] [CrossRef]
- Tjong, F.V.Y.; Beurskens, N.E.G.; de Groot, J.R.; Waweru, C.; Liu, S.; Ritter, P.; Reynolds, D.; Wilde, A.A.M.; Knops, R.E. Health-Related Quality of Life Impact of a Transcatheter Pacing System. J. Cardiovasc. Electrophysiol. 2018, 29, 1697–1704. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Calvert, M.J.; Verboven, Y.; Freemantle, N. Effects of Cardiac Resynchronization Therapy on Long-Term Quality of Life: An Analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) Study. Am. Heart J. 2009, 157, 457–466. [Google Scholar] [CrossRef]
- Healey, J.S.; Toff, W.D.; Lamas, G.A.; Andersen, H.R.; Thorpe, K.E.; Ellenbogen, K.A.; Lee, K.L.; Skene, A.M.; Schron, E.B.; Skehan, J.D.; et al. Cardiovascular Outcomes with Atrial-Based Pacing Compared with Ventricular Pacing: Meta-Analysis of Randomized Trials, Using Individual Patient Data. Circulation 2006, 114, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.O.; Bank, A.J.; Nsah, E.; Koullick, M.; Zeng, Q.C.; Hettrick, D.; Sheldon, T.; Lamas, G.A. Minimizing Ventricular Pacing to Reduce Atrial Fibrillation in Sinus-Node Disease. N. Engl. J. Med. 2007, 357, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, E.P.; Sanders, G.D.; Singh, K.; Greenfield, R.A.; Gillis, A.M.; Wilkoff, B.L.; Piccini, J.P.; Al-Khatib, S.M. Single vs. Dual Chamber Implantable Cardioverter-Defibrillators or Programming of Implantable Cardioverter-Defibrillators in Patients without a Bradycardia Pacing Indication: Systematic Review and Meta-Analysis. Europace 2018, 20, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Mond, H.G.; Proclemer, A. The 11th World Survey of Cardiac Pacing and Implantable Cardioverter-Defibrillators: Calendar Year 2009—A World Society of Arrhythmia’s Project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef]
- Mond, H.G. The World Survey of Cardiac Pacing and Cardioverter Defibrillators: Calendar Year 1997. Pacing Clin. Electrophysiol. 2001, 24, 869–870. [Google Scholar] [CrossRef]
- Vaidya, V.R.; Asirvatham, R.; Kowlgi, G.N.; Dai, M.Y.; Cochuyt, J.J.; Hodge, D.O.; Deshmukh, A.J.; Cha, Y.M. Trends in Cardiovascular Implantable Electronic Device Insertion Between 1988 and 2018 in Olmsted County. JACC Clin. Electrophysiol. 2022, 8, 88–100. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–577. [Google Scholar] [CrossRef]
- Raatikainen, M.J.P.; Arnar, D.O.; Merkely, B.; Nielsen, J.C.; Hindricks, G.; Heidbuchel, H.; Camm, J. A Decade of Information on the Use of Cardiac Implantable Electronic Devices and Interventional Electrophysiological Procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace 2017, 19, II1–II90. [Google Scholar] [CrossRef]
- Bannehr, M.; Reiners, D.; Lichtenauer, M.; Kopp, K.; Jirak, P.; Georgi, C.; Butter, C.; Edlinger, C. Impact of Socioeconomic Aspects on Cardiac Implantable Electronic Device Treatment and Application of the EHRA Guidelines: A European Comparison. Wien. Klin. Wochenschr. 2022, 134, 646–653. [Google Scholar] [CrossRef]
- Connolly, S.J.; Kerr, C.R.; Gent, M.; Roberts, R.S.; Yusuf, S.; Gillis, A.M.; Sami, M.H.; Talajic, M.; Tang, A.S.L.; Klein, G.J.; et al. Effects of Physiologic Pacing versus Ventricular Pacing on the Risk of Stroke and Death Due to Cardiovascular Causes. Canadian Trial of Physiologic Pacing Investigators. N. Engl. J. Med. 2000, 342, 1385–1391. [Google Scholar] [CrossRef]
- Lamas, G.A.; Lee, K.; Sweeney, M.; Leon, A.; Yee, R.; Ellenbogen, K.; Greer, S.; Wilber, D.; Silverman, R.; Marinchak, R.; et al. The Mode Selection Trial (MOST) in Sinus Node Dysfunction: Design, Rationale, and Baseline Characteristics of the First 1000 Patients. Am. Heart J. 2000, 140, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.L.; Cook, J.R.; Epstein, A.E.; Greene, L.; Hallstrom, A.P.; Hsia, H.; Kutalek, S.P.; Sharma, A.; Blatt, B.; Karas, B.; et al. Dual-Chamber Pacing or Ventricular Backup Pacing in Patients with an Implantable Defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002, 288, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Toff, W.D.; Camm, A.J.; Skehan, J.D. Single-Chamber versus Dual-Chamber Pacing for High-Grade Atrioventricular Block. N. Engl. J. Med. 2005, 353, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Thomsen, P.E.B.; Højberg, S.; Møller, M.; Vesterlund, T.; Dalsgaard, D.; Mortensen, L.S.; Nielsen, T.; Asklund, M.; Friis, E.V.; et al. A Comparison of Single-Lead Atrial Pacing with Dual-Chamber Pacing in Sick Sinus Syndrome. Eur. Heart J. 2011, 32, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Starck, C.; Auricchio, A.; Biffi, M.; Burri, M.; D’avila, A.; Deharo, J.C.; Glikson, M.; Israel, C.; Lau, C.P.; et al. EHRA Expert Consensus Statement and Practical Guide on Optimal Implantation Technique for Conventional Pacemakers and Implantable Cardioverter-Defibrillators: Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace 2021, 23, 983–1008. [Google Scholar] [CrossRef] [PubMed]
- Armaganijan, L.V.; Toff, W.D.; Nielsen, J.C.; Andersen, H.R.; Connolly, S.J.; Ellenbogen, K.A.; Healey, J.S. Are Elderly Patients at Increased Risk of Complications Following Pacemaker Implantation? A Meta-Analysis of Randomized Trials. Pacing Clin. Electrophysiol. 2012, 35, 131–134. [Google Scholar] [CrossRef]
- Persson, R.; Earley, A.; Garlitski, A.C.; Balk, E.M.; Uhlig, K. Adverse Events Following Implantable Cardioverter Defibrillator Implantation: A Systematic Review. J. Interv. Card. Electrophysiol. 2014, 40, 191–205. [Google Scholar] [CrossRef]
- Witt, C.M.; Lenz, C.J.; Shih, H.H.; Ebrille, E.; Rosenbaum, A.N.; Aung, H.; van Zyl, M.; Manocha, K.K.; Deshmukh, A.J.; Hodge, D.O.; et al. Right Atrial Lead Fixation Type and Lead Position Are Associated with Significant Variation in Complications. J. Interv. Card. Electrophysiol. 2016, 47, 313–319. [Google Scholar] [CrossRef]
- Chen, S.; Ling, Z.; Kiuchi, M.G.; Yin, Y.; Krucoff, M.W. The Efficacy and Safety of Cardiac Resynchronization Therapy Combined with Implantable Cardioverter Defibrillator for Heart Failure: A Meta-Analysis of 5674 Patients. Europace 2013, 15, 992–1001. [Google Scholar] [CrossRef]
- Witt, C.T.; Ng Kam Chuen, M.J.; Kronborg, M.B.; Kristensen, J.; Gerdes, C.; Nielsen, J.C. Non-Infective Left Ventricular Lead Complications Requiring Re-Intervention Following Cardiac Resynchronization Therapy: Prevalence, Causes and Outcomes. J. Interv. Card. Electrophysiol. 2022, 63, 69–75. [Google Scholar] [CrossRef]
- Sohail, M.R.; Henrikson, C.A.; Braid-Forbes, M.J.; Forbes, K.F.; Lerner, D.J. Mortality and Cost Associated with Cardiovascular Implantable Electronic Device Infections. Arch. Intern Med. 2011, 171, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Greenspon, A.J.; Eby, E.L.; Petrilla, A.A.; Sohail, M.R. Treatment Patterns, Costs, and Mortality among Medicare Beneficiaries with CIED Infection. Pacing Clin. Electrophysiol. 2018, 41, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Tarakji, K.G.; Mittal, S.; Kennergren, C.; Corey, R.; Poole, J.E.; Schloss, E.; Gallastegui, J.; Pickett, R.A.; Evonich, R.; Philippon, F.; et al. Antibacterial Envelope to Prevent Cardiac Implantable Device Infection. N. Engl. J. Med. 2019, 380, 1895–1905. [Google Scholar] [CrossRef]
- Boriani, G.; Kennergren, C.; Tarakji, K.G.; Wright, D.J.; Ahmed, F.Z.; McComb, J.M.; Goette, A.; Blum, T.; Biffi, M.; Green, M.; et al. Cost-Effectiveness Analyses of an Absorbable Antibacterial Envelope for Use in Patients at Increased Risk of Cardiac Implantable Electronic Device Infection in Germany, Italy, and England. Value Health 2021, 24, 930–938. [Google Scholar] [CrossRef]
- Özcan, C.; Raunsø, J.; Lamberts, M.; Køber, L.; Lindhardt, T.B.; Bruun, N.E.; Laursen, M.L.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, M.L. Infective Endocarditis and Risk of Death after Cardiac Implantable Electronic Device Implantation: A Nationwide Cohort Study. Europace 2017, 19, 1007–1014. [Google Scholar] [CrossRef]
- Hasan, F.; Nedios, S.; Karosiene, Z.; Scholten, M.; Lemke, B.; Tulka, S.; Knippschild, S.; Macher-Heidrich, S.; Adomeit, H.J.; Zarse, M.; et al. Perioperative Complications after Pacemaker Implantation: Higher Complication Rates with Subclavian Vein Puncture than with Cephalic Vein Cutdown. J. Interv. Card. Electrophysiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Chan, N.Y.; Kwong, N.P.; Cheong, A.P. Venous Access and Long-Term Pacemaker Lead Failure: Comparing Contrast-Guided Axillary Vein Puncture with Subclavian Puncture and Cephalic Cutdown. Europace 2017, 19, 1193–1197. [Google Scholar] [CrossRef]
- Tagliari, A.P.; Kochi, A.N.; Mastella, B.; Saadi, R.P.; di Leoni Ferrari, A.; Dussin, L.H.; de Moura, L.; Martins, M.R.; Saadi, E.K.; Polanczyk, C.A. Ultrasound-Guided Axillary Vein Puncture in Cardiac Lead Implantation: Time to Move to a New Standard Access? Arrhythm. Electrophysiol. Rev. 2020, 9, 78. [Google Scholar] [CrossRef]
- Tagliari, A.P.; Kochi, A.N.; Mastella, B.; Saadi, R.P.; di Leoni Ferrari, A.; Saadi, E.K.; Polanczyk, C.A. Axillary Vein Puncture Guided by Ultrasound vs Cephalic Vein Dissection in Pacemaker and Defibrillator Implant: A Multicenter Randomized Clinical Trial. Heart Rhythm 2020, 17, 1554–1560. [Google Scholar] [CrossRef]
- Iuliano, S.; Fisher, S.G.; Karasik, P.E.; Fletcher, R.D.; Singh, S.N. Qrs Duration and Mortality in Patients with Congestive Heart Failure. Am. Heart J. 2002, 143, 1085–1091. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; de Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Abraham, W.T.; Linde, C.; Gold, M.R.; Young, J.B.; Claude Daubert, J.; Sherfesee, L.; Wells, G.A.; Tang, A.S.L. An Individual Patient Meta-Analysis of Five Randomized Trials Assessing the Effects of Cardiac Resynchronization Therapy on Morbidity and Mortality in Patients with Symptomatic Heart Failure. Eur. Heart J. 2013, 34, 3547–3556. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.A.; Brown-Mahoney, C.; Kay, G.N.; Ellenbogen, K.A. Clinical Outcomes after Ablation and Pacing Therapy for Atrial Fibrillation: A Meta-Analysis. Circulation 2000, 101, 1138–1144. [Google Scholar] [CrossRef]
- Brignole, M.; Pokushalov, E.; Pentimalli, F.; Palmisano, P.; Chieffo, E.; Occhetta, E.; Quartieri, F.; Calò, L.; Ungar, A.; Mont, L. A Randomized Controlled Trial of Atrioventricular Junction Ablation and Cardiac Resynchronization Therapy in Patients with Permanent Atrial Fibrillation and Narrow QRS. Eur. Heart J. 2018, 39, 3999–4008. [Google Scholar] [CrossRef]
- Brignole, M.; Pentimalli, F.; Palmisano, P.; Landolina, M.; Quartieri, F.; Occhetta, E.; Calò, L.; Mascia, G.; Mont, L.; Vernooy, K.; et al. AV Junction Ablation and Cardiac Resynchronization for Patients with Permanent Atrial Fibrillation and Narrow QRS: The APAF-CRT Mortality Trial. Eur. Heart J. 2021, 42, 4731–4739. [Google Scholar] [CrossRef]
- Linde, C.; Abraham, W.T.; Gold, M.R.; St. John Sutton, M.; Ghio, S.; Daubert, C. Randomized Trial of Cardiac Resynchronization in Mildly Symptomatic Heart Failure Patients and in Asymptomatic Patients with Left Ventricular Dysfunction and Previous Heart Failure Symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef]
- Jastrzebski, M.; Baranchuk, A.; Fijorek, K.; Kisiel, R.; Kukla, P.; Sondej, T.; Czarnecka, D. Cardiac Resynchronization Therapy-Induced Acute Shortening of QRS Duration Predicts Long-Term Mortality Only in Patients with Left Bundle Branch Block. Europace 2019, 21, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Appert, L.; Menet, A.; Altes, A.; Ennezat, P.V.; Bardet-Bouchery, H.; Binda, C.; Guyomar, Y.; Delelis, F.; Castel, A.L.; Le Goffic, C.; et al. Clinical Significance of Electromechanical Dyssynchrony and QRS Narrowing in Patients With Heart Failure Receiving Cardiac Resynchronization Therapy. Can. J. Cardiol. 2019, 35, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Field, M.E.; Yu, N.; Wold, N.; Gold, M.R. Comparison of Measures of Ventricular Delay on Cardiac Resynchronization Therapy Response. Heart Rhythm 2020, 17, 615–620. [Google Scholar] [CrossRef]
- Singh, J.P.; Fan, D.; Heist, E.K.; Alabiad, C.R.; Taub, C.; Reddy, V.; Mansour, M.; Picard, M.H.; Ruskin, J.N.; Mela, T. Left Ventricular Lead Electrical Delay Predicts Response to Cardiac Resynchronization Therapy. Heart Rhythm 2006, 3, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Botto, G.; Mantica, M.; La Rosa, C.; Occhetta, E.; Verlato, R.; Molon, G.; Ammendola, E.; Villani, G.Q.; Bongiorni, M.G.; et al. Incremental Value of Larger Interventricular Conduction Time in Improving Cardiac Resynchronization Therapy Outcome in Patients with Different QRS Duration. J. Cardiovasc. Electrophysiol. 2014, 25, 500–506. [Google Scholar] [CrossRef]
- Gamble, J.H.P.; Herring, N.; Ginks, M.; Rajappan, K.; Bashir, Y.; Betts, T.R. Procedural Success of Left Ventricular Lead Placement for Cardiac Resynchronization Therapy: A Meta-Analysis. JACC Clin. Electrophysiol. 2016, 2, 69–77. [Google Scholar] [CrossRef]
- Crossley, G.H.; Exner, D.; Mead, R.H.; Sorrentino, R.A.; Hokanson, R.; Li, S.; Adler, S. Chronic Performance of an Active Fixation Coronary Sinus Lead. Heart Rhythm 2010, 7, 472–478. [Google Scholar] [CrossRef]
- Chapman, M.; Bates, M.G.D.; Behar, N.M.; Williams, I.; Dewhurst, M.; Monkhouse, C.; Hayward, C.; Muthumala, A.; Chow, A.; Linker, N.J.; et al. A Novel Quadripolar Active Fixation Left-Ventricular Pacing Lead for Cardiac Resynchronization Therapy: Initial United Kingdom Experience. JACC Clin. Electrophysiol. 2019, 5, 1028–1035. [Google Scholar] [CrossRef]
- Cronin, E.M.; Ingelmo, C.P.; Rickard, J.; Wazni, O.M.; Martin, D.O.; Wilkoff, B.L.; Baranowski, B. Active Fixation Mechanism Complicates Coronary Sinus Lead Extraction and Limits Subsequent Reimplantation Targets. J. Interv. Card. Electrophysiol. 2013, 36, 81–86. [Google Scholar] [CrossRef]
- Daubert, C.; Behar, N.; Martins, R.P.; Mabo, P.; Leclercq, C. Avoiding Non-Responders to Cardiac Resynchronization Therapy: A Practical Guide. Eur. Heart J. 2017, 38, 1463–1472. [Google Scholar] [CrossRef]
- Varma, N.; Boehmer, J.; Bhargava, K.; Yoo, D.; Leonelli, F.; Costanzo, M.; Saxena, A.; Sun, L.; Gold, M.R.; Singh, J.; et al. Evaluation, Management, and Outcomes of Patients Poorly Responsive to Cardiac Resynchronization Device Therapy. J. Am. Coll. Cardiol. 2019, 74, 2588–2603. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Kim, J.; Lerman, B.B. Improving Cardiac Resynchronisation Therapy. Arrhythm. Electrophysiol. Rev. 2019, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.O.; Lemke, B.; Birnie, D.; Krum, H.; Lee, K.L.F.; Aonuma, K.; Gasparini, M.; Starling, R.C.; Milasinovic, G.; Rogers, T.; et al. Investigation of a Novel Algorithm for Synchronized Left-Ventricular Pacing and Ambulatory Optimization of Cardiac Resynchronization Therapy: Results of the Adaptive CRT Trial. Heart Rhythm 2012, 9, 1807–1814.e1. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, S.; Huang, D.; Xue, X.; Xu, J.; Tao, Q.; Zhang, W.; Zhang, Z.; Hua, W.; Liang, Y.; et al. Evaluation of the Therapeutic Effects of QuickOpt Optimization in Chinese Patients with Chronic Heart Failure Treated by Cardiac Resynchronization. Sci. Rep. 2018, 8, 4259. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, K.A.; Gold, M.R.; Meyer, T.E.; Fernández Lozano, I.; Mittal, S.; Waggoner, A.D.; Lemke, B.; Singh, J.P.; Spinale, F.G.; van Eyk, J.E.; et al. Primary Results from the SmartDelay Determined AV Optimization: A Comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) Trial: A Randomized Trial Comparing Empirical, Echocardiography-Guided, and Algorithmic Atrioventricular Delay Programming in Cardiac Resynchronization Therapy. Circulation 2010, 122, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Delnoy, P.P.; Brachmann, J.; Reynolds, D.; Padeletti, L.; Noelker, G.; Kantipudi, C.; Lopez, J.M.R.; Dichtl, W.; Borri-Brunetto, A.; et al. Contractility Sensor-Guided Optimization of Cardiac Resynchronization Therapy: Results from the RESPOND-CRT Trial. Eur. Heart J. 2017, 38, 730–738. [Google Scholar] [CrossRef]
- Trucco, E.; Tolosana, J.M.; Arbelo, E.; Doltra, A.; Castel, M.Á.; Benito, E.; Borràs, R.; Guasch, E.; Vidorreta, S.; Vidal, B.; et al. Improvement of Reverse Remodeling Using Electrocardiogram Fusion-Optimized Intervals in Cardiac Resynchronization Therapy: A Randomized Study. JACC Clin. Electrophysiol. 2018, 4, 181–189. [Google Scholar] [CrossRef]
- Rinaldi, C.A.; Burri, H.; Thibault, B.; Curnis, A.; Rao, A.; Gras, D.; Sperzel, J.; Singh, J.P.; Biffi, M.; Bordachar, P.; et al. A Review of Multisite Pacing to Achieve Cardiac Resynchronization Therapy. Europace 2015, 17, 7–17. [Google Scholar] [CrossRef]
- Leclercq, C.; Burri, H.; Curnis, A.; Delnoy, P.P.; Rinaldi, C.A.; Sperzel, J.; Lee, K.; Calò, L.; Vicentini, A.; Concha, J.F.; et al. Cardiac Resynchronization Therapy Non-Responder to Responder Conversion Rate in the More Response to Cardiac Resynchronization Therapy with MultiPoint Pacing (MORE-CRT MPP) Study: Results from Phase I. Eur. Heart J. 2019, 40, 2979–2987. [Google Scholar] [CrossRef]
- Bleeker, G.B.; Kaandorp, T.A.M.; Lamb, H.J.; Boersma, E.; Steendijk, P.; de Roos, A.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Effect of Posterolateral Scar Tissue on Clinical and Echocardiographic Improvement after Cardiac Resynchronization Therapy. Circulation 2006, 113, 969–976. [Google Scholar] [CrossRef]
- Bilchick, K.C.; Kuruvilla, S.; Hamirani, Y.S.; Ramachandran, R.; Clarke, S.A.; Parker, K.M.; Stukenborg, G.J.; Mason, P.; Ferguson, J.D.; Moorman, J.R.; et al. Impact of Mechanical Activation, Scar, and Electrical Timing on Cardiac Resynchronization Therapy Response and Clinical Outcomes. J. Am. Coll. Cardiol. 2014, 63, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.Z.; Virdee, M.S.; Palmer, C.R.; Pugh, P.J.; O’Halloran, D.; Elsik, M.; Read, P.A.; Begley, D.; Fynn, S.P.; Dutka, D.P. Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy: The TARGET Study: A Randomized, Controlled Trial. J. Am. Coll. Cardiol. 2012, 59, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Kydd, A.C.; Khan, F.Z.; Watson, W.D.; Pugh, P.J.; Virdee, M.S.; Dutka, D.P. Prognostic Benefit of Optimum Left Ventricular Lead Position in Cardiac Resynchronization Therapy: Follow-up of the TARGET Study Cohort (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy). JACC Heart Fail. 2014, 2, 205–212. [Google Scholar] [CrossRef]
- Saba, S.; Marek, J.; Schwartzman, D.; Jain, S.; Adelstein, E.; White, P.; Oyenuga, O.A.; Onishi, T.; Soman, P.; Gorcsan, J. Echocardiography-Guided Left Ventricular Lead Placement for Cardiac Resynchronization Therapy: Results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region Trial. Circ. Heart Fail. 2013, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Laksman, Z.; Yee, R.; Stirrat, J.; Gula, L.J.; Skanes, A.C.; Leong-Sit, P.; Manlucu, J.; McCarty, D.; Turkistani, Y.; Scholl, D.; et al. Model-Based Navigation of Left and Right Ventricular Leads to Optimal Targets for Cardiac Resynchronization Therapy: A Single-Center Feasibility Study. Circ. Arrhythm. Electrophysiol. 2014, 7, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. The Effects of Right Ventricular Apical Pacing on Ventricular Function and Dyssynchrony Implications for Therapy. J. Am. Coll. Cardiol. 2009, 54, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Epstein, A.E.; Verdino, R.J.; Lin, D.; Goldberg, L.R.; Marchlinski, F.E.; Frankel, D.S. Incidence and Predictors of Right Ventricular Pacing-Induced Cardiomyopathy. Heart Rhythm 2014, 11, 1619–1625. [Google Scholar] [CrossRef]
- Kaye, G.C.; Linker, N.J.; Marwick, T.H.; Pollock, L.; Graham, L.; Pouliot, E.; Poloniecki, J.; Gammage, M. Effect of Right Ventricular Pacing Lead Site on Left Ventricular Function in Patients with High-Grade Atrioventricular Block: Results of the Protect-Pace Study. Eur. Heart J. 2015, 36, 856–862. [Google Scholar] [CrossRef]
- Cheung, J.W.; Ip, J.E.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Feldman, D.N.; Swaminathan, R.V.; Lerman, B.B.; Kim, L.K. Trends and Outcomes of Cardiac Resynchronization Therapy Upgrade Procedures: A Comparative Analysis Using a United States National Database 2003-2013. Heart Rhythm 2017, 14, 1043–1050. [Google Scholar] [CrossRef]
- James, T.N.; Sherf, L. Fine Structure of the His Bundle. Circulation 1971, 44, 9–28. [Google Scholar] [CrossRef]
- Narula, O.S. Longitudinal Dissociation in the His Bundle. Bundle Branch Block Due to Asynchronous Conduction within the His Bundle in Man. Circulation 1977, 56, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, N.; Amay-Y-Leon, F.; Schonfield, C.; Scherlag, B.J.; Rosen, K.; Lazzara, R.; Wyndham, C. Normalization of Bundle Branch Block Patterns by Distal His Bundle Pacing. Clinical and Experimental Evidence of Longitudinal Dissociation in the Pathologic His Bundle. Circulation 1978, 57, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Scherlag, B.J.; El-Sherif, N.; Hope, R.R.; Lazzara, R. The Significance of Dissociation of Conduction in the Canine His Bundle. Electrophysiological Studies in Vivo and in Vitro. J. Electrocardiol. 1978, 11, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Casavant, D.A.; Romanyshyn, M.; Anderson, K. Permanent, Direct His-Bundle Pacing: A Novel Approach to Cardiac Pacing in Patients with Normal His-Purkinje Activation. Circulation 2000, 101, 869–877. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Naperkowski, A.; Ellenbogen, K.A.; Dandamudi, G. Electrophysiologic Insights Into Site of Atrioventricular Block: Lessons From Permanent His Bundle Pacing. JACC Clin. Electrophysiol. 2015, 1, 571–581. [Google Scholar] [CrossRef]
- Barba-Pichardo, R.; Moriña-Vázquez, P.; Fernández-Gómez, J.M.; Venegas-Gamero, J.; Herrera-Carranza, M. Permanent His-Bundle Pacing: Seeking Physiological Ventricular Pacing. Europace 2010, 12, 527–533. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; Oren, J.W.; Dandamudi, G.; Vijayaraman, P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018, 71, 2319–2330. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Vijayaraman, P.; Nayak, H.M.; Verma, N.; Dandamudi, G.; Sharma, P.S.; Saleem, M.; Mandrola, J.; Genovese, D.; Tung, R. His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 157–159. [Google Scholar] [CrossRef]
- Vinther, M.; Risum, N.; Svendsen, J.H.; Møgelvang, R.; Philbert, B.T. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients With Left Bundle Branch Block (His-Alternative). JACC Clin. Electrophysiol. 2021, 7, 1422–1432. [Google Scholar] [CrossRef]
- Margarida Pujol-Lopez, M.; Rafael Jiménez-Arjona, M.; Paz Garre, B.; Eduard Guasch, M.P.; Roger Borràs, M.; Adelina Doltra, M.P.; Elisenda Ferró, M.; Cora García-Ribas, M.P.; Mireia Niebla, R.; Esther Carro, R.; et al. Conduction System Pacing vs Biventricular Pacing in Heart Failure and Wide QRS Patients: LEVEL-AT Trial. Clin. Electrophysiol. 2022, 8, 1431–1445. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, D.L.; Calame, S.; Crespo, E.M.; Calame, J.; Lobel, R.; Spector, P.S. Electrical Resynchronization Induced by Direct His-Bundle Pacing. Heart Rhythm 2010, 7, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Mao, G.; Vijayaraman, P.; Ellenbogen, K.A. Long-Term Outcomes of His Bundle Pacing in Patients with Heart Failure with Left Bundle Branch Block. Heart 2019, 105, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Boczar, K.; Sławuta, A.; Ząbek, A.; Dębski, M.; Vijayaraman, P.; Gajek, J.; Lelakowski, J.; Małecka, B. Cardiac Resynchronization Therapy with His Bundle Pacing. Pacing Clin. Electrophysiol. 2019, 42, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Moriña-Vázquez, P.; Moraleda-Salas, M.T.; Rodríguez-Albarrán, A.; Arce-León, Á.; Venegas-Gamero, J.; Fernández-Gómez, J.M.; Esteve-Ruiz, I.; Barba-Pichardo, R. Cardiac Resynchronization Therapy in Non-Ischemic Cardiomyopathy: A Comparative Non-Randomized Study of His Bundle Pacing versus Biventricular Pacing. J. Interv. Card. Electrophysiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Lustgarten, D.L.; Crespo, E.M.; Arkhipova-Jenkins, I.; Lobel, R.; Winget, J.; Koehler, J.; Liberman, E.; Sheldon, T. His-Bundle Pacing versus Biventricular Pacing in Cardiac Resynchronization Therapy Patients: A Crossover Design Comparison. Heart Rhythm 2015, 12, 1548–1557. [Google Scholar] [CrossRef]
- Zanon, F.; Abdelrahman, M.; Marcantoni, L.; Naperkowski, A.; Subzposh, F.A.; Pastore, G.; Baracca, E.; Boaretto, G.; Raffagnato, P.; Tiribello, A.; et al. Long Term Performance and Safety of His Bundle Pacing: A Multicenter Experience. J. Cardiovasc. Electrophysiol. 2019, 30, 1594–1601. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Naperkowski, A.; Subzposh, F.A.; Abdelrahman, M.; Sharma, P.S.; Oren, J.W.; Dandamudi, G.; Ellenbogen, K.A. Permanent His-Bundle Pacing: Long-Term Lead Performance and Clinical Outcomes. Heart Rhythm 2018, 15, 696–702. [Google Scholar] [CrossRef]
- Sharma, P.S.; Naperkowski, A.; Bauch, T.D.; Chan, J.Y.S.; Arnold, A.D.; Whinnett, Z.I.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His Bundle Pacing for Cardiac Resynchronization Therapy in Patients With Heart Failure and Right Bundle Branch Block. Circ. Arrhythm. Electrophysiol. 2018, 11, e006613. [Google Scholar] [CrossRef]
- Zheng, R.; Yao, H.; Lian, L. His-Purkinje Conduction System Pacing for Pacing-Induced Cardiomyopathy: A Systematic Literature Review and Meta-Analysis. J. Interv. Card. Electrophysiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Toding Labi, D.N.; Rampengan, P.S.; Rampengan, D. Comparison between His-Bundle Pacing and Biventricular Pacing as Cardiac Resynchronization Therapy for Heart Failure Patients: A Systematic Review and Meta-Analysis. EP Eur. 2022, 24, euac053.479. [Google Scholar] [CrossRef]
- Qi, J.; Jia, X.; Wang, Z. His Bundle Pacing for Cardiac Resynchronization Therapy: A Systematic Literature Review and Meta-Analysis. J. Interv. Card. Electrophysiol. 2020, 59, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A Novel Pacing Strategy With Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can. J. Cardiol. 2017, 33, 1736.e1–1736.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, J.; Qi, Y.; Wang, F.; Guo, L.; Shi, X.; Wu, W.; Zhou, X.; Li, R. Cardiac Resynchronization Therapy by Left Bundle Branch Area Pacing in Patients with Heart Failure and Left Bundle Branch Block. Heart Rhythm 2019, 16, 1783–1790. [Google Scholar] [CrossRef]
- Huang, W.; Wu, S.; Vijayaraman, P.; Su, L.; Chen, X.; Cai, B.; Zou, J.; Lan, R.; Fu, G.; Mao, G.; et al. Cardiac Resynchronization Therapy in Patients With Nonischemic Cardiomyopathy Using Left Bundle Branch Pacing. JACC Clin. Electrophysiol. 2020, 6, 849–858. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Ponnusamy, S.S.; Cano, Ó.; Sharma, P.S.; Naperkowski, A.; Subsposh, F.A.; Moskal, P.; Bednarek, A.; Dal Forno, A.R.; Young, W.; et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin. Electrophysiol. 2021, 7, 135–147. [Google Scholar] [CrossRef]
- Su, L.; Wang, S.; Wu, S.; Xu, L.; Huang, Z.; Chen, X.; Zheng, R.; Jiang, L.; Ellenbogen, K.A.; Whinnett, Z.I.; et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythm. Electrophysiol. 2021, 14, E009261. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Herweg, B.; Verma, A.; Sharma, P.S.; Batul, S.A.; Ponnusamy, S.S.; Schaller, R.D.; Cano, O.; Molina-Lerma, M.; Curila, K.; et al. Rescue Left Bundle Branch Area Pacing in Coronary Venous Lead Failure or Nonresponse to Biventricular Pacing: Results from International LBBAP Collaborative Study Group. Heart Rhythm 2022, 19, 1272–1280. [Google Scholar] [CrossRef]
- Grieco, D.; Bressi, E.; Sedláček, K.; Čurila, K.; Vernooy, K.; Fedele, E.; De Ruvo, E.; Fagagnini, A.; Kron, J.; Padala, S.K.; et al. Feasibility and Safety of Left Bundle Branch Area Pacing-Cardiac Resynchronization Therapy in Elderly Patients. J. Interv. Card. Electrophysiol. 2023, 66, 311–321. [Google Scholar] [CrossRef]
- Chen, X.; Ye, Y.; Wang, Z.; Jin, Q.; Qiu, Z.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Liang, Y.; et al. Cardiac Resynchronization Therapy via Left Bundle Branch Pacing vs. Optimized Biventricular Pacing with Adaptive Algorithm in Heart Failure with Left Bundle Branch Block: A Prospective, Multi-Centre, Observational Study. Europace 2022, 24, 807–816. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Zalavadia, D.; Haseeb, A.; Dye, C.; Madan, N.; Skeete, J.R.; Vipparthy, S.C.; Young, W.; Ravi, V.; Rajakumar, C.; et al. Clinical Outcomes of Conduction System Pacing Compared to Biventricular Pacing in Patients Requiring Cardiac Resynchronization Therapy. Heart Rhythm 2022, 19, 1263–1271. [Google Scholar] [CrossRef]
- Wu, S.; Su, L.; Vijayaraman, P.; Zheng, R.; Cai, M.; Xu, L.; Shi, R.; Huang, Z.; Whinnett, Z.I.; Huang, W. Left Bundle Branch Pacing for Cardiac Resynchronization Therapy: Nonrandomized On-Treatment Comparison With His Bundle Pacing and Biventricular Pacing. Can. J. Cardiol. 2021, 37, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Jastrzȩbski, M.; Kiełbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left Bundle Branch Area Pacing Outcomes: The Multicentre European MELOS Study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Niu, H.; Hu, Y.; Liu, X.; Zhang, N.; Cai, M.; Chen, X.; Zhou, X.; Gold, M.R.; Hua, W.; et al. Permanent His Bundle Pacing Implantation Facilitated by Visualization of the Tricuspid Valve Annulus. Circ. Arrhythm. Electrophysiol. 2020, 13, E008370. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Marcantoni, L.; Zuin, M.; Pastore, G.; Baracca, E.; Tiribello, A.; Raffagnato, P.; Boaretto, G.; Roncon, L.; Vijayaraman, P. Electrogram-Only Guided Approach to His Bundle Pacing with Minimal Fluoroscopy: A Single-Center Experience. J. Cardiovasc. Electrophysiol. 2020, 31, 805–812. [Google Scholar] [CrossRef]
- Sharma, P.S.; Huang, H.D.; Trohman, R.G.; Naperkowski, A.; Ellenbogen, K.A.; Vijayaraman, P. Low Fluoroscopy Permanent His Bundle Pacing Using Electroanatomic Mapping: A Feasibility Study. Circ. Arrhythm. Electrophysiol. 2019, 12, e006967. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Hou, X.; Wang, Y.; Qian, Z.; Li, K.; Ge, P.; Zou, J. Comparison of the Effects of Selective and Non-Selective His Bundle Pacing on Cardiac Electrical and Mechanical Synchrony. Europace 2018, 20, 1010–1017. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Tung, R. Selective versus Non-Selective His Bundle Pacing for Cardiac Resynchronization Therapy. J. Electrocardiol. 2017, 50, 191–194. [Google Scholar] [CrossRef]
- Ramos-Maqueda, J.; Alarcón, F.; Cabrera-Ramos, M. Zero Fluoroscopy Approach for Cardiac Resynchronization Therapy Using Left Bundle Branch Area Pacing. J. Interv. Card. Electrophysiol. 2022, 65, 327–328. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Moskal, P.; Bednarek, A.; Kiełbasa, G.; Kusiak, A.; Sondej, T.; Bednarski, A.; Vijayaraman, P.; Czarnecka, D. Programmed Deep Septal Stimulation: A Novel Maneuver for the Diagnosis of Left Bundle Branch Capture during Permanent Pacing. J. Cardiovasc. Electrophysiol. 2020, 31, 485–493. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Knops, R.E.; Sperzel, J.; Miller, M.A.; Petru, J.; Simon, J.; Sediva, L.; de Groot, J.R.; Tjong, F.V.Y.; Jacobson, P.; et al. Permanent Leadless Cardiac Pacing: Results of the LEADLESS Trial. Circulation 2014, 129, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Exner, D.V.; Cantillon, D.J.; Doshi, R.; Bunch, T.J.; Tomassoni, G.F.; Friedman, P.A.; Estes, N.A.M.; Ip, J.; Niazi, I.; et al. Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker. N. Engl. J. Med. 2015, 373, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; Narasimhan, C.; Steinwender, C.; Brugada, J.; Lloyd, M.; et al. A Leadless Intracardiac Transcatheter Pacing System. N. Engl. J. Med. 2016, 374, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Exner, D.V.; Doshi, R.; Tomassoni, G.; Bunch, T.J.; Estes, N.A.M.; Neužil, P.; Paulin, F.L.; Garcia Guerrero, J.J.; Cantillon, D.J. Primary Results on Safety and Efficacy From the LEADLESS II–Phase 2 Worldwide Clinical Trial. Clin. Electrophysiol. 2022, 8, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Chinitz, L.; Ritter, P.; Khelae, S.K.; Iacopino, S.; Garweg, C.; Grazia-Bongiorni, M.; Neuzil, P.; Johansen, J.B.; Mont, L.; Gonzalez, E.; et al. Accelerometer-Based Atrioventricular Synchronous Pacing with a Ventricular Leadless Pacemaker: Results from the Micra Atrioventricular Feasibility Studies. Heart Rhythm 2018, 15, 1363–1371. [Google Scholar] [CrossRef]
- Steinwender, C.; Khelae, S.K.; Garweg, C.; Chan, J.Y.S.; Ritter, P.; Johansen, J.B.; Sagi, V.; Epstein, L.M.; Piccini, J.P.; Pascual, M.; et al. Atrioventricular Synchronous Pacing Using a Leadless Ventricular Pacemaker: Results From the MARVEL 2 Study. JACC Clin. Electrophysiol. 2020, 6, 94–106. [Google Scholar] [CrossRef]
- Chinitz, L.A.; El-Chami, M.F.; Sagi, V.; Garcia, H.; Hackett, F.K.; Leal, M.; Whalen, P.; Henrikson, C.A.; Greenspon, A.J.; Sheldon, T.; et al. Ambulatory Atrioventricular Synchronous Pacing over Time Using a Leadless Ventricular Pacemaker: Primary Results from the AccelAV Study. Heart Rhythm 2022, 20, 46–54. [Google Scholar] [CrossRef]
- Neugebauer, F.; Noti, F.; van Gool, S.; Roten, L.; Baldinger, S.H.; Seiler, J.; Madaffari, A.; Servatius, H.; Ryser, A.; Tanner, H.; et al. Leadless Atrioventricular Synchronous Pacing in an Outpatient Setting: Early Lessons Learned on Factors Affecting Atrioventricular Synchrony. Heart Rhythm 2022, 19, 748–756. [Google Scholar] [CrossRef]
- Garweg, C.; Khelae, S.K.; Steinwender, C.; Chan, J.Y.S.; Ritter, P.; Johansen, J.B.; Sagi, V.; Epstein, L.M.; Piccini, J.P.; Pascual, M.; et al. Predictors of Atrial Mechanical Sensing and Atrioventricular Synchrony with a Leadless Ventricular Pacemaker: Results from the MARVEL 2 Study. Heart Rhythm 2020, 17, 2037–2045. [Google Scholar] [CrossRef]
- Ngo, L.; Nour, D.; Denman, R.A.; Walters, T.E.; Haqqani, H.M.; Woodman, R.J.; Ranasinghe, I. Safety and Efficacy of Leadless Pacemakers: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, 19212. [Google Scholar] [CrossRef]
- Garweg, C.; Vandenberk, B.; Jentjens, S.; Foulon, S.; Hermans, P.; Poels, P.; Haemers, P.; Ector, J.; Willems, R. Bacteraemia after Leadless Pacemaker Implantation. J. Cardiovasc. Electrophysiol. 2020, 31, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Johansen, J.B.; Zaidi, A.; Faerestrand, S.; Reynolds, D.; Garcia-Seara, J.; Mansourati, J.; Pasquie, J.L.; McElderry, H.T.; Roberts, P.R.; et al. Leadless Pacemaker Implant in Patients with Pre-Existing Infections: Results from the Micra Postapproval Registry. J. Cardiovasc. Electrophysiol. 2019, 30, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Koay, A.; Khelae, S.; Wei, K.K.; Muhammad, Z.; Mohd Ali, R.; Omar, R. Treating an Infected Transcatheter Pacemaker System via Percutaneous Extraction. Heart Rhythm Case Rep. 2016, 2, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Gabriels, J.K.; Soo Kim, B.; Ismail, H.; Willner, J.; Beldner, S.J.; John, R.M.; Epstein, L.M. Concomitant Leadless Pacemaker Implantation and Lead Extraction during an Active Infection. J. Cardiovasc. Electrophysiol. 2020, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Bicong, L.; Allen, J.C.; Arps, K.; Al-Khatib, S.M.; Bahnson, T.D.; Daubert, J.P.; Frazier-Mills, C.; Hegland, D.D.; Jackson, K.P.; Jackson, L.R.; et al. Leadless Pacemaker Implantation after Lead Extraction for Cardiac Implanted Electronic Device Infection. J. Cardiovasc. Electrophysiol. 2022, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Breeman, K.T.N.; Beurskens, N.E.G.; Driessen, A.H.G.; Wilde, A.A.M.; Tjong, F.V.Y.; Knops, R.E. Timing and Mid-Term Outcomes of Using Leadless Pacemakers as Replacement for Infected Cardiac Implantable Electronic Devices. J. Interv. Card. Electrophysiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Bonner, M.; Holbrook, R.; Stromberg, K.; Mayotte, J.; Molan, A.; Sohail, M.R.; Epstein, L.M. Leadless Pacemakers Reduce Risk of Device-Related Infection: Review of the Potential Mechanisms. Heart Rhythm 2020, 17, 1393–1397. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Xu, L.; Xiao, F.; Whinnett, Z.I.; Vijayaraman, P.; Su, L.; Huang, W. Feasibility and Efficacy of His Bundle Pacing or Left Bundle Pacing Combined With Atrioventricular Node Ablation in Patients With Persistent Atrial Fibrillation and Implantable Cardioverter-Defibrillator Therapy. J. Am. Heart Assoc. 2019, 8, e014253. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A. Atrioventricular Node Ablation and His Bundle Pacing. Europace 2017, 19, iv10–iv16. [Google Scholar] [CrossRef]
- Su, L.; Cai, M.; Wu, S.; Wang, S.; Xu, T.; Vijayaraman, P.; Huang, W. Long-Term Performance and Risk Factors Analysis after Permanent His-Bundle Pacing and Atrioventricular Node Ablation in Patients with Atrial Fibrillation and Heart Failure. Europace 2020, 22, II19–II26. [Google Scholar] [CrossRef]
- Jin, Q.-Q.; Zheng, C.; Wang, Y.-J.; Lin, J.-X.; Wu, D.-Z.; Lin, J.-F.; Guan, X.-Q. Feasibility of Left Bundle Branch Area Pacing Combined with Atrioventricular Node Ablation in Atrial Fibrillation Patients with Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Mathew, A.J.; Naperkowski, A.; Young, W.; Pokharel, P.; Batul, S.A.; Storm, R.; Oren, J.W.; Subzposh, F.A. Conduction System Pacing versus Conventional Pacing in Patients Undergoing Atrioventricular Node Ablation: Nonrandomized, on-Treatment Comparison. Heart Rhythm O2 2022, 3, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, M.; Mrak, M.; Mežnar, A.Z.; Žižek, D. Biventricular versus Conduction System Pacing after Atrioventricular Node Ablation in Heart Failure Patients with Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2022, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P. Extraction of Left Bundle Branch Pacing Lead. JACC Clin. Electrophysiol. 2020, 6, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Migliore, F.; Aruta, P.; Cecchetto, A.; Iliceto, S.; Gerosa, G.; Catanzariti, D. Extraction of Left Bundle Branch Pacing Lead: A Safe Procedure? Europace 2021, 23, 1921. [Google Scholar] [CrossRef]
- Ponnusamy, S.S.; Vijayaraman, P. Late Dislodgement of Left Bundle Branch Pacing Lead and Successful Extraction. J. Cardiovasc. Electrophysiol. 2021, 32, 2346–2349. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A. Extraction of the Permanent His Bundle Pacing Lead: Safety Outcomes and Feasibility of Reimplantation. Heart Rhythm 2019, 16, 1196–1203. [Google Scholar] [CrossRef]
- De Pooter, J.; Calle, S.; Timmermans, F.; Van Heuverswyn, F. Left Bundle Branch Area Pacing Using Stylet-Driven Pacing Leads with a New Delivery Sheath: A Comparison with Lumen-Less Leads. J. Cardiovasc. Electrophysiol. 2021, 32, 439–448. [Google Scholar] [CrossRef]
- Thaler, R.; Sinner, M.F.; Joghetaei, N.; Fichtner, S. Early Sudden Distal Conductor Fracture of a Stylet-Driven Lead Implanted for Left Bundle Branch Area Pacing. Heart Rhythm Case Rep. 2022, 9, 28–30. [Google Scholar] [CrossRef]
- Agudo, C.A.; Jaén, E.G.I.; Sánchez, D.J.; Urda, V.C.; Ramos, J.T.; Lozano, I.F. Extraction of a Fractured Pacemaker Lead in the Left Bundle Branch Area Using a Snare via a Femoral Approach. J. Interv. Card. Electrophysiol. 2023, 66, 239–240. [Google Scholar] [CrossRef]
- Knops, R.E.; Olde Nordkamp, L.R.A.; Delnoy, P.-P.H.M.; Boersma, L.V.A.; Kuschyk, J.; El-Chami, M.F.; Bonnemeier, H.; Behr, E.R.; Brouwer, T.F.; Kääb, S.; et al. Subcutaneous or Transvenous Defibrillator Therapy. N. Engl. J. Med. 2020, 383, 526–536. [Google Scholar] [CrossRef]
- Rordorf, R. The ATLAS Randomised Clinical Trial: What Do the Superiority Results Mean for Subcutaneous ICD Therapy and Sudden Cardiac Death Prevention as a Whole? Arrhythm. Electrophysiol. Rev. 2022, 11, 36313240. [Google Scholar] [CrossRef]
- Breeman, K.T.N.; Swackhamer, B.; Brisben, A.J.; Quast, A.F.B.E.; Carter, N.; Shuros, A.; Soltis, B.; Koop, B.E.; Burke, M.C.; Wilde, A.A.M.; et al. Long-Term Performance of a Novel Communicating Antitachycardia Pacing-Enabled Leadless Pacemaker and Subcutaneous Implantable Cardioverter-Defibrillator System: A Comprehensive Preclinical Study. Heart Rhythm 2022, 19, 837–846. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Herweg, B.; Ellenbogen, K.A.; Gajek, J. His-Optimized Cardiac Resynchronization Therapy to Maximize Electrical Resynchronization: A Feasibility Study. Circ. Arrhythm. Electrophysiol. 2019, 12, e006934. [Google Scholar] [CrossRef]
- Zweerink, A.; Zubarev, S.; Bakelants, E.; Potyagaylo, D.; Stettler, C.; Chmelevsky, M.; Lozeron, E.D.; Hachulla, A.L.; Vallée, J.P.; Burri, H. His-Optimized Cardiac Resynchronization Therapy With Ventricular Fusion Pacing for Electrical Resynchronization in Heart Failure. JACC Clin. Electrophysiol. 2021, 7, 881–892. [Google Scholar] [CrossRef]
- Vijayaraman, P. Left Bundle Branch Pacing Optimized Cardiac Resynchronization Therapy: A Novel Approach. JACC Clin. Electrophysiol. 2021, 7, 1076–1078. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Moskal, P.; Huybrechts, W.; Curila, K.; Sreekumar, P.; Rademakers, L.M.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.S.; Bednarek, A.; et al. Left Bundle Branch-Optimized Cardiac Resynchronization Therapy (LOT-CRT): Results from an International LBBAP Collaborative Study Group. Heart Rhythm 2022, 19, 13–21. [Google Scholar] [CrossRef]
- Elliott, M.K.; Vergara, P.; Wijesuriya, N.; Mehta, V.S.; Bosco, P.; Jacon, P.; Lee, M.; Taloni, S.; Niederer, S.; Alison, J.; et al. Feasibility of Leadless Left Ventricular Septal Pacing with the WiSE-CRT System to Target the Left Bundle Branch Area: A Porcine Model and Multicenter Patient Experience. Heart Rhythm 2022, 19, 1974–1983. [Google Scholar] [CrossRef]
- Okabe, T.; Hummel, J.D.; Bank, A.J.; Niazi, I.K.; McGrew, F.A.; Kindsvater, S.; Oza, S.R.; Scherschel, J.A.; Walsh, M.N.; Singh, J.P. Leadless Left Ventricular Stimulation with WiSE-CRT System-Initial Experience and Results from Phase I of SOLVE-CRT Study (Nonrandomized, Roll-in Phase). Heart Rhythm 2022, 19, 22–29. [Google Scholar] [CrossRef]
- Drastic Falls in Cost Are Powering Another Computer Revolution | The Economist. Available online: https://www.economist.com/technology-quarterly/2019/09/12/drastic-falls-in-cost-are-powering-another-computer-revolution (accessed on 5 April 2023).
- Na, J.S.; Sokolow, M.; Childress, J.; Han, P.; Patel, S.; Rottman, J. Recent Temporal Trends in Hospital Costs for Non-Surgical Patients Receiving Implantable Cardioverter Defibrillators. J. Interv. Card. Electrophysiol. 2022, 63, 231–237. [Google Scholar] [CrossRef]
- Rück, A.; Saleh, N.; Glaser, N. Outcomes Following Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: SWEDEHEART Observational Study. JACC Cardiovasc. Interv. 2021, 14, 2173–2181. [Google Scholar] [CrossRef]
- Levack, M.M.; Kapadia, S.R.; Soltesz, E.G.; Gillinov, A.M.; Houghtaling, P.L.; Navia, J.L.; Krishnaswamy, A.; Blackstone, E.H.; Svensson, L.G.; Mick, S.L. Prevalence of and Risk Factors for Permanent Pacemaker Implantation After Aortic Valve Replacement. Ann. Thorac. Surg. 2019, 108, 700–707. [Google Scholar] [CrossRef]


| Complication | Incidence |
|---|---|
| Mortality (procedure-related) | <0.1% |
| Pneumothorax | 0.4–2.8% |
| Perforation | 0.1–1.5% |
| Cardiac tamponade | 0.5–1.5% |
| Pocket hematoma | 0.2–16.0% |
| Infection | 0.6–3.4% |
| Lead dislodgement | 1.2–3.3% |
| Other | <0.5% |
| Algorithm | Manufacturer | Optimization | Mode | Programmable? | Dynamic? | Safety Endpoint Met? | Trial Efficacy Endpoint Met? * |
|---|---|---|---|---|---|---|---|
| AdaptivCRT [63] | Medtronic | AV, VV | IEGM | No | Yes (1/min) | Yes | Non-inferior |
| QuickOpt [64] | Abbott | AV, VV | IEGM | Yes | No | Yes | Non-inferior |
| SmartDelay [65] | Boston | AV | IEGM | No | No | Yes | Equivalent |
| SonR [66] | Sorin | AV, VV | Hemodynamic sensor | No | Yes (1/week) | Yes | Non-inferior |
| SyncAV [67] | Abbott | AV | IEGM | Yes | Yes (1/256 beats) | NR | NR |
| AutoAdapt † | Biotronik | AV, VV | IEGM | NR | Yes (1/min) | NR | NR |
| Trial | Year | Device | N | Mean Age | % Female | Follow-up | Primary Outcome | Implant Success Rate, n/N (%) | Complication Rate | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| LEADLESS [121] | 2014 | NanoStim | 33 | 76.5 ± 8.4 | 33 | 3 m | 31/33 (94%) * | 32/33 (97%) | 3% (1 perforation requiring surgery; died of stroke) | 5 patients require > 1 LP during procedure |
| LEADLESS II [122] | 2015 | NanoStim | 526 | 75.8 ± 12.1 | 38.2 | 6 m | 270/300 (90.0%) † | 504/526 (95.8%) | 6.7% (22 events in 20 patients) | 1.7% dislodgements 1.3% tamponade 1.3% elevated thresholds |
| Micra IDE [123] | 2016 | Micra | 725 | 75.9 ± 10.9 | 41.2 | 6 m | 292/297 (98.3%) ‡ | 719/725 (99.2%) | 4.0% (28 events in 25 patients) | 1.6% perforation/effusion |
| LEADLESS II-Phase 2 [124] | 2022 | Aveir | 200 | 75.6 ± 11.3 | 37.5 | 6 w | 188/196 (95.9%) ¶ | 196/200 (98%) | 4.0% (9 events in 8 patients) | 1.5% tamponade |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballantyne, B.A.; Chew, D.S.; Vandenberk, B. Paradigm Shifts in Cardiac Pacing: Where Have We Been and What Lies Ahead? J. Clin. Med. 2023, 12, 2938. https://doi.org/10.3390/jcm12082938
Ballantyne BA, Chew DS, Vandenberk B. Paradigm Shifts in Cardiac Pacing: Where Have We Been and What Lies Ahead? Journal of Clinical Medicine. 2023; 12(8):2938. https://doi.org/10.3390/jcm12082938
Chicago/Turabian StyleBallantyne, Brennan A., Derek S. Chew, and Bert Vandenberk. 2023. "Paradigm Shifts in Cardiac Pacing: Where Have We Been and What Lies Ahead?" Journal of Clinical Medicine 12, no. 8: 2938. https://doi.org/10.3390/jcm12082938
APA StyleBallantyne, B. A., Chew, D. S., & Vandenberk, B. (2023). Paradigm Shifts in Cardiac Pacing: Where Have We Been and What Lies Ahead? Journal of Clinical Medicine, 12(8), 2938. https://doi.org/10.3390/jcm12082938






